-
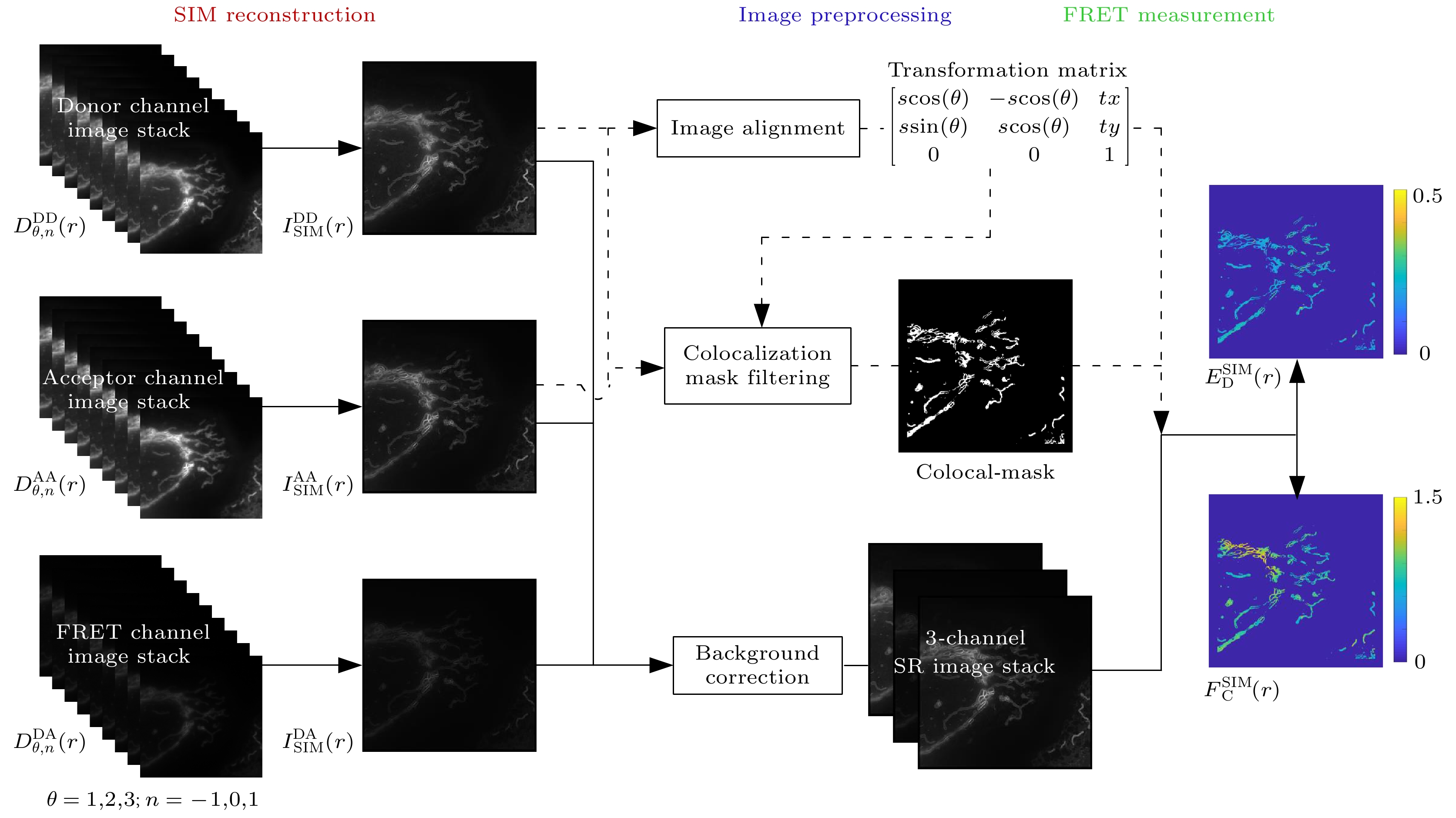
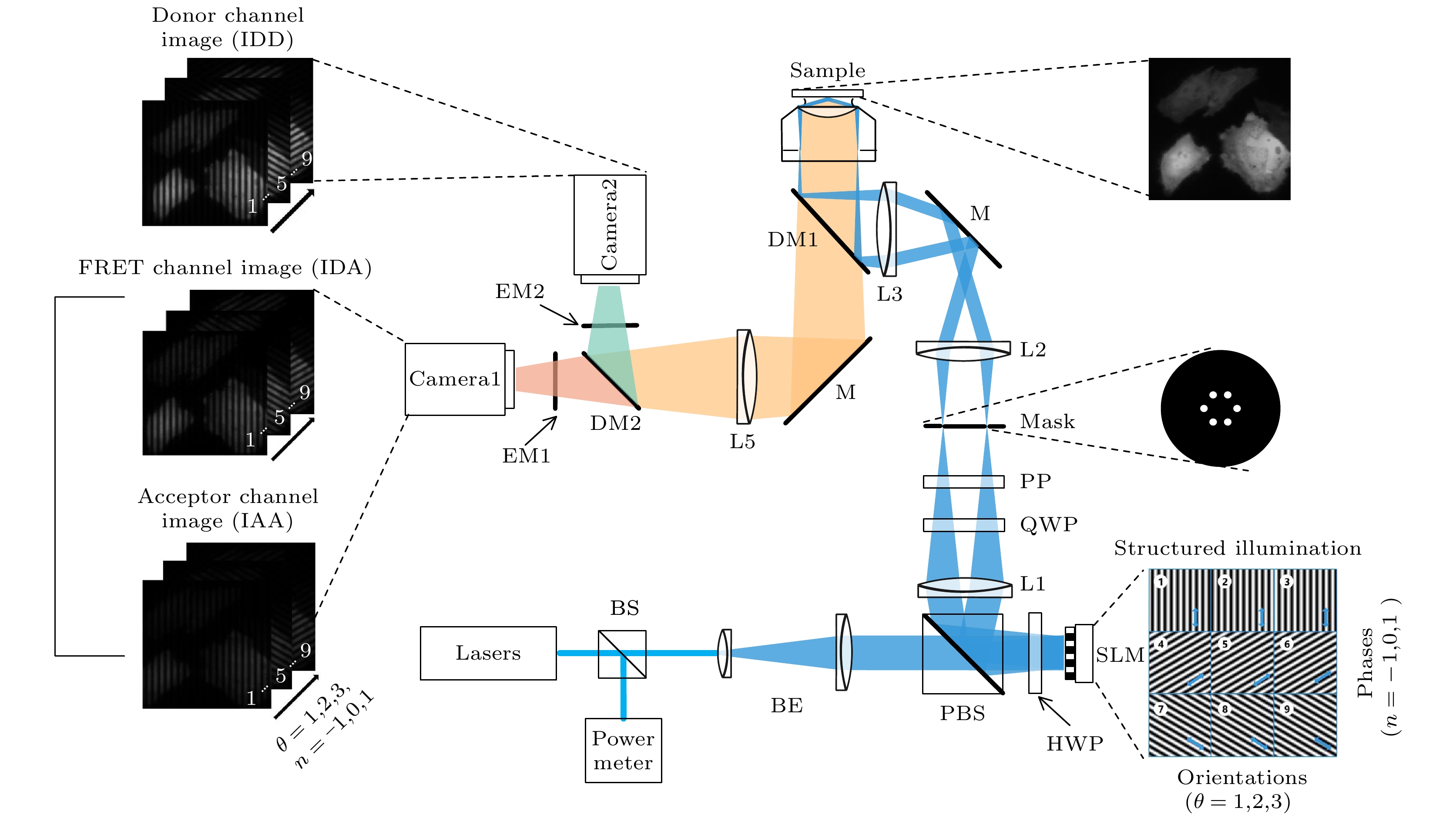
The Structured illumination (SI)-based super resolution fluorescence resonance energy transfer (SR-FRET) imaging technique, known as SISR-FRET, enables the investigation of molecular structures and functions in cellular organelles by resolving sub-diffraction FRET signals within living cells. The FRET microscopy offers unique advantages for quantitatively detecting dynamic interactions and spatial distribution of biomolecules within living cells. The spatial resolution of conventional FRET microscopy is limited by the diffraction limit, and it can only capture the average behavior of these events within the resolution limits of conventional fluorescence microscopy. The SISR-FRET performs sequential linear reconstruction of the three-channel SIM images followed by FRET quantitative analysis by using a common localization mask-based filtering approach. This two-step process ensures the fidelity of the reconstructed SR-FRET signals while effectively removing false-positive FRET signals caused by SIM artifacts. However, the slow imaging speed resulting from the switching of excitation-emission channels in SISR-FRET imaging limits its application in fast imaging scenarios. To address this issue, this study proposes a dual-channel structured illumination super-resolution quantitative FRET imaging system and method. By incorporating an FRET dual-channel imaging and registration module into the imaging pathway, the spatial switching and channel multiplexing of the SISR-FRET excitation-emission channels are achieved. Combining the image reconstruction algorithm with channel sub-pixel registration correction, the dual-channel SISR-FRET technique enhances the temporal resolution by 3.5 times while preserving the quantitative super-resolution FRET analysis. Experimental results are obtained by using a multi-color SIM system to perform super-resolution imaging of living cells expressing mitochondria outer membrane FRET standard plasmids. These experiments validate the improved spatial and temporal resolution of dual-channel SISR-FRET and the fidelity of FRET quantification analysis. In summary, this research presents a novel dual-channel structured illumination super-resolution FRET imaging system and method. It overcomes the limitations of slow imaging speed in SISR-FRET by realizing the spatial switching and channel multiplexing of excitation-emission channels. The proposed technique enhances the temporal resolution while maintaining quantitative analysis of super-resolution FRET. Experimental validation demonstrates the increased spatial and temporal resolution of dual-channel SISR-FRET and the accuracy of FRET quantification analysis. This advancement contributes to the study of molecular structures and functions in cellular organelles, providing valuable insights into the intricate mechanisms of living cells.
-
Keywords:
- fluorescence resonance energy transfer /
- structured illumination /
- super-resolution /
- living cells
[1] Rao V S, Srinivas K, Sujini G N, Kumar G N S 2014 Int. J. Proteomics 2014 1
[2] Acuner Ozbabacan S E, Engin H B, Gursoy A, Keskin O 2011 Protein Eng. Des. Sel. 24 635
 Google Scholar
Google Scholar
[3] Xing S, Wallmeroth N, Berendzen K W, Grefen C 2016 Plant Physiol. 171 727
[4] Algar W R, Hildebrandt N, Vogel S S, Medintz I L 2019 Nat. Methods 16 815
 Google Scholar
Google Scholar
[5] Jares-Erijman E A, Jovin T M 2003 Nat. Biotechnol. 21 1387
 Google Scholar
Google Scholar
[6] Ben-Johny M, Yue D N, Yue D T 2016 Nat. Commun. 7 13709
 Google Scholar
Google Scholar
[7] Chen H C, Sun B N, Sun H, Xu L J, Wu G H, Tu Z, Cheng X C, Fan X H, Mai Z H, Tang Q L, Wang X P, Chen T S 2021 Cell Death Discov. 7 363
 Google Scholar
Google Scholar
[8] Sun B N, Chen H C, Wang X P, Chen T S 2023 Cell Death Discov. 9 37
[9] Yang F F, Qu W F, Du M Y, Mai Z Y, Wang B, Ma Y Y, Wang X P, Chen T S 2020 Cell. Mol. Life Sci. 77 2387
 Google Scholar
Google Scholar
[10] Szalai A M, Zaza C, Stefani F D 2021 Nanoscale 13 18421
 Google Scholar
Google Scholar
[11] Szabó Á, Szendi-Szatmári T, Szöllősi J, Nagy P 2020 Methods Appl. Fluoresc. 8 032003
 Google Scholar
Google Scholar
[12] Grecco H E, Verveer P J 2011 ChemPhysChem 12 484
 Google Scholar
Google Scholar
[13] Deußner-Helfmann N S, Auer A, Strauss M T, Malkusch S, Dietz M S, Barth H D, Jungmann R, Heilemann M 2018 Nano Lett. 18 4626
 Google Scholar
Google Scholar
[14] Tardif C, Nadeau G, Labrecque S, Côté D, Lavoie-Cardinal F 2019 Neurophotonics 6 1
[15] Szalai A M, Siarry B, Lukin J, Giusti S, Unsain N, Cáceres A, Steiner F, Tinnefeld P, Refojo D, Jovin T M, Stefani F D 2021 Nano Lett. 21 2296
 Google Scholar
Google Scholar
[16] Liu Z, Luo Z W, Chen H C, Yin A, Sun H, Zhuang Z F, Chen T S 2022 Cytom. Part A 101 264
 Google Scholar
Google Scholar
[17] Zhao T Y, Wang Z J, Cai Y N, Liang Y S, Wang SW, Zhang J X, Chen T S, Lei M 2023 Opt. Lasers Eng. 167 107606
 Google Scholar
Google Scholar
[18] Zhao W S, Zhao S Q, Li L J, et al. 2022 Nat. Biotechnol. 40 606
 Google Scholar
Google Scholar
[19] Huang X S, Fan J C, Li L J, Liu H S, Wu R L, Wu Y, Wei L S, Mao H, Lal A, Xi P, Tang L Q, Zhang Y F, Liu Y M, Tan S, Chen L Y 2018 Nat. Biotechnol. 36 451
 Google Scholar
Google Scholar
[20] Li D, Shao L, Chen B C, Zhang X, Zhang M, Moses B, Milkie D E, Beach J R, Hammer J A, Pasham M, Kirchhausen T, Baird M A, Davidson M W, Xu P, Betzig E 2015 Science 349 aab3500
 Google Scholar
Google Scholar
[21] Kner P, Chhun B B, Griffis E R, Winoto L, Gustafsson M G L 2009 Nat. Methods 6 339
 Google Scholar
Google Scholar
[22] Wen G, Li S M, Wang L B, et al. 2021 Light Sci. Appl. 10 70
 Google Scholar
Google Scholar
[23] Luo Z W, Wu G, Kong M, Chen Z, Zhuang Z F, Fan J C, Chen T S 2023 Photonics Res. 11 887
 Google Scholar
Google Scholar
[24] Fan J C, Huang X S, Li L, Tan S, Chen L 2019 Biophys. Rep. 5 80
 Google Scholar
Google Scholar
[25] Sun H, Zhang C, Ma Y, Du M, Chen T S 2019 Biomed. Signal Process. Control 53 101585
 Google Scholar
Google Scholar
-
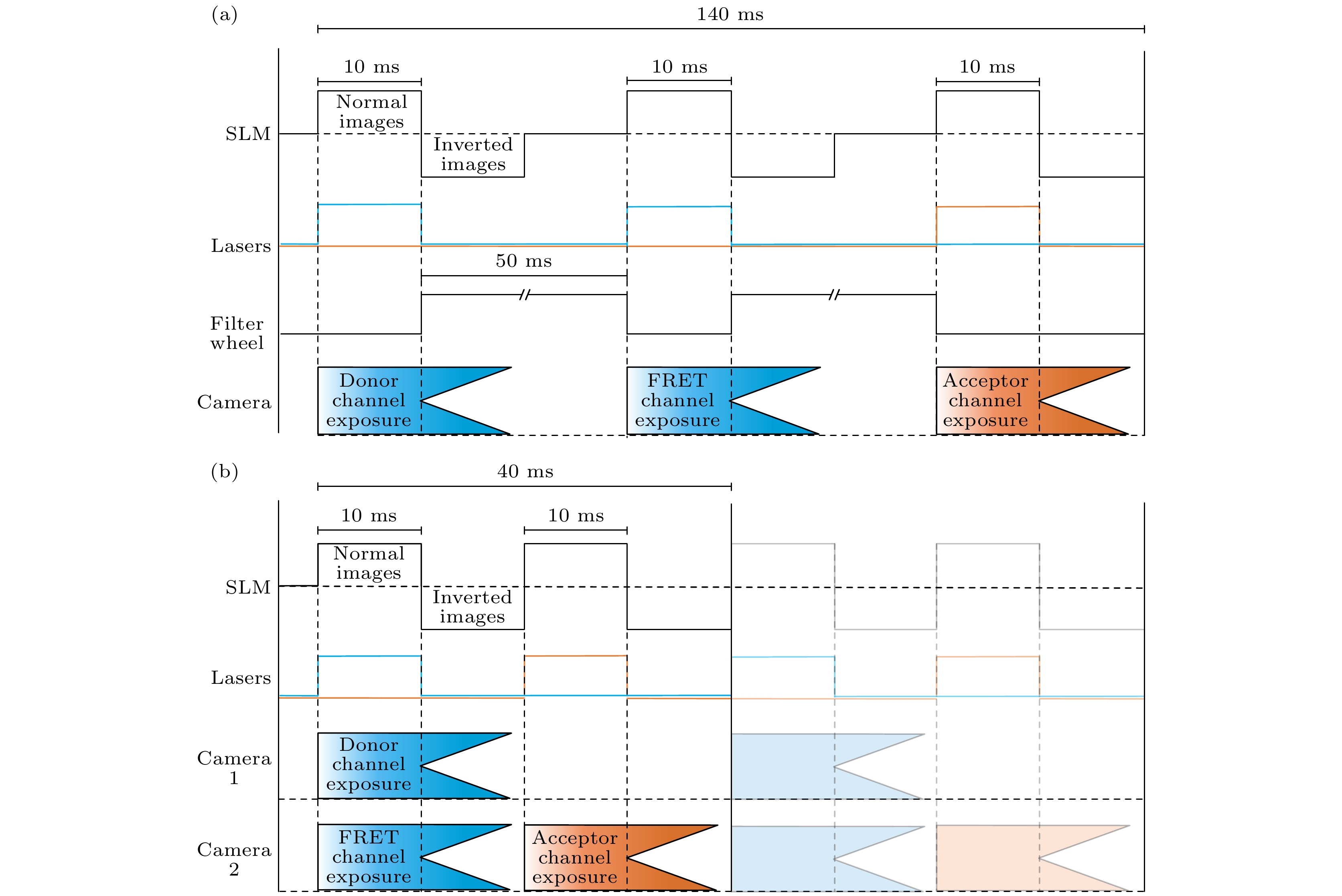
图 3 双通道SISR-FRET与单通道SISR-FRET时序图对比 (a)单通道SISR-FRET在一个FRET采集周期的时序图; (b)双通道SISR-FRET相同周期的时序图
Figure 3. Comparison of timing sequence of dual-channel SISR-FRET and single-channel SISR-FRET: (a) Timing sequence of single-channel SISR-FRET in one FRET acquisition cycle; (b) timing sequence of dual-channel SISR-FRET in the same cycle.
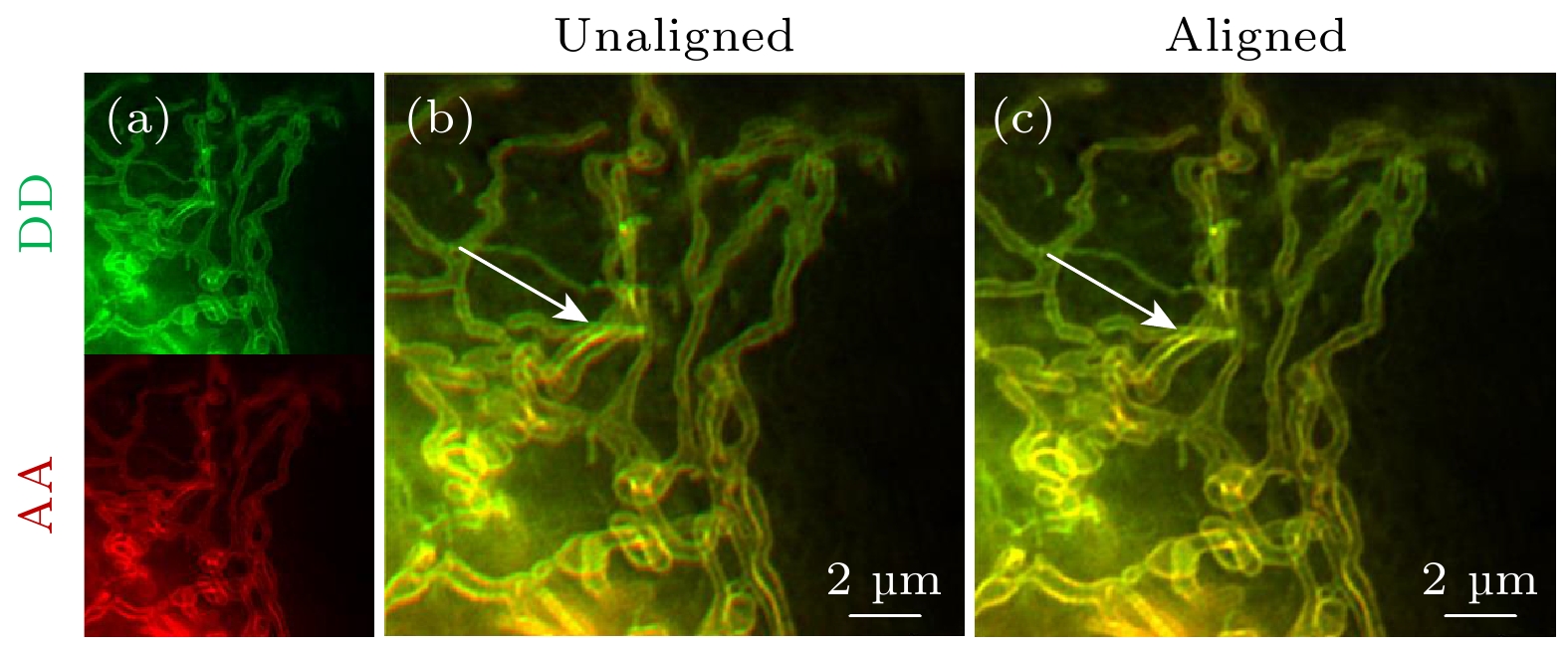
图 4 双通道SISR-FRET通道对准效果 (a) DD通道和AA通道成像结果的伪彩图, 绿色为DD通道, 红色为AA通道;(b)未经过算法对准的DD通道和AA通道成像结果叠加图; (c)经过仿射变换矩阵对准的DD通道和AA通道成像结果叠加图. 比例尺: 2 μm
Figure 4. Dual-channel SISR-FRET alignment results: (a) Pseudo-color image of DD channel and AA channel imaging results, green is DD channel, red is AA channel; (b) overlay of the imaging results of DD channel and AA channel without algorithm alignment; (c) overlay of DD channel and AA channel imaging results after affine transformation matrix alignment. Scale bar: 2 μm.
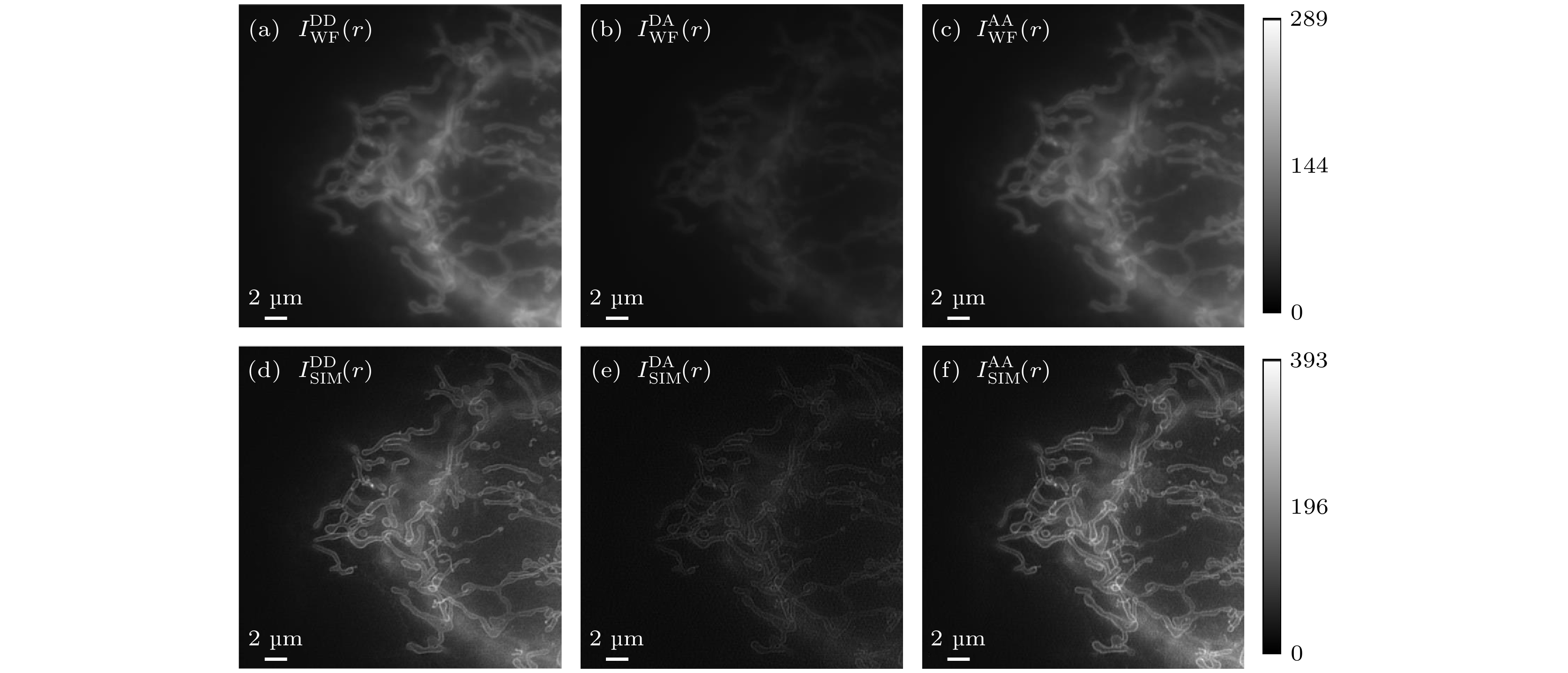
图 5 双通道SISR-FRET成像系统对ActA-G17 M样本成像结果 (a)—(c) DD, DA, AA通道宽场成像结果; (d)—(f) DD, DA, AA通道超分辨成像结果. 比例尺: 2 μm
Figure 5. Imaging results of the dual-channel SISR-FRET imaging system for the ActA-G17M sample: (a)–(c) Wide-field imaging results in the DD, DA, and AA channels; (d)–(f) super-resolution imaging results in the DD, DA, and AA channels. Scale bar: 2 μm.
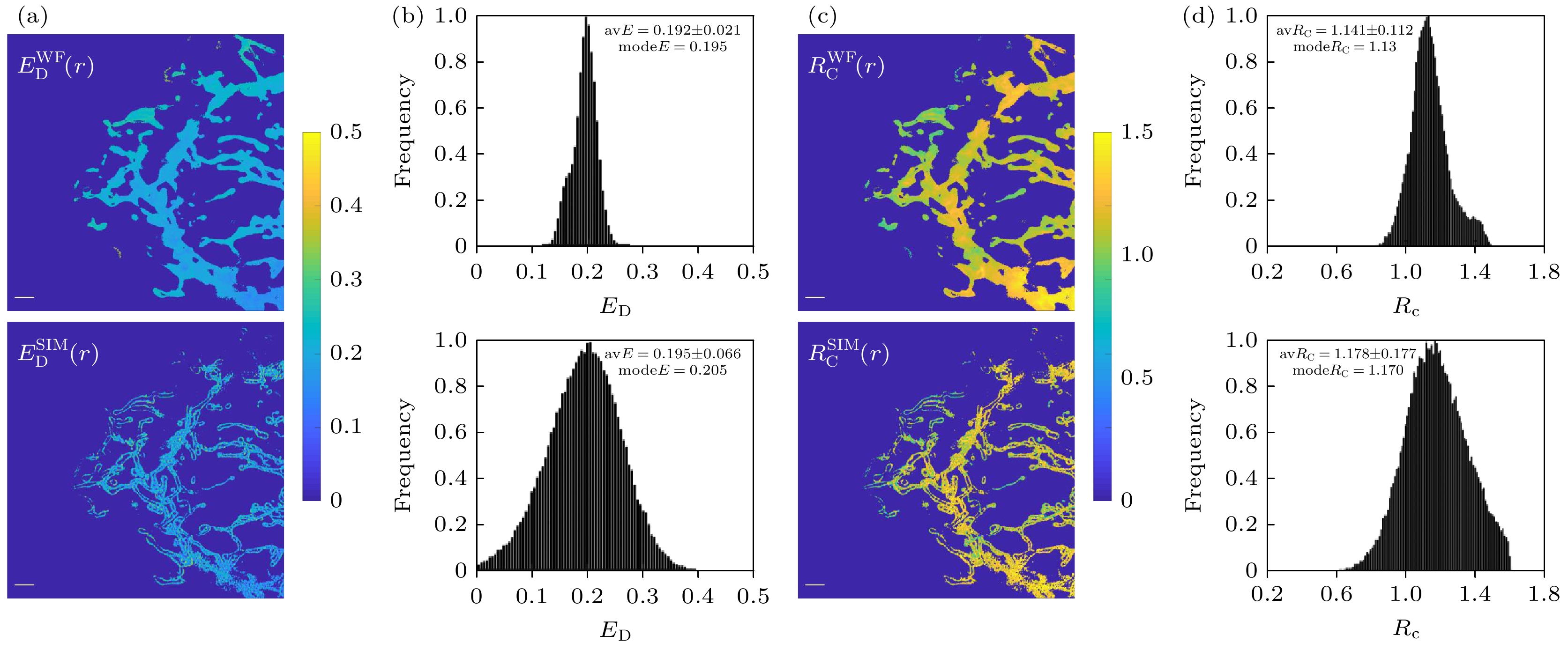
图 6 双通道SISR-FRET成像定量FRET分析结果对比 (a)宽场FRET和超分辨FRET效率ED的伪彩图; (b)图(a)中结果的统计直方图; (c)宽场FRET和超分辨FRET受供体浓度比 RC的伪彩图; (d) 图(c)中结果的统计直方图. 比例尺: 2 μm
Figure 6. Comparison of quantitative FRET analysis results from dual-channel SISR-FRET imaging: (a) Pseudo-color map of ED for wide-field FRET and super-resolution FRET; (b) corresponding statistical histograms; (c) pseudo-color plot of RC for wide-field FRET and super-resolution FRET; (d) corresponding statistical histograms. Scale bars: 2 μm.
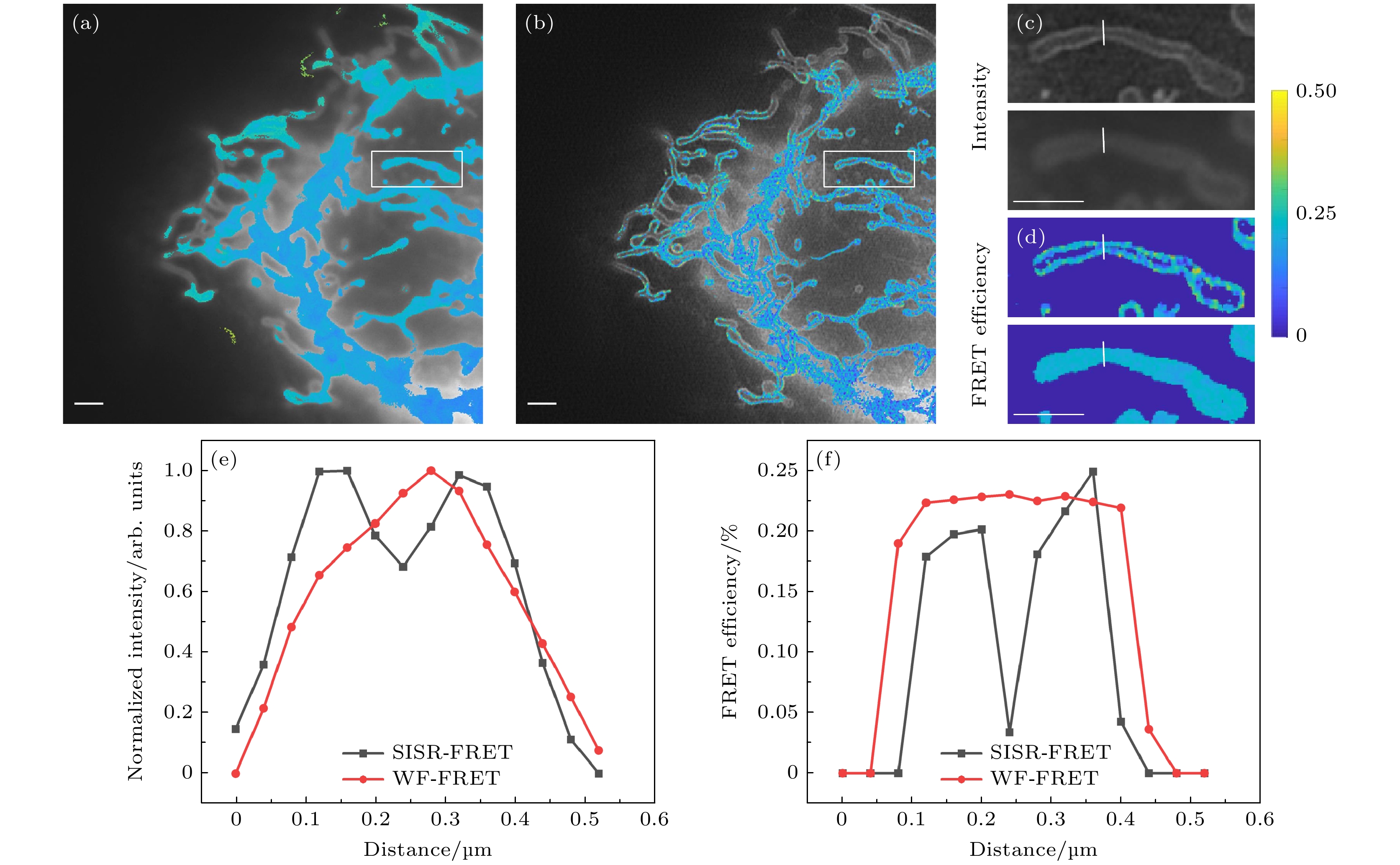
图 7 双通道SISR-FRET分辨率提升的定量分析 (a) 宽场FRET效率ED的伪彩图和DD通道灰度图像的重叠结果; (b) SISR-FRET效率ED的伪彩图和DD通道灰度图像的重叠结果; (c) 宽场FRET与SISR-FRET中DD通道灰度图像的局部细节对比; (d) 宽场FRET与SISR-FRET中效率ED的伪彩图的局部细节对比; (e), (f) 图 (c)和图 (d)中划线位置的横切面强度图. 比例尺: 2 μm
Figure 7. Quantitative analysis of dual-channel SISR-FRET resolution enhancement: (a) The merge of intensity and ED map images of wide-field FRET; (b) the merge of intensity and ED map images of SISR-FRET; (c) partly enlarged view of intensity images of wide-field FRET and SISR-FRET in the DD channel; (d) partly enlarged view of ED map images of wide-field FRET and SISR-FRET; (e), (f) normalized intensity and FRET profiles along the marked lines in (c) and (d). Scale bars: 2 μm.
-
[1] Rao V S, Srinivas K, Sujini G N, Kumar G N S 2014 Int. J. Proteomics 2014 1
[2] Acuner Ozbabacan S E, Engin H B, Gursoy A, Keskin O 2011 Protein Eng. Des. Sel. 24 635
 Google Scholar
Google Scholar
[3] Xing S, Wallmeroth N, Berendzen K W, Grefen C 2016 Plant Physiol. 171 727
[4] Algar W R, Hildebrandt N, Vogel S S, Medintz I L 2019 Nat. Methods 16 815
 Google Scholar
Google Scholar
[5] Jares-Erijman E A, Jovin T M 2003 Nat. Biotechnol. 21 1387
 Google Scholar
Google Scholar
[6] Ben-Johny M, Yue D N, Yue D T 2016 Nat. Commun. 7 13709
 Google Scholar
Google Scholar
[7] Chen H C, Sun B N, Sun H, Xu L J, Wu G H, Tu Z, Cheng X C, Fan X H, Mai Z H, Tang Q L, Wang X P, Chen T S 2021 Cell Death Discov. 7 363
 Google Scholar
Google Scholar
[8] Sun B N, Chen H C, Wang X P, Chen T S 2023 Cell Death Discov. 9 37
[9] Yang F F, Qu W F, Du M Y, Mai Z Y, Wang B, Ma Y Y, Wang X P, Chen T S 2020 Cell. Mol. Life Sci. 77 2387
 Google Scholar
Google Scholar
[10] Szalai A M, Zaza C, Stefani F D 2021 Nanoscale 13 18421
 Google Scholar
Google Scholar
[11] Szabó Á, Szendi-Szatmári T, Szöllősi J, Nagy P 2020 Methods Appl. Fluoresc. 8 032003
 Google Scholar
Google Scholar
[12] Grecco H E, Verveer P J 2011 ChemPhysChem 12 484
 Google Scholar
Google Scholar
[13] Deußner-Helfmann N S, Auer A, Strauss M T, Malkusch S, Dietz M S, Barth H D, Jungmann R, Heilemann M 2018 Nano Lett. 18 4626
 Google Scholar
Google Scholar
[14] Tardif C, Nadeau G, Labrecque S, Côté D, Lavoie-Cardinal F 2019 Neurophotonics 6 1
[15] Szalai A M, Siarry B, Lukin J, Giusti S, Unsain N, Cáceres A, Steiner F, Tinnefeld P, Refojo D, Jovin T M, Stefani F D 2021 Nano Lett. 21 2296
 Google Scholar
Google Scholar
[16] Liu Z, Luo Z W, Chen H C, Yin A, Sun H, Zhuang Z F, Chen T S 2022 Cytom. Part A 101 264
 Google Scholar
Google Scholar
[17] Zhao T Y, Wang Z J, Cai Y N, Liang Y S, Wang SW, Zhang J X, Chen T S, Lei M 2023 Opt. Lasers Eng. 167 107606
 Google Scholar
Google Scholar
[18] Zhao W S, Zhao S Q, Li L J, et al. 2022 Nat. Biotechnol. 40 606
 Google Scholar
Google Scholar
[19] Huang X S, Fan J C, Li L J, Liu H S, Wu R L, Wu Y, Wei L S, Mao H, Lal A, Xi P, Tang L Q, Zhang Y F, Liu Y M, Tan S, Chen L Y 2018 Nat. Biotechnol. 36 451
 Google Scholar
Google Scholar
[20] Li D, Shao L, Chen B C, Zhang X, Zhang M, Moses B, Milkie D E, Beach J R, Hammer J A, Pasham M, Kirchhausen T, Baird M A, Davidson M W, Xu P, Betzig E 2015 Science 349 aab3500
 Google Scholar
Google Scholar
[21] Kner P, Chhun B B, Griffis E R, Winoto L, Gustafsson M G L 2009 Nat. Methods 6 339
 Google Scholar
Google Scholar
[22] Wen G, Li S M, Wang L B, et al. 2021 Light Sci. Appl. 10 70
 Google Scholar
Google Scholar
[23] Luo Z W, Wu G, Kong M, Chen Z, Zhuang Z F, Fan J C, Chen T S 2023 Photonics Res. 11 887
 Google Scholar
Google Scholar
[24] Fan J C, Huang X S, Li L, Tan S, Chen L 2019 Biophys. Rep. 5 80
 Google Scholar
Google Scholar
[25] Sun H, Zhang C, Ma Y, Du M, Chen T S 2019 Biomed. Signal Process. Control 53 101585
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 5766
- PDF Downloads: 82
- Cited By: 0














 DownLoad:
DownLoad: