-
Quantitative phase microscopy (QPM) is a label-free imaging technique often employed for long-term, high-contrast imaging of live bio-samples. Yet, QPM is not specific to a certain subcellular organelle. As a remedy, fluorescence microscopy can visualize specific subcellular organelles once being labeled with fluorescent markers. In this paper, a high-resolution phase/fluorescence dual-modality microscopic imaging method based on structured light illumination is proposed. In a dual-modality microscopic system, periodic stripes are generated by a digital micromirror array (DMD), and are used as the common illumination for both modalities. For QPM imaging, the holograms of the sample under structured light illuminations from different directions and phase shifts are recorded, from which a quantitative phase image with resolution enhancement can be reconstructed via a synthetic aperture procedure. Furthermore, a numerical approach is proposed to compensate for the environmental disturbances that often challenge aperture synthesis of phase imaging. This method determines each time the phase distortions caused by environmental disturbances through using the spectrum of the 0th order of the structured light illumination, and the phase distortions are removed from the phase distributions of the waves along the 0th and the ±1st diffraction orders. Resolution enhancement of QPM imaging is realized by synthesizing the spectra of all the waves along different diffraction orders of the structured light illuminations of different orientations. With phase images, three-dimensional shapes, inner structures, or refractive index distributions of transparent and translucent samples can be obtained. For fluorescence imaging, intensity images (morie patterns) of the sample under different structured light illuminations are recorded. The spectra along different diffraction orders are separated by using a phase shifting reconstruction algorithm, and are shifted to their original positions, forming a synthesized spectrum that is much broader than the spectra of raw intensity images (NA-limited spectra). An inverse Fourier transform on the synthesized spectrum yields a super-resolution fluorescence image of the sample. With the reconstructed fluorescence images, specific subcellular organelles labeled with fluorescent markers can be visualized. The combination of quantitative phase microscopy and fluorescence microscopy can obtain multidimensional information about the sample. In this dual-mode imaging system, the spatial resolution of quantitative phase imaging and fluorescence imaging are 840 nm and 440 nm, respectively. The proposed dual-mode microscopy imaging technique has been demonstrated for imaging fluorescent beads, fly wings, spring/rice leaves, mouse tail transection, and fluorescence-stained SiHa cells. We envisage that this method can be further applied to many fields, such as biomedicine, industry, and chemistry.
-
Keywords:
- structured illumination /
- dual-modality imaging /
- quantitative phase microscopy /
- resolution enhancement /
- phase compensation
[1] Shin S, Kim K, Yoon J, Park Y K 2015 Opt. Lett. 40 5410
 Google Scholar
Google Scholar
[2] Popescu G, Ikeda T, Dasari R R, Feld M S 2006 Opt. Lett. 31 775
 Google Scholar
Google Scholar
[3] Yoon J, Kim K, Park H, Choi C, Jang S, Park Y 2015 Biomed. Opt. Express 6 3856
 Google Scholar
Google Scholar
[4] Yoon J, Kim K, Kim M H, Jo Y, Kang S J, Park Y 2015 Asia Communications and Photonics Conference 2015, Hong Kong, China, November 19, 2015 ASu2A.159
[5] Ma Y, Dai T, Lei Y, Zheng J, Liu M, Sui B, Smith Z J, Chu K, Kong L, Gao P 2022 Opt. Express 30 9505
 Google Scholar
Google Scholar
[6] Zhuo K Q, Wang Y, Wang Y, Wen K, Liu M, Ma Y, Zheng J J, Gao P 2021 Front. Phys. 9 689
 Google Scholar
Google Scholar
[7] Schnars U, Jüptner W 1994 Appl. Opt. 33 179
 Google Scholar
Google Scholar
[8] Goodman J W, Lawrence R 1967 Appl. Phys. Lett. 11 77
 Google Scholar
Google Scholar
[9] Kim M K 2009 J. Photon. Energy 1 8005
 Google Scholar
Google Scholar
[10] Osten W, Faridian A, Gao P, Körner K, Naik D, Pedrini G, Singh A K, Takeda M, Wilke M 2014 Appl. Opt. 53 G44
 Google Scholar
Google Scholar
[11] Gao P, Yuan C J 2022 Light: Adv. Manuf. 3 105
 Google Scholar
Google Scholar
[12] Kuznetsova Y, Neumann A, Brueck S R J 2008 J. Opt. Soc. Am. A 25 811
 Google Scholar
Google Scholar
[13] Schwarz C J, Kuznetsova Y, Brueck S R J 2003 Opt. Lett. 28 1424
 Google Scholar
Google Scholar
[14] Cheng C J, Lai X J, Lin Y C, Tu H Y 2013 IEEE 4th International Conference on Photonics (ICP), Malaysia Melaka, October 28–30, 2013 pp215–217
[15] Xin W Y, Sha G, Yong W D, Wen L Q, Rong L, Jie Z 2016 Opt. Commun. 366 81
 Google Scholar
Google Scholar
[16] Gao P, Pedrini G, Osten W 2013 Opt. Lett. 38 1328
 Google Scholar
Google Scholar
[17] Chowdhury S, Eldridge W J, Wax A, Izatt J 2017 Optica 4 537
 Google Scholar
Google Scholar
[18] Zheng J, Pedrini G, Gao P, Yao B, Osten W 2015 J. Opt. 17 085301
 Google Scholar
Google Scholar
[19] Latychevskaia T, Fink H W 2013 Opt. Express 21 7726
 Google Scholar
Google Scholar
[20] Rong L, Latychevskaia T, Wang D, Zhou X, Huang H, Li Z, Wang Y 2014 Opt. Express 22 17236
 Google Scholar
Google Scholar
[21] Di J, Zhao J, Jiang H, Zhang P, Fan Q, Sun W 2008 Appl. Opt. 47 5654
 Google Scholar
Google Scholar
[22] Conchello J A, Lichtman J W 2005 Nat. Methods 2 920
 Google Scholar
Google Scholar
[23] Rust M J, Bates M, Zhuang X 2006 Nat. Methods 3 793
 Google Scholar
Google Scholar
[24] Fölling J, Bossi M, Bock H, Medda R, Wurm C A, Hein B, Jakobs S, Eggeling C, Hell S W 2008 Nat. Methods 5 943
 Google Scholar
Google Scholar
[25] Liu Y J, Lu Y Q, Yang X S, Zheng X L, Wen S H, Wang F, Vidal X, Zhao J B, Liu D M, Zhou Z G, Ma C S, Zhou J J, Xi P, Jin D Y 2017 Nature 543 229
 Google Scholar
Google Scholar
[26] Török P, Munro P 2004 Opt. Express 12 3605
 Google Scholar
Google Scholar
[27] Kim Y D, Ahn M K, Kim T, Yoo H, Gweon D G 2012 Meas. Sci. Technol. 23 105403
 Google Scholar
Google Scholar
[28] Chen L, Huang X, Fan J, Li L, Shan T 2018 Nat. Biotechnol. 36 451
 Google Scholar
Google Scholar
[29] Wen K, Gao Z, Fang X, Liu M, Zheng J, Ma Y, Zalevsky Z, Gao P 2021 Opt. Express 29 33679
 Google Scholar
Google Scholar
[30] Lal A, Shan C, Xi P 2016 IEEE J. Sel. Top. Quantum Electron. 22 50
 Google Scholar
Google Scholar
[31] Brown P T, Kruithoff R, Seedorf G J, Shepherd D P 2021 Biomed. Opt. Express 12 3700
 Google Scholar
Google Scholar
-
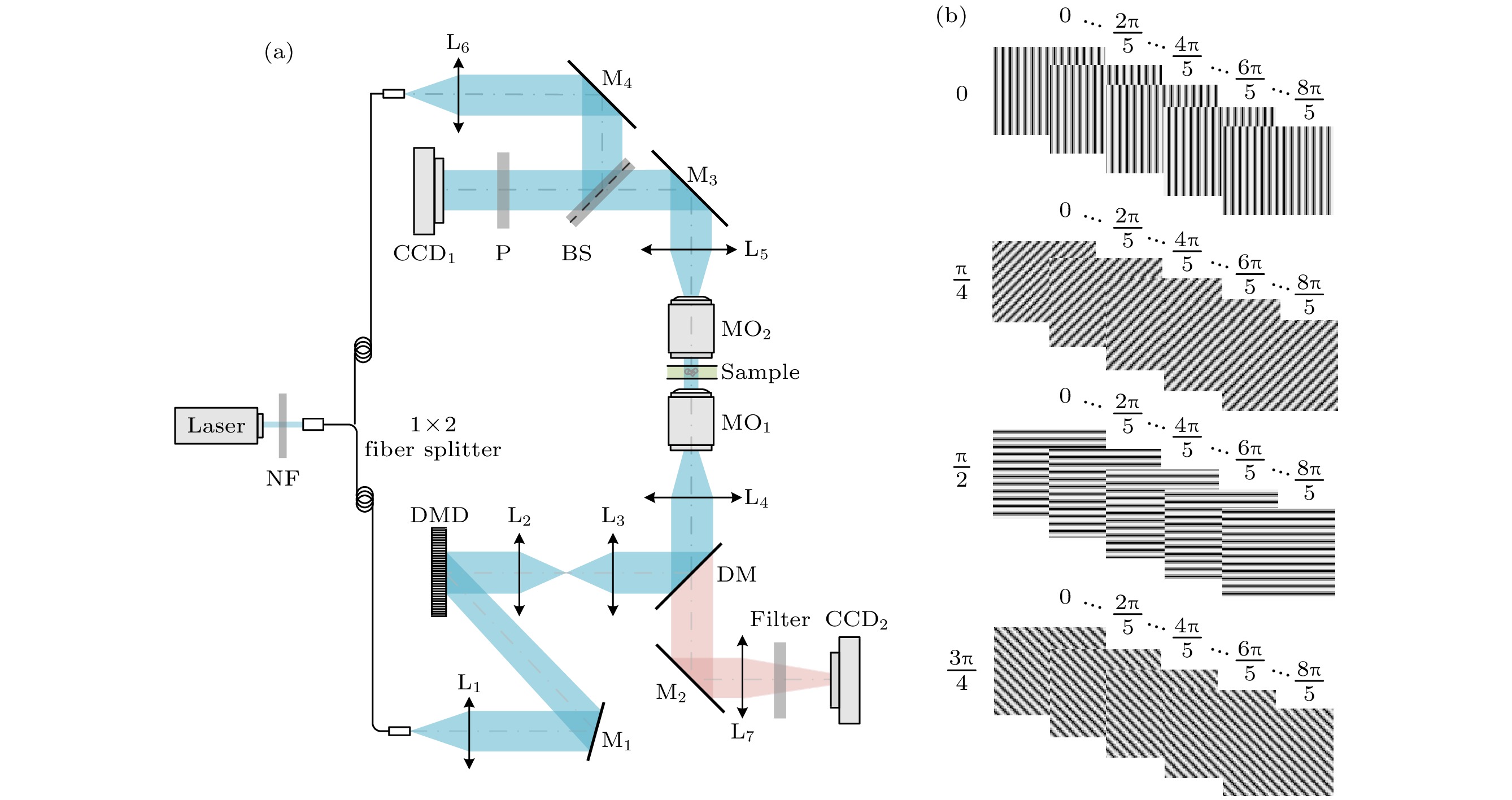
图 1 结构光照明相位/荧光双模式显微成像示意图 (a) 光路原理图; (b) 在DMD上加载的正弦条纹图案
Figure 1. Experimental setup for dual-modality optical microscopy (including quantitative phase microscopy and super-resolution fluorescence microscopy) based on structured light illumination: (a) Optical setup of dual modality; (b) fringe patterns loaded on DMD.
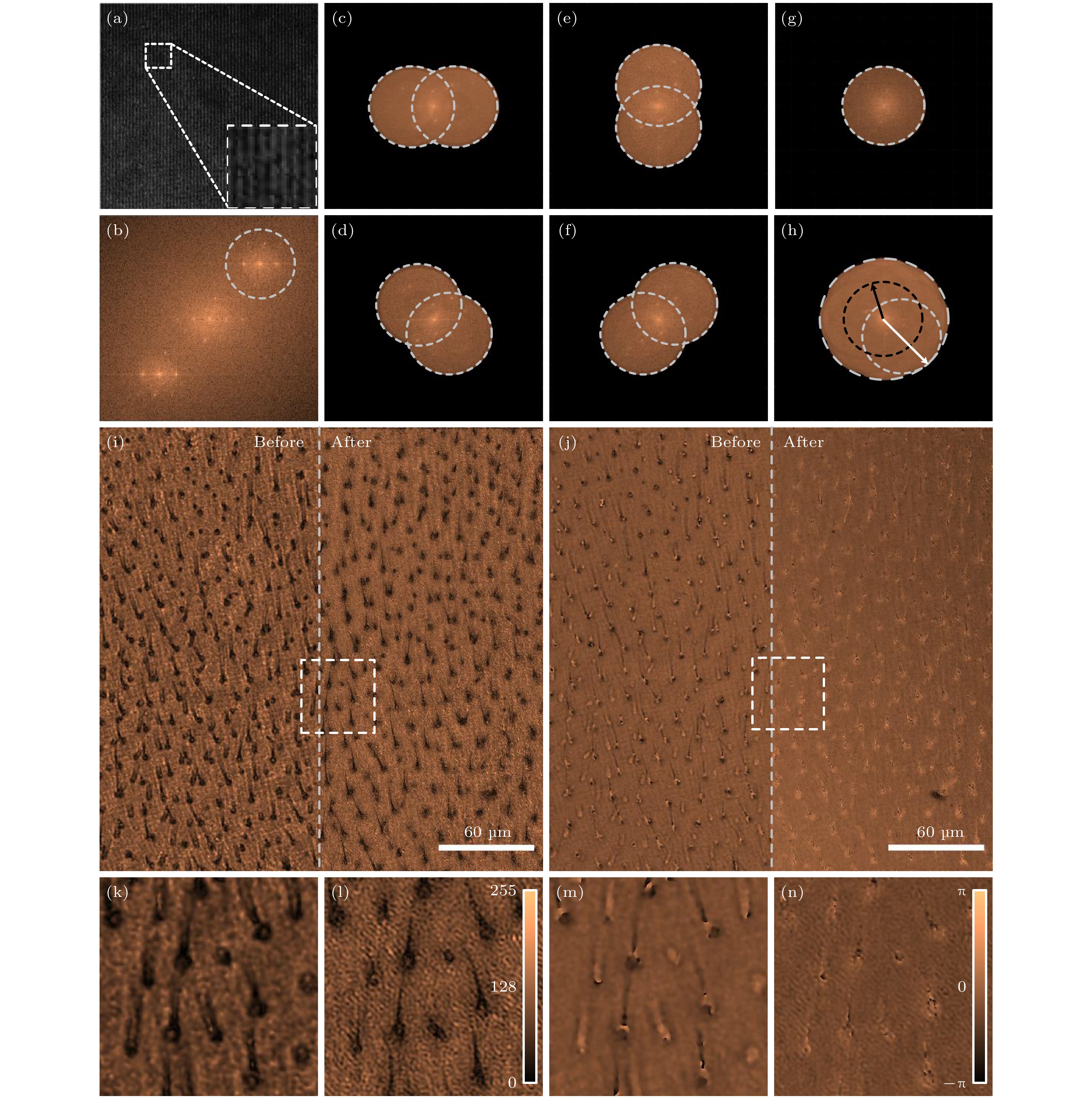
图 2 结构光照明定量相位显微的图像重构以及对苍蝇翅切片的成像结果 (a) 结构光照明数字全息显微的全息图; (b) 图(a)中全息图的频谱分布; (c)−(f) 条纹方向为0°, 45°, 90°和135°的结构光照明下得到的物光频谱分布; (g)和(h) 平行光照明和结构光照明下的样品频谱分布; (i)和(j) 苍蝇翅切片的振幅图和相位图, 其中左/右半部分别为使用平行光照明/结构光照明对应的图像; (k)和(l)图(i)中虚框部位在平行光照明和结构光照明下恢复的振幅结果; (m)和(n)图(j)中虚框部位在平行光照明和结构光照明下恢复的相位结果
Figure 2. Image reconstruction of quantitative phase microscopy with structured light illumination and imaging results on fly wing sections: (a) Hologram of digital holographic microscopy (DHM) illuminated by structured light; (b) spectral distribution of the hologram in Fig. 2(a); (c)−(f) spectra of the object waves obtained using 0°, 45°, 90° and 135° structured illumination; (g) and (h) frequency spectrum of samples under parallel and structured light illumination; (i) and (j) amplitude and phase images of a fly wing slice, where the left/right halves are images using parallel/structured light illumination, respectively; (k) and (l) the reconstructed amplitude results of the virtual box in the Fig. 2(i) under plane-wave illumination and structured illumination; (m) and (n) reconstructed phase results of the dashed box in the Fig. 2(j) under plane-wave illumination and structured illumination.
图 3 结构光照明荧光显微成像的图像重构以及女贞叶切片的成像结果 (a)−(d) 条纹方向为0°, 45°, 90°和135°的结构光照明下得到的物光频谱分布; (e)和(f) 平行光照明和结构光照明下的样品频谱分布; (g) 该图左侧和右侧为使用平行光照明和结构光照明女贞叶切片的荧光图像; (h)和(i)图(g)中方框部位在使用平行光照明和结构光照明的荧光图像放大图
Figure 3. Image reconstruction of structured light-illuminated fluorescence microscopy and imaging results of privet leaf slices: (a)−(d) Spectral distribution of object waves under structured light illumination with fringe directions of 0°, 45°, 90° and 135°; (e) and (f) spectral distribution of samples under parallel and structured light illumination; (g) the left and right halves of the figure are fluorescence images of privet leaf sections illuminated using parallel and structured light illumination; (h) and (i) magnified wide-field and SIM images of the sample within the white-dash box in Fig. 3(g).
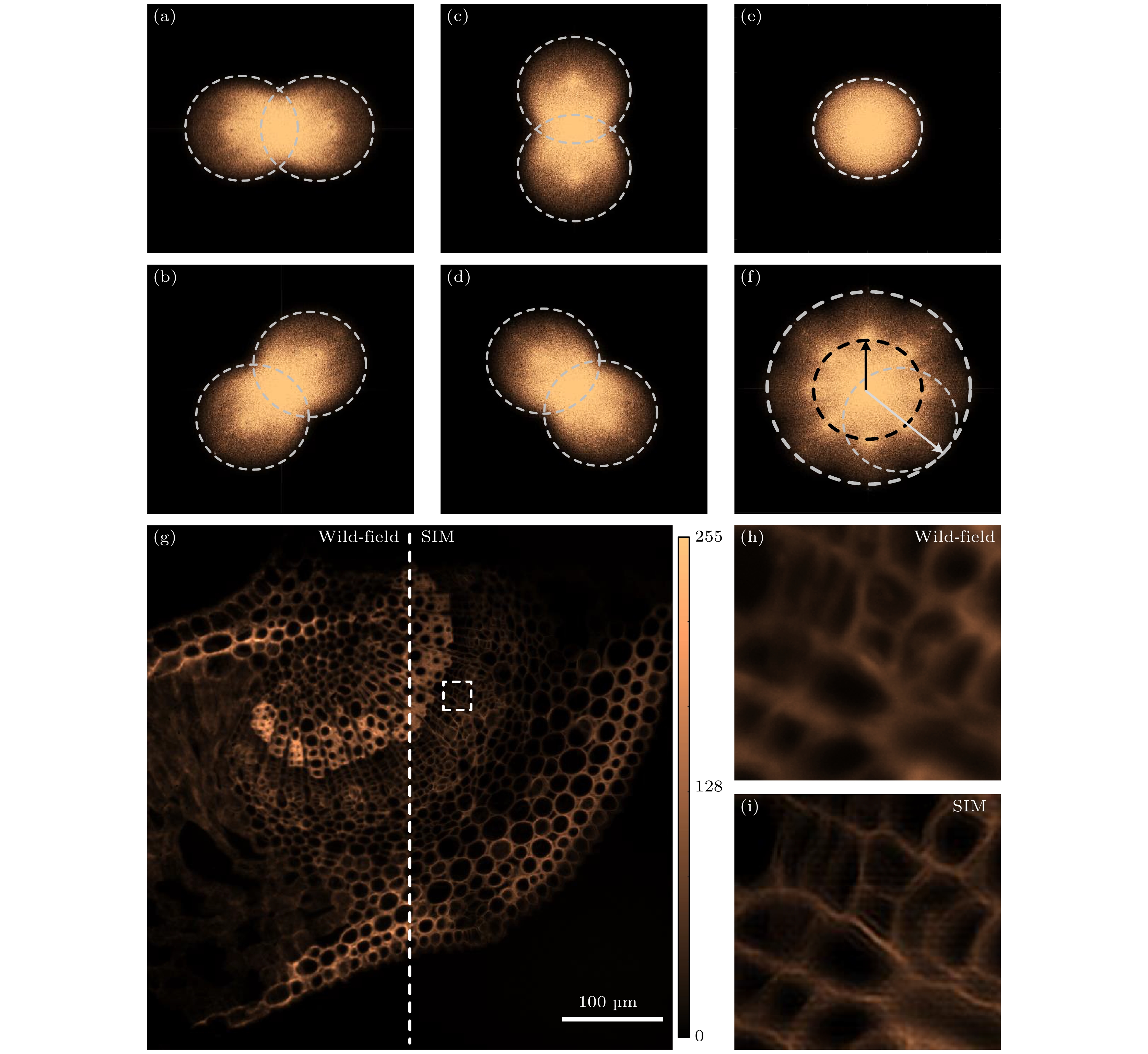
图 4 基于平行光/结构光照明数字全息显微对二氧化硅小球的成像结果 (a) 平行光(左)和结构光(右)照明时DHM获得的振幅图像; (b)和(c)图(a)中白色方框区域在使用平行光照明和结构光照明的重构结果; (d) 图(b)和(c)中沿白色虚线的强度分布; (e) 平行光/结构光照明DHM的空间分辨率比较, 在图(a)中随机选择35个小球, 并对其在两个成像模式下半高全宽进行统计
Figure 4. Plane-wave/structured-illumination based DHM imaging of SiO2 beads: (a) Images of SiO2 beads obtained by DHM with parallel illumination and structure illumination, respectively; (b) and (c) the magnified images within the white-box area in panel (a) obtained by plane-wave and structured illumination based DHM, respectively; (d) the intensity distributions along the white lines in panel (b) and (c); (e) statistics of lateral resolution of the two imaging modalities by randomly choosing and FWHM analysis of 35 beads for in panel (a).
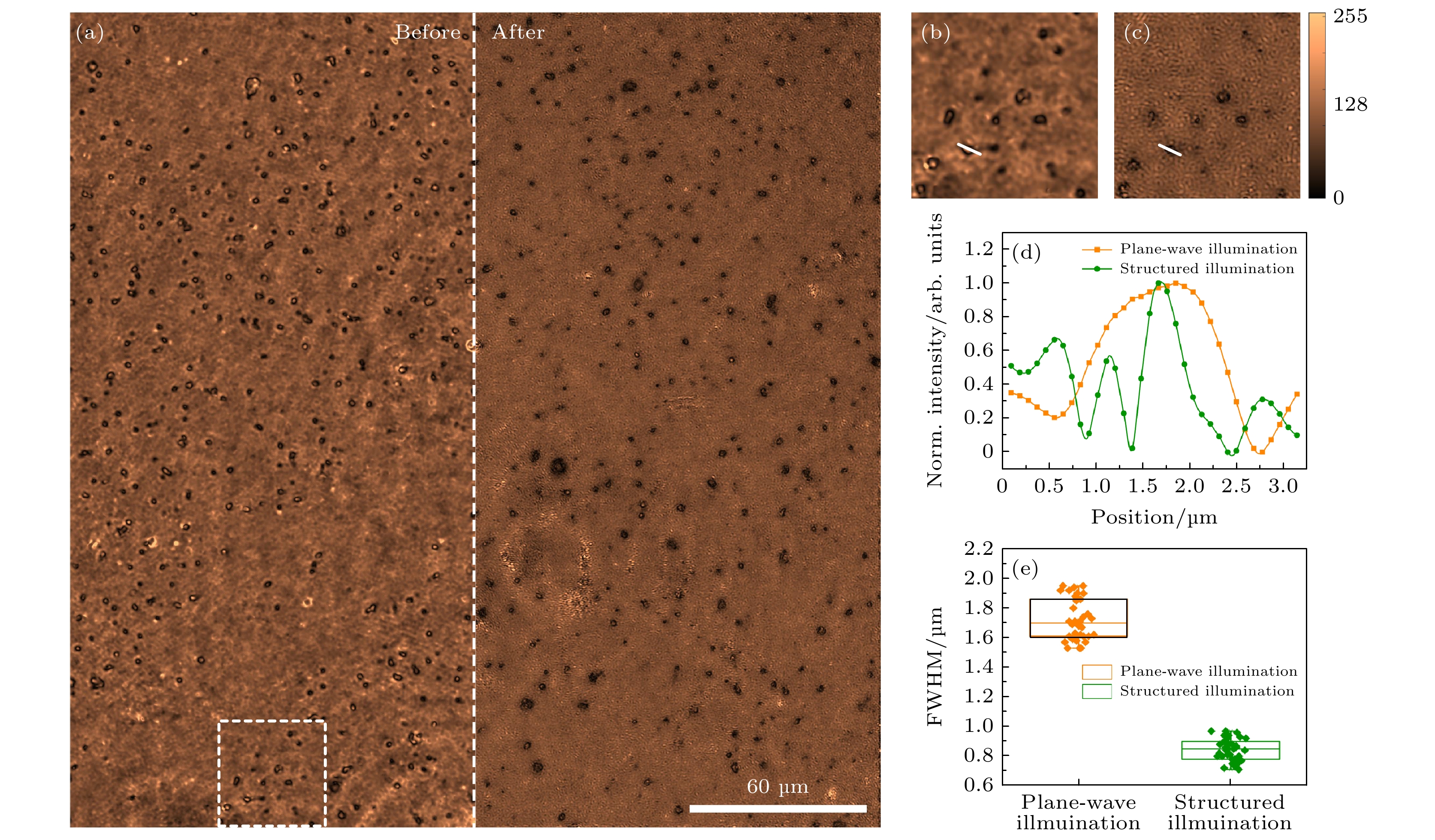
图 5 宽场显微和SIM对荧光小球的成像结果 (a) 宽场显微(左)和SIM(右)图像; (b)和(c)图(a)中白色方框区域对应的宽场和SIM图像的放大图; (d)图(b)和(c)中沿白色虚线的强度分布; (e)图(a)宽场和SIM模式空间分辨率的统计, 在图(a)中随机选择35个小球, 并对其在两个成像模式下半高全宽进行统计
Figure 5. Wide-field and SIM imaging of fluorescent beads: (a) Wide-field (left) and SIM (right) images of the sample; (b) and (c) the magnified wide-field and SIM images of the sample within the white-box in panel (a); (d) the intensity distribution along the white lines in panel (b) and (c); (e) statistics of lateral resolution of the two imaging modalities by randomly choosing and FWHM analysis of 35 beads for in panel (a).
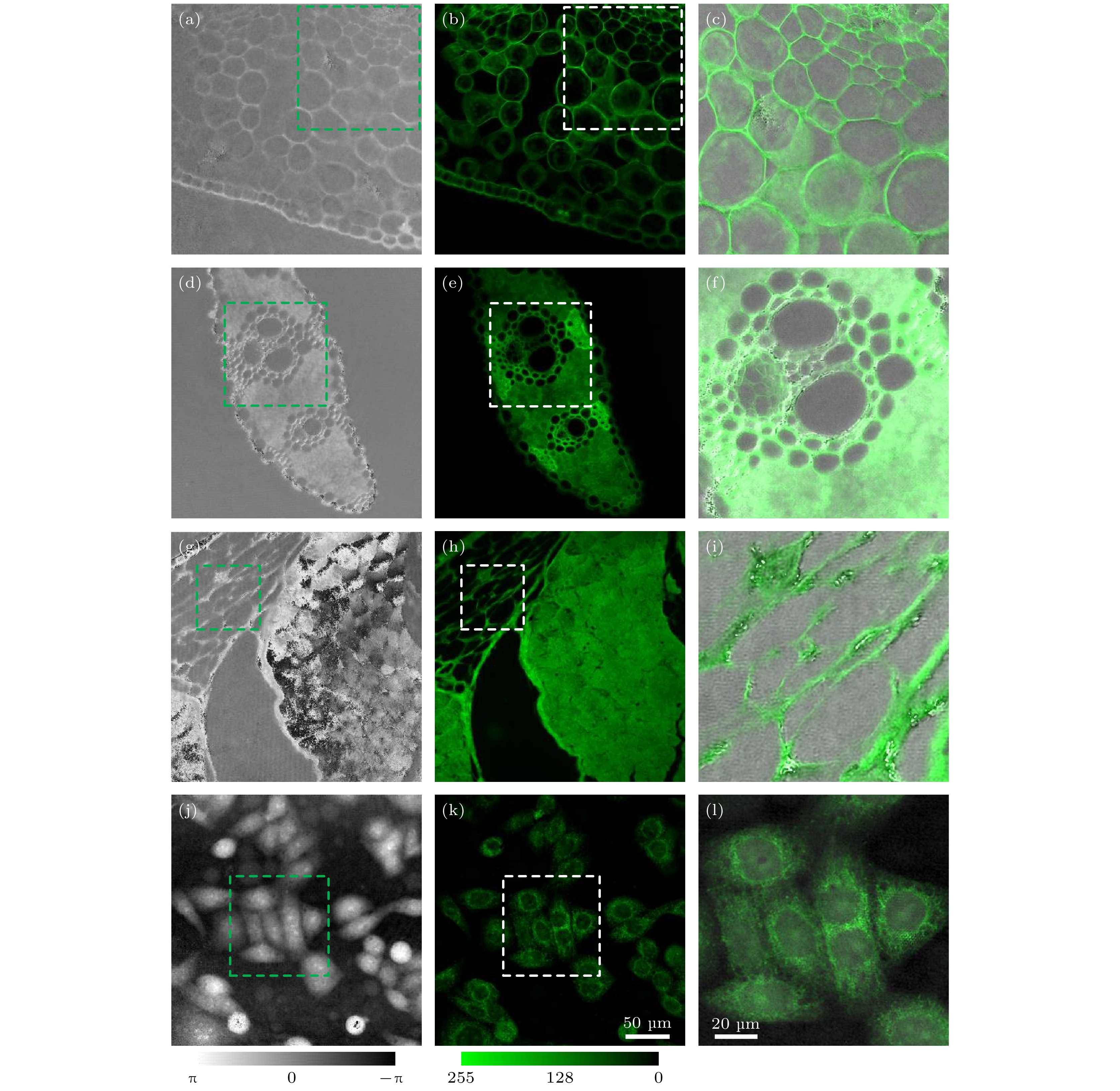
图 6 结构光照明相位/荧光双模式显微成像 (a)和(b) 迎春叶切片的相位和荧光图像; (d)和(e)水稻叶的相位和荧光图像; (g)和(h)鼠尾切片的相位和荧光图像; (j) SiHa细胞的相位图像; (k) SiHa细胞中的线粒体的荧光图像; (c), (f), (i)和(l) 4种样品的相位/荧光融合图
Figure 6. Structured illumination based phase/fluorescence dual-mode microscopic imaging of spring leaves, rice leaves, and mouse tail transection: (a) and (b) Phase and fluorescence images of cross-sections of spring leaves; (d) and (e) phase and fluorescence images of rice leaves; (g) and (h) phase and fluorescence images of mouse tail transection; (j) phase image of SiHa cells; (k) fluorescence image of mitochondria in SiHa cells; (c), (f), (i) and (l) combined phase/fluorescence images of the four samples.
-
[1] Shin S, Kim K, Yoon J, Park Y K 2015 Opt. Lett. 40 5410
 Google Scholar
Google Scholar
[2] Popescu G, Ikeda T, Dasari R R, Feld M S 2006 Opt. Lett. 31 775
 Google Scholar
Google Scholar
[3] Yoon J, Kim K, Park H, Choi C, Jang S, Park Y 2015 Biomed. Opt. Express 6 3856
 Google Scholar
Google Scholar
[4] Yoon J, Kim K, Kim M H, Jo Y, Kang S J, Park Y 2015 Asia Communications and Photonics Conference 2015, Hong Kong, China, November 19, 2015 ASu2A.159
[5] Ma Y, Dai T, Lei Y, Zheng J, Liu M, Sui B, Smith Z J, Chu K, Kong L, Gao P 2022 Opt. Express 30 9505
 Google Scholar
Google Scholar
[6] Zhuo K Q, Wang Y, Wang Y, Wen K, Liu M, Ma Y, Zheng J J, Gao P 2021 Front. Phys. 9 689
 Google Scholar
Google Scholar
[7] Schnars U, Jüptner W 1994 Appl. Opt. 33 179
 Google Scholar
Google Scholar
[8] Goodman J W, Lawrence R 1967 Appl. Phys. Lett. 11 77
 Google Scholar
Google Scholar
[9] Kim M K 2009 J. Photon. Energy 1 8005
 Google Scholar
Google Scholar
[10] Osten W, Faridian A, Gao P, Körner K, Naik D, Pedrini G, Singh A K, Takeda M, Wilke M 2014 Appl. Opt. 53 G44
 Google Scholar
Google Scholar
[11] Gao P, Yuan C J 2022 Light: Adv. Manuf. 3 105
 Google Scholar
Google Scholar
[12] Kuznetsova Y, Neumann A, Brueck S R J 2008 J. Opt. Soc. Am. A 25 811
 Google Scholar
Google Scholar
[13] Schwarz C J, Kuznetsova Y, Brueck S R J 2003 Opt. Lett. 28 1424
 Google Scholar
Google Scholar
[14] Cheng C J, Lai X J, Lin Y C, Tu H Y 2013 IEEE 4th International Conference on Photonics (ICP), Malaysia Melaka, October 28–30, 2013 pp215–217
[15] Xin W Y, Sha G, Yong W D, Wen L Q, Rong L, Jie Z 2016 Opt. Commun. 366 81
 Google Scholar
Google Scholar
[16] Gao P, Pedrini G, Osten W 2013 Opt. Lett. 38 1328
 Google Scholar
Google Scholar
[17] Chowdhury S, Eldridge W J, Wax A, Izatt J 2017 Optica 4 537
 Google Scholar
Google Scholar
[18] Zheng J, Pedrini G, Gao P, Yao B, Osten W 2015 J. Opt. 17 085301
 Google Scholar
Google Scholar
[19] Latychevskaia T, Fink H W 2013 Opt. Express 21 7726
 Google Scholar
Google Scholar
[20] Rong L, Latychevskaia T, Wang D, Zhou X, Huang H, Li Z, Wang Y 2014 Opt. Express 22 17236
 Google Scholar
Google Scholar
[21] Di J, Zhao J, Jiang H, Zhang P, Fan Q, Sun W 2008 Appl. Opt. 47 5654
 Google Scholar
Google Scholar
[22] Conchello J A, Lichtman J W 2005 Nat. Methods 2 920
 Google Scholar
Google Scholar
[23] Rust M J, Bates M, Zhuang X 2006 Nat. Methods 3 793
 Google Scholar
Google Scholar
[24] Fölling J, Bossi M, Bock H, Medda R, Wurm C A, Hein B, Jakobs S, Eggeling C, Hell S W 2008 Nat. Methods 5 943
 Google Scholar
Google Scholar
[25] Liu Y J, Lu Y Q, Yang X S, Zheng X L, Wen S H, Wang F, Vidal X, Zhao J B, Liu D M, Zhou Z G, Ma C S, Zhou J J, Xi P, Jin D Y 2017 Nature 543 229
 Google Scholar
Google Scholar
[26] Török P, Munro P 2004 Opt. Express 12 3605
 Google Scholar
Google Scholar
[27] Kim Y D, Ahn M K, Kim T, Yoo H, Gweon D G 2012 Meas. Sci. Technol. 23 105403
 Google Scholar
Google Scholar
[28] Chen L, Huang X, Fan J, Li L, Shan T 2018 Nat. Biotechnol. 36 451
 Google Scholar
Google Scholar
[29] Wen K, Gao Z, Fang X, Liu M, Zheng J, Ma Y, Zalevsky Z, Gao P 2021 Opt. Express 29 33679
 Google Scholar
Google Scholar
[30] Lal A, Shan C, Xi P 2016 IEEE J. Sel. Top. Quantum Electron. 22 50
 Google Scholar
Google Scholar
[31] Brown P T, Kruithoff R, Seedorf G J, Shepherd D P 2021 Biomed. Opt. Express 12 3700
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 8566
- PDF Downloads: 179
- Cited By: 0














 DownLoad:
DownLoad: