-
The pH-sensitive organic electrochemical transistors are expected to be widely used in wearable electronic devices for in-situ physiological monitoring. However, the unclear current-voltage relationship seriously hinders it from developing in design, optimization, and application. In the present work, the current-voltage characteristic of pH-sensitive organic electrochemical transistor is investigated by combining the electrochemical equilibrium equation with the series model of differential capacitances formed at gate electrode/electrolyte and semiconductor channel/electrolyte interface. Moreover, a pH-sensitive organic electrochemical transistor is constructed by using poly (3,4-ethylenedioxythiophene)/polystyrene sulfonate as the semiconductor layer material and modifying the gate electrode with pH-sensitive polymer (poly (3,4-ethylenedioxythiophene)/bromothymol blue). The effectiveness of the theoretical model is verified by investigating the output, transfer, and pH response characteristics of the pH-sensitive organic electrochemical transistor. The experimental results show that the detection sensitivity can reach up to 0.22 mA·pH·unit–1, and the pH response is gate-bias dependent. Then, a polynomial indicating the gate bias effect is introduced to modify the current-voltage characteristic equation. The goodness of fitting the theoretical model to the experimental results of transfer curves is found to be 0.998. The comparison between experimental and theoretical results of the gate bias corresponding to the peak transconductance and pH sensitivity responding to gate bias can also verify the effectiveness of the modified theoretical model. The results can provide theoretical support for the design and manufacture of pH-sensitive organic electrochemical transistors based flexible biosensors.
[1] Rivnay J, Inal S, Salleo A, Owens R M, Berggren M, Malliaras G G 2018 Nat. Rev. Mater. 3 1
 Google Scholar
Google Scholar
[2] Donahue M J, Williamson A, Strakosas X, Friedlein J T, McLeod R R, Gleskova H, Malliaras G G 2018 Adv. Mater. 30 1705031
 Google Scholar
Google Scholar
[3] Liang Y, Wu C, Figueroa-Miranda G, Offenhaeusser A, Mayer D 2019 Biosens. Bioelectron. 144 111668
 Google Scholar
Google Scholar
[4] Guo K, Wustoni S, Koklu A, Díaz-Galicia E, Moser M, Hama A, Alqahtani A A, Ahmad A N, Alhamlan F S, Shuaib M, Pain A, McCulloch I, Arold S T, Grünberg R, Inal S 2021 Nat. Biomed. Eng. 5 666
 Google Scholar
Google Scholar
[5] White K A, Grillo-Hill B K, Barber D L 2017 J. Cell Sci. 130 663
 Google Scholar
Google Scholar
[6] Mariani F, Gualandi I, Tessarolo M, Fraboni B, Scavetta E 2018 Nat. Rev. Mater. 10 22474
[7] Scheiblin G, Coppard R, Owens R M, Mailley P, Malliaras G G 2017 Adv. Mater. Technol. 2 1600141
 Google Scholar
Google Scholar
[8] Demuru S, Kunnel B P, Briand D 2020 Adv. Mater. Technol. 5 2000328
 Google Scholar
Google Scholar
[9] Demuru S, Kunnel B P, Briand D 2021 Biosens. Bioelectron. X 7 100065
[10] Mariani F, Gualandi I, Tonelli D, Decataldo F, Possanzini L, Fraboni B, Scavetta E 2020 Electrochem. Commun. 116 106763
 Google Scholar
Google Scholar
[11] Bernards D A, Malliaras G G 2007 Adv. Funct. Mater. 17 3538
 Google Scholar
Google Scholar
[12] Ji J L, Li M M, Chen Z W, Wang H W, Jiang X N, Zhuo K, Liu Y, Yang X, Gu Z, Sang S B, Shu Y 2019 Nano Res. 12 1943
 Google Scholar
Google Scholar
[13] Moser M, Hidalgo T C, Surgailis J, Gladisch J, Ghosh S, Sheelamanthula R, Thiburce Q, Giovannitti A, Salleo A, Gasparini N, Wadsworth A, Zozoulenko I, Berggren M, Stavrinidou E, Inal S, McCulloch I 2020 Adv. Mater. 32 2002748
 Google Scholar
Google Scholar
[14] Wu X, Surendran A, Ko J, Filonik O, Herzig E M, Müller-Buschbaum P, Leong W L 2019 Adv. Mater. 31 1805544
 Google Scholar
Google Scholar
[15] Bidinger S L, Han S, Malliaras G G, Hasan T 2022 Appl. Phys. Lett. 120 073302
 Google Scholar
Google Scholar
[16] He Y, Kukhta N A, Marks A, Luscombe C K 2022 J. Mater. Chem. C 10 2314
 Google Scholar
Google Scholar
[17] Kim S H, Hong K, Xie W, Lee K H, Zhang S, Lodge T P, Frisbie C D 2013 Adv. Mater. 25 1822
 Google Scholar
Google Scholar
[18] Yan Y, Chen Q, Wu X, Wang X, Li E, Ke Y, Liu Y, Chen H, Guo T 2020 ACS Appl. Mater. Interfaces 12 49915
 Google Scholar
Google Scholar
[19] Rivnay J, Leleux P, Sessolo M, Khodagholy D, Hervé T, Fiocchi M, Malliaras G G 2013 Adv. Mater. 25 7010
 Google Scholar
Google Scholar
-
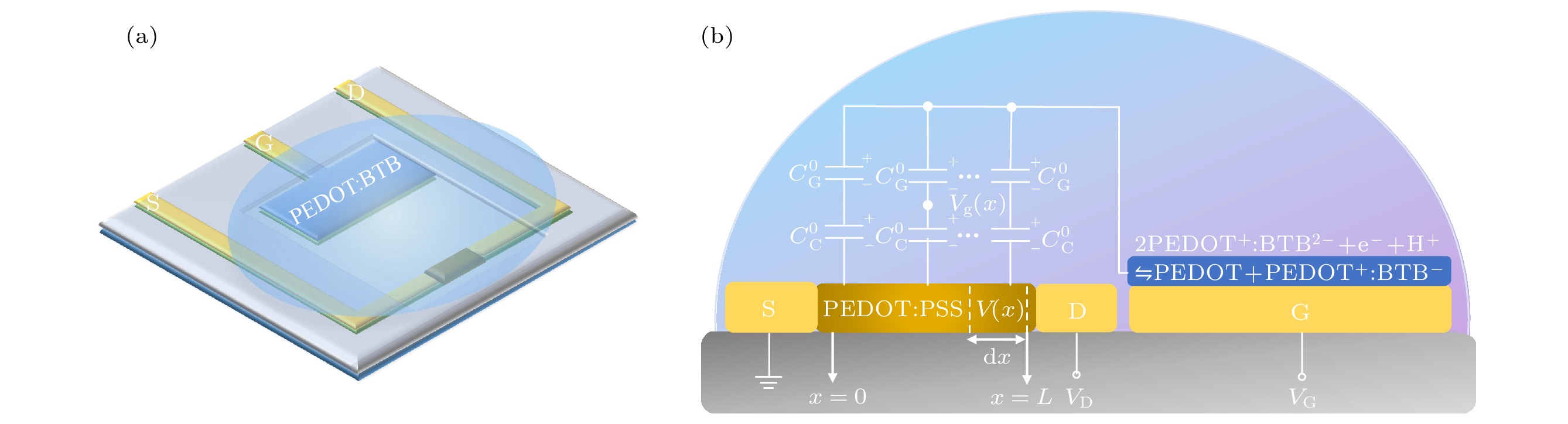
图 1 有机电化学晶体管(OECT) (a)结构制备示意图, 其中S, D, G分别表示OECT的源极、漏极与栅极; (b)OECT半导体沟道的原子力显微镜测试图; (c)修饰有PEDOT:BTB膜的OECT栅极激光共聚焦扫描显微镜测试图
Figure 1. The organic electrochemical transistors (OECTs): (a) Schematic of structure preparation, S, D, and G represents the source, drain, and gate, respectively; (b) the atomic force microscope image of the semiconductor channel; (c) the laser confocal scanning microscope image of the gate electrode modified with PEDOT:BTB films.
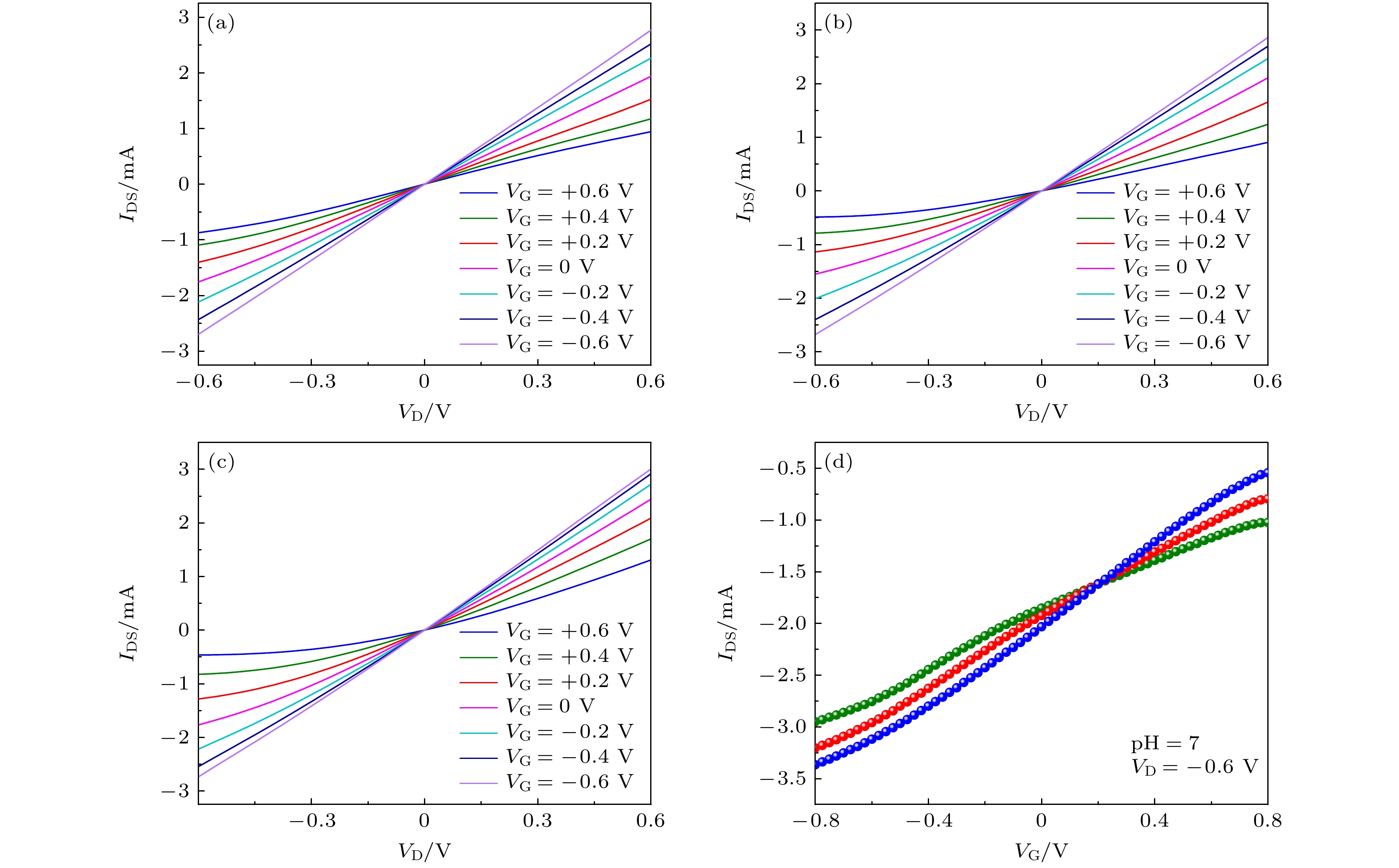
图 3 使用 (a) 5次、(b) 10次、(c) 20次循环伏安法(CV)电沉积制备PEDOT:BTB膜, 并以此修饰栅极获得的晶体管输出曲线与(d)转移曲线, 其中绿色、红色、蓝色转移曲线分别对应于图(a)、图(b)与图(c)
Figure 3. Output curves of OECTs, PEDOT:BTB film modifying the gate electrode is obtained by (a) 5 cycles, (b) 10 cycles, and (c) 20 cycles of CV electrodepositions; (d) transfer curves of OECTs, in which the green, red, and blue curves correspond to (a), (b) and (c), respectively.
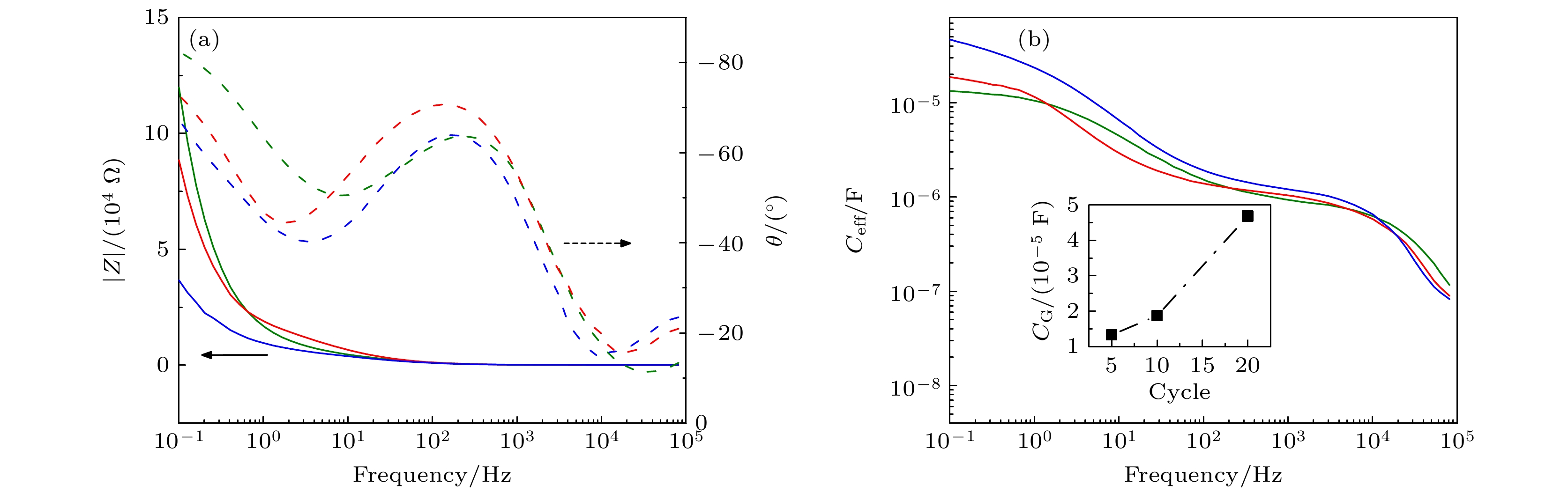
图 4 栅极/电解质界面电容(CG)与CV次数关系 (a)电化学阻抗谱(Electrochemical impedance spectroscopy, EIS)波特图; (b)由EIS获得的CG等效电容(Ceff), 插图用10–1 Hz处Ceff表示CG, 其值随CV次数增大而增大. 绿色, 红色、蓝色曲线分别表示5, 10, 20次CV循环的测试结果
Figure 4. Relationship between gate/electrolyte interface capacitance (CG) and CV times: (a) The Bode electrochemical impedance spectroscopy (EIS); (b) the equivalent capacitance of CG (Ceff). The inset shows that CG, indicated by Ceff at 10–1 Hz, increases with CV cycles. The green, red and blue curves represent the experimental results using 5, 10, and 20 CV cycles.
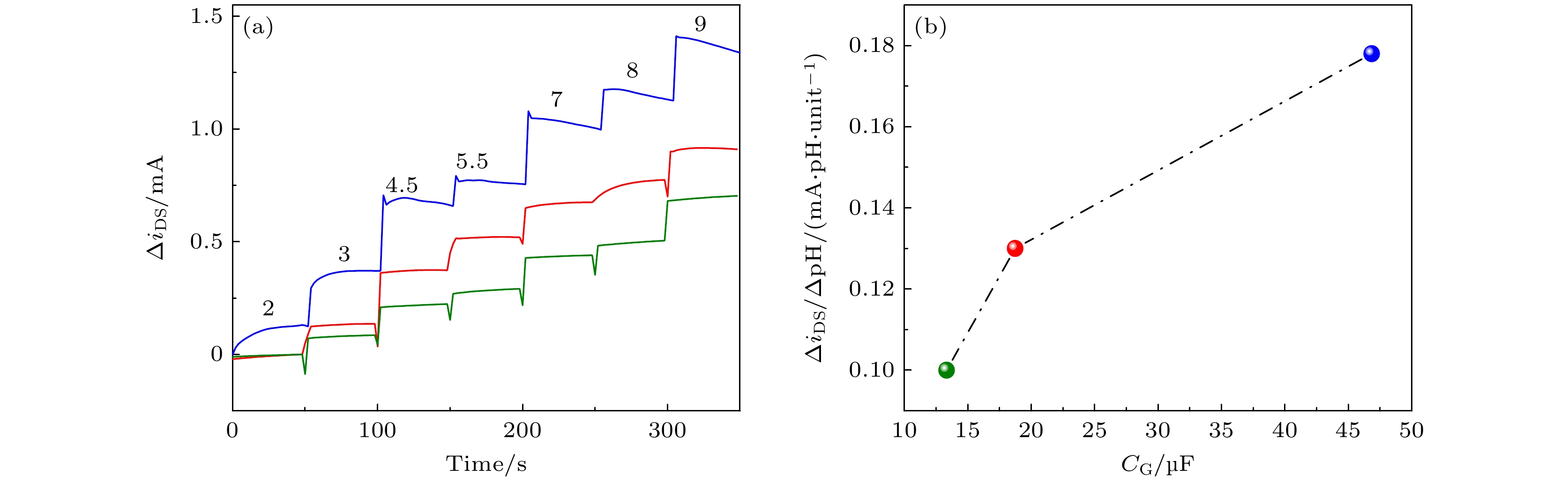
图 5 晶体管pH响应与CG的关系 (a)ΔiDS是基于pH = 2的源漏极瞬态电流(iDS)归一化的pH响应; (b)ΔiDS/ΔpH随着CG的增大而增大. 图中绿色, 红色、蓝色数据分别表示采用5, 10, 20次CV循环的测试结果
Figure 5. The relationship between the pH sensitivity and CV cycle numbers: (a) ΔiDS represents the pH response normalized by the source-drain transient current (iDS) obtained at pH = 2; (b) ΔiDS/ΔpH increases with the CG increment. The green, red and blue data represent the experimental results using 5, 10, and 20 CV cycles, respectively.
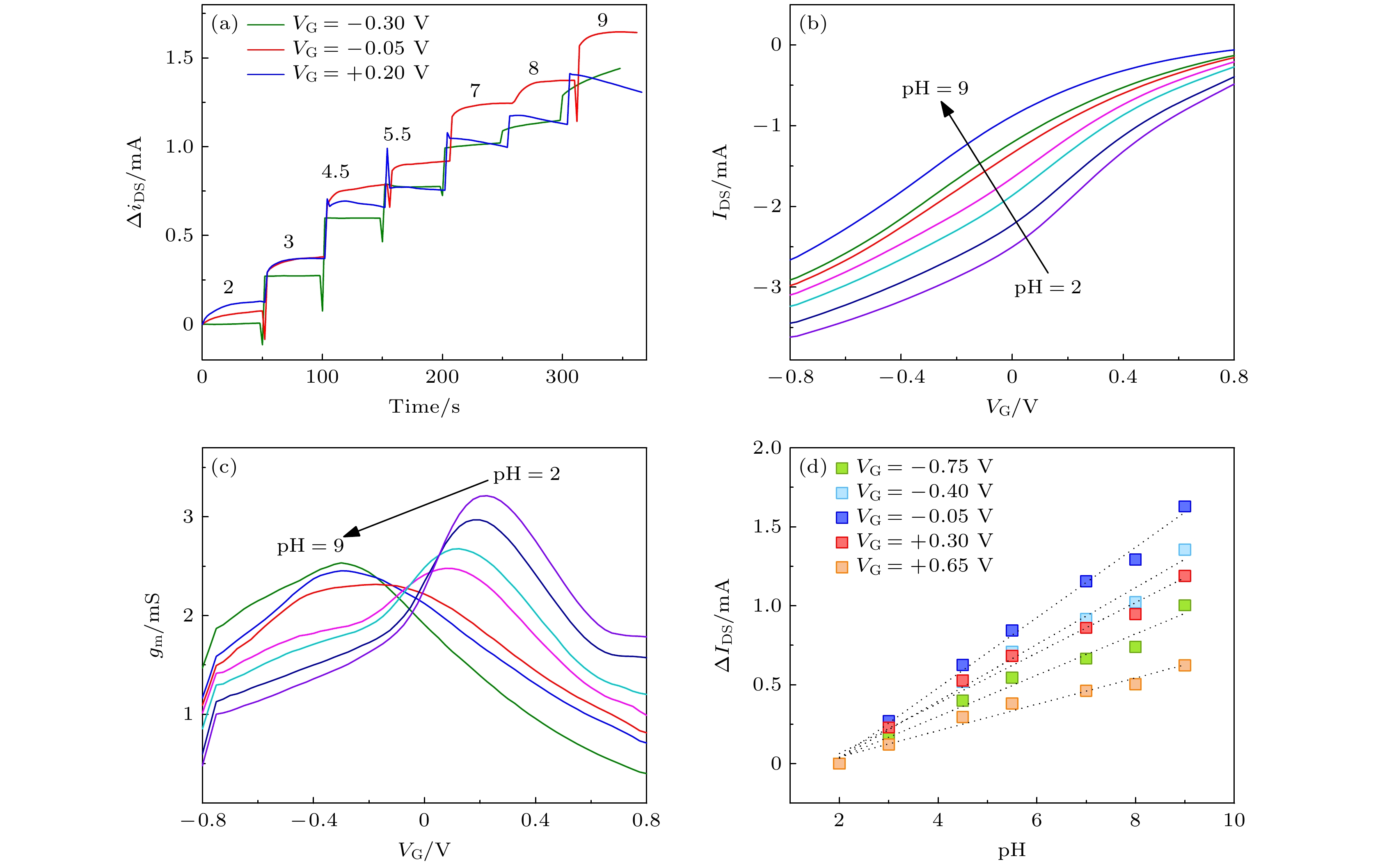
图 6 OECT对于pH值的响应 (a)不同VG条件下OECT瞬态响应电流iDS对于pH的响应, ΔiDS由任意pH条件下的iDS与pH = 2的值归一化所得; (b)不同pH条件下晶体管的转移曲线; (c)不同pH条件下晶体管的跨导曲线; (d)不同VG条件下OECT稳态响应电流IDS对于pH的响应, ΔIDS由任意pH条件下的IDS与pH = 2时的值归一化所得
Figure 6. The response of OECT to the ambient pH: (a) the effect of VG on OECTs transient response (iDS), ΔiDS is obtained by normalizing iDS under specific pH level and the value at pH = 2; (b) OECTs transfer curves under different pH levels; (c) OECTs transconductance curves under different pH levels; (d) the effect of VG on OECTs steady-state response (IDS), ΔIDS is obtained by normalizing IDS under specific pH level and the value at pH = 2.
图 7 实验结果与拟合数据比较 (a) OECT转移曲线; (b)由拟合转移曲线获得的跨导曲线; (c)不同pH条件下的峰值跨导对应的栅极电压, 蓝色与红色分别代表实验与拟合结果; (d) VG对于晶体管pH响应的影响, 蓝色与红色分别代表实验与拟合结果
Figure 7. Comparisons of experimental and fitting results: (a) Transfer curves; (b) transconductance curves derived by the fitting transfer curves; (c) the gate bias corresponding to the peak transconductance under different pH levels; (d) the effect of VG on the pH response of transistors. The blue and red data in (c) and (d) represent the experimental and fitting results, respectively.
表 1 不同pH敏感OECT的性能对比
Table 1. Performance comparison of pH sensitive OECTs
-
[1] Rivnay J, Inal S, Salleo A, Owens R M, Berggren M, Malliaras G G 2018 Nat. Rev. Mater. 3 1
 Google Scholar
Google Scholar
[2] Donahue M J, Williamson A, Strakosas X, Friedlein J T, McLeod R R, Gleskova H, Malliaras G G 2018 Adv. Mater. 30 1705031
 Google Scholar
Google Scholar
[3] Liang Y, Wu C, Figueroa-Miranda G, Offenhaeusser A, Mayer D 2019 Biosens. Bioelectron. 144 111668
 Google Scholar
Google Scholar
[4] Guo K, Wustoni S, Koklu A, Díaz-Galicia E, Moser M, Hama A, Alqahtani A A, Ahmad A N, Alhamlan F S, Shuaib M, Pain A, McCulloch I, Arold S T, Grünberg R, Inal S 2021 Nat. Biomed. Eng. 5 666
 Google Scholar
Google Scholar
[5] White K A, Grillo-Hill B K, Barber D L 2017 J. Cell Sci. 130 663
 Google Scholar
Google Scholar
[6] Mariani F, Gualandi I, Tessarolo M, Fraboni B, Scavetta E 2018 Nat. Rev. Mater. 10 22474
[7] Scheiblin G, Coppard R, Owens R M, Mailley P, Malliaras G G 2017 Adv. Mater. Technol. 2 1600141
 Google Scholar
Google Scholar
[8] Demuru S, Kunnel B P, Briand D 2020 Adv. Mater. Technol. 5 2000328
 Google Scholar
Google Scholar
[9] Demuru S, Kunnel B P, Briand D 2021 Biosens. Bioelectron. X 7 100065
[10] Mariani F, Gualandi I, Tonelli D, Decataldo F, Possanzini L, Fraboni B, Scavetta E 2020 Electrochem. Commun. 116 106763
 Google Scholar
Google Scholar
[11] Bernards D A, Malliaras G G 2007 Adv. Funct. Mater. 17 3538
 Google Scholar
Google Scholar
[12] Ji J L, Li M M, Chen Z W, Wang H W, Jiang X N, Zhuo K, Liu Y, Yang X, Gu Z, Sang S B, Shu Y 2019 Nano Res. 12 1943
 Google Scholar
Google Scholar
[13] Moser M, Hidalgo T C, Surgailis J, Gladisch J, Ghosh S, Sheelamanthula R, Thiburce Q, Giovannitti A, Salleo A, Gasparini N, Wadsworth A, Zozoulenko I, Berggren M, Stavrinidou E, Inal S, McCulloch I 2020 Adv. Mater. 32 2002748
 Google Scholar
Google Scholar
[14] Wu X, Surendran A, Ko J, Filonik O, Herzig E M, Müller-Buschbaum P, Leong W L 2019 Adv. Mater. 31 1805544
 Google Scholar
Google Scholar
[15] Bidinger S L, Han S, Malliaras G G, Hasan T 2022 Appl. Phys. Lett. 120 073302
 Google Scholar
Google Scholar
[16] He Y, Kukhta N A, Marks A, Luscombe C K 2022 J. Mater. Chem. C 10 2314
 Google Scholar
Google Scholar
[17] Kim S H, Hong K, Xie W, Lee K H, Zhang S, Lodge T P, Frisbie C D 2013 Adv. Mater. 25 1822
 Google Scholar
Google Scholar
[18] Yan Y, Chen Q, Wu X, Wang X, Li E, Ke Y, Liu Y, Chen H, Guo T 2020 ACS Appl. Mater. Interfaces 12 49915
 Google Scholar
Google Scholar
[19] Rivnay J, Leleux P, Sessolo M, Khodagholy D, Hervé T, Fiocchi M, Malliaras G G 2013 Adv. Mater. 25 7010
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 9185
- PDF Downloads: 117
- Cited By: 0















 DownLoad:
DownLoad: