-
Single-molecular surface-induced fluorescence attenuation (smSIFA) is a precise method of studying the vertical movement of biological macromolecules based on two-dimensional material receptors. This method is not affected by two-dimensional planar motion of membrane or proteins. However, the detection range and accuracy of vertical movement are determined by the properties of two-dimensional materials as receptors. In recent years, surface induced fluorescence attenuation based on graphene oxide and graphene has played an important role in studying biomacromolecules. However, the detection range of graphene and graphene oxide are limited owing to the fixed and limited characteristic quenching distance. Adjusting the detection range requires replacing the medium material, which poses difficulties in selecting and preparing materials. Therefore, it is urgently needed to develop controllable materials for single-molecular SIFA. In this study, the single-molecule SIFA with graphene oxide as the medium acceptor is improved by reducing graphene oxide through thermal reduction. By controlling the reduction temperature, reduced graphene oxides to different reduction degrees are prepared and the characteristic quenching distances are adjusted. The characteristic quenching distance is measured by fluorescent labeled DNA. Single-molecule SIFA based on reduced graphene oxide is used to observe the conformational changes of Holliday junction, and the detection range of reduced graphene oxide is demonstrated.
-
Keywords:
- reduced graphene oxide /
- surface-induced fluorescence attenuation /
- characteristic quenching distance /
- fluorescence resonance energy transfer
[1] Lerner E, Barth A, Hendrix J, et al. 2021 Elife 10 e60416
 Google Scholar
Google Scholar
[2] Keller A M, DeVore M S, Stich D G, Vu D M, Causgrove T, Werner J H 2018 Anal. Chem. 90 6109
 Google Scholar
Google Scholar
[3] Ishikawa-Ankerhold H C, Ankerhold R, Drummen G P 2012 Molecules 17 4047
 Google Scholar
Google Scholar
[4] 贾棋, 樊秦凯, 侯文清, 杨晨光, 王利邦, 王浩, 徐春华, 李明, 陆颍 2021 70 158701
 Google Scholar
Google Scholar
Jia Q, Fan Q K, Hou W Q, Yang C G, Wang L B, Wang H, Xu C H, Li M, Lu Y 2021 Acta Phys. Sin. 70 158701
 Google Scholar
Google Scholar
[5] Almen M S, Nordstrom K J V, Fredriksson R, Schioth H B 2009 Bmc. Biology. 7 50
 Google Scholar
Google Scholar
[6] White S H, Wimley W C 1999 Annu. Rev. Bioph. Biom. 28 319
 Google Scholar
Google Scholar
[7] 马东飞, 侯文清, 徐春华, 赵春雨, 马建兵, 黄星榞, 贾棋, 马璐, 刘聪, 李明, 陆颖 2020 69 038701
 Google Scholar
Google Scholar
Ma D F, Hou W Q, Xu C H, Zhao C Y, Ma J B, Huang X Y, Jia Q, Ma L, Liu C, Li M, Lu Y 2020 Acta Phys. Sin. 69 038701
 Google Scholar
Google Scholar
[8] Ponmalar, II, Cheerla R, Ayappa K G, Basu J K 2019 Proc. Natl. Acad. Sci. USA 116 12839
 Google Scholar
Google Scholar
[9] King C, Raicu V, Hristova K 2017 J. Biol. Chem. 292 5291
 Google Scholar
Google Scholar
[10] King C, Sarabipour S, Byrne P, Leahy D J, Hristova K 2014 Biophys. J. 106 1309
 Google Scholar
Google Scholar
[11] Li Y, Qian Z, Ma L, Hu S, Nong D, Xu C, Ye F, Lu Y, Wei G, Li M 2016 Nat. Commun. 7 12906
 Google Scholar
Google Scholar
[12] Ma L, Li Y, Ma J B, Hu S X, Li M 2018 Biochemistry 57 4735
 Google Scholar
Google Scholar
[13] Jiang X, Yang C G, Qiu J, Ma D F, Xu C, Hu S X, Han W J, Yuan B, Lu Y 2022 Nanoscale 14 17654
 Google Scholar
Google Scholar
[14] Ma L, Hu S X, He X L, Yang N, Chen L C, Yang C G, Ye F F, Wei T T, Li M 2019 Nano. Lett. 19 6937
 Google Scholar
Google Scholar
[15] Kaminska I, Bohlen J, Yaadav R, Schuler P, Raab M, Schroder T, Zahringer J, Zielonka K, Krause S, Tinnefeld P 2021 Adv. Mater. 33 e2101099
 Google Scholar
Google Scholar
[16] Kaminska I, Bohlen J, Rocchetti S, Selbach F, Acuna G P, Tinnefeld P 2019 Nano. Lett. 19 4257
 Google Scholar
Google Scholar
[17] Federspiel F, Froehlicher G, Nasilowski M, Pedetti S, Mahmood A, Doudin B, Park S, Lee J O, Halley D, Dubertret B, Gilliot P, Berciaud S 2015 Nano. Lett. 15 1252
 Google Scholar
Google Scholar
[18] Gaudreau L, Tielrooij K J, Prawiroatmodjo G E D K, Osmond J, de Abajo F J G, Koppens F H L 2013 Nano. Lett. 13 2030
 Google Scholar
Google Scholar
[19] Li W, Wojcik M, Xu K 2019 Nano. Lett. 19 983
 Google Scholar
Google Scholar
[20] Eda G, Fanchini G, Chhowalla M 2008 Nat. Nanotechnol. 3 270
 Google Scholar
Google Scholar
[21] Pei S F, Cheng H M 2012 Carbon 50 3210
 Google Scholar
Google Scholar
[22] Stankovich S, Dikin D A, Piner R D, Kohlhaas K A, Kleinhammes A, Jia Y, Wu Y, Nguyen S T, Ruoff R S 2007 Carbon 45 1558
 Google Scholar
Google Scholar
[23] Sulowska K, Wiwatowski K, Szustakiewicz P, Grzelak J, Lewandowski W, Mackowski S 2018 Materials (Basel) 11 1567
 Google Scholar
Google Scholar
[24] Kim J, Cote L J, Kim F, Huang J X 2010 J. Am. Chem. Soc. 132 260
 Google Scholar
Google Scholar
[25] Kovtyukhova N I, Ollivier P J, Martin B R, Mallouk T E, Chizhik S A, Buzaneva E V, Gorchinskiy A D 1999 Chem. Mater. 11 771
 Google Scholar
Google Scholar
[26] Hummers W S, Offeman R E 1958 J. Am. Chem. Soc. 80 1339
 Google Scholar
Google Scholar
[27] Chen X, Meng D, Wang B, Li B W, Li W, Bielawski C W, Ruoff R S 2016 Carbon 101 71
 Google Scholar
Google Scholar
[28] Lazauskas A, Baltrusaitis J, Grigaliūnas V, Guobienė A, Prosyčevas I, Narmontas P, Abakevičienė B, Tamulevičius S 2014 Superlattices Microstruct. 75 461
 Google Scholar
Google Scholar
[29] Li J, Ma J, Kumar V, Fu H, Xu C, Wang S, Jia Q, Fan Q, Xi X, Li M, Liu H, Lu Y 2022 Nucleic. Acids. Res. 50 7002
 Google Scholar
Google Scholar
[30] Ma J B, Chen Z, Xu C H, Huang X Y, Jia Q, Zou Z Y, Mi C Y, Ma D F, Lu Y, Zhang H D, Li M 2020 Nucleic. Acids. Res. 48 3156
 Google Scholar
Google Scholar
[31] 陈 泽, 马建兵, 黄星榞, 贾棋, 徐春华, 张慧东, 陆颖 2018 67 118201
 Google Scholar
Google Scholar
Chen Z, Ma J B, Huang X Y, Jia Q, Xu C H, Zhang H D, Lu Y 2018 Acta Phys. Sin. 67 118201
 Google Scholar
Google Scholar
[32] Wei A, Wang J X, Long Q, Liu X M, Li X G, Dong X C, Huang W 2011 Mater. Res. Bull. 46 2131
 Google Scholar
Google Scholar
[33] Luo D, Zhang G, Liu J, Sun X 2011 J. Phys. Chem. C. 115 11327
 Google Scholar
Google Scholar
[34] Xu S T, Liu J K, Xue Y, Wu T Y, Zhang Z F 2017 Fuller. Nanotub. Car. N. 25 40
 Google Scholar
Google Scholar
[35] Zhen X J, Huang Y F, Yang S S, Feng Z Z, Wang Y, Li C H, Miao Y J, Yin H 2020 Mater. Lett. 260 126880
[36] Dessinges M N, Maier B, Zhang Y, Peliti M, Bensimon D, Croquette V 2002 Phys. Rev. Lett. 89 248102
 Google Scholar
Google Scholar
[37] Baumann C G, Smith S B, Bloomfield V A, Bustamante C 1997 Proc. Natl. Acad. Sci. U. S. A. 94 6185
 Google Scholar
Google Scholar
[38] Son S, Takatori S C, Belardi B, Podolski M, Bakalar M H, Fletcher D A 2020 Proc. Natl. Acad. Sci. U. S. A. 117 14209
 Google Scholar
Google Scholar
[39] Demirel G B, Caykara T 2009 Appl. Surf. Sci. 255 6571
 Google Scholar
Google Scholar
[40] Lu J R, Su T J, Thomas R K 1999 J. Colloid. Interf. Sci. 213 426
 Google Scholar
Google Scholar
[41] P. C. Weber J J W, f M. W. Pantoliano, and F. R. Salemme 1992 J. Am. Chem. Soc. 114 3197
 Google Scholar
Google Scholar
[42] Liu Y L, West S C 2004 Nat. Rev. Mol. Cell. Bio. 5 937
 Google Scholar
Google Scholar
[43] Clegg R M, Murchie A I, Lilley D M 1994 Biophysical. J. 66 99
 Google Scholar
Google Scholar
[44] McKinney S A, Tan E, Wilson T J, Nahas M K, Declais A C, Clegg R M, Lilley D M J, Ha T 2004 Biochem. Soc. T 32 41
 Google Scholar
Google Scholar
[45] McKinney S A, Declais A C, Lilley D M J, Ha T 2003 Nat. Struct. Biol. 10 93
 Google Scholar
Google Scholar
[46] Hohng S, Joo C, Ha T 2004 Biophys. J. 87 1328
 Google Scholar
Google Scholar
[47] Lee J, Lee S, Ragunathan K, Joo C, Ha T, Hohng S 2010 Angew. Chem. Int. Ed. Engl. 49 9922
 Google Scholar
Google Scholar
[48] Uphoff S, Holden S J, Le Reste L, Periz J, van de Linde S, Heilemann M, Kapanidis A N 2010 Nat. Methods. 7 831
 Google Scholar
Google Scholar
-
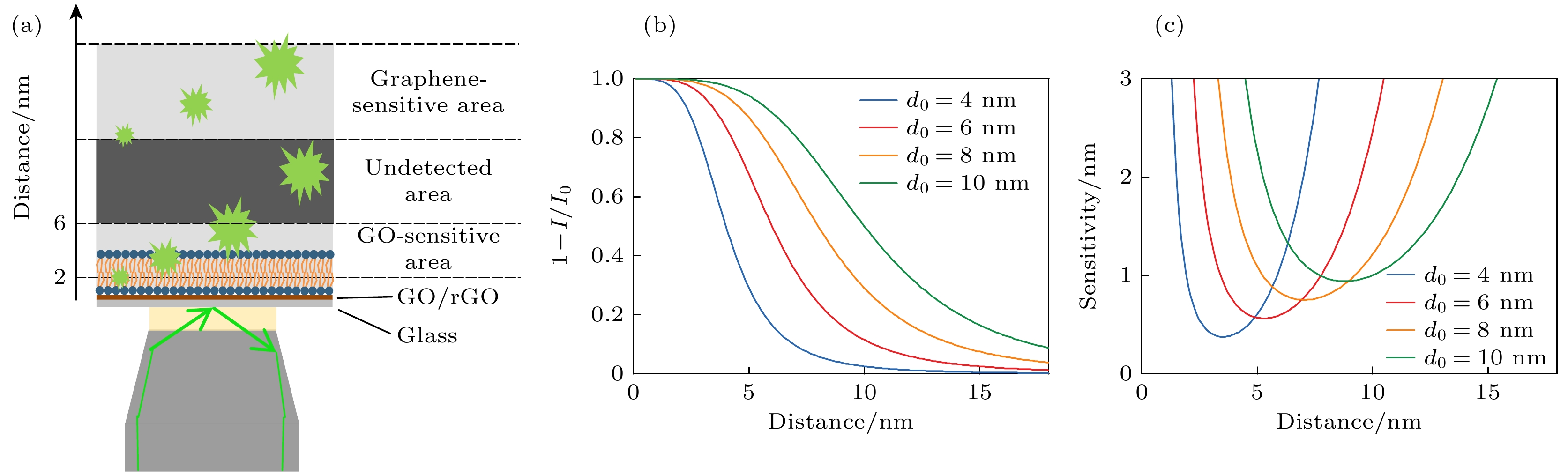
图 1 调控d0的SIFA方法 (a) SIFA方法示意图; (b) 荧光供体光强衰减和表面距离的关系; (c) SIFA探测灵敏度和荧光供体距表面距离的关系
Figure 1. SIFA method of adjustable d0: (a) Schematic representation of SIFA method; (b) relationship between degree of attenuation of a fluorescent donor and donor-surface distance; (c) relationship between detection sensitivity of SIFA and donor-surface distance.
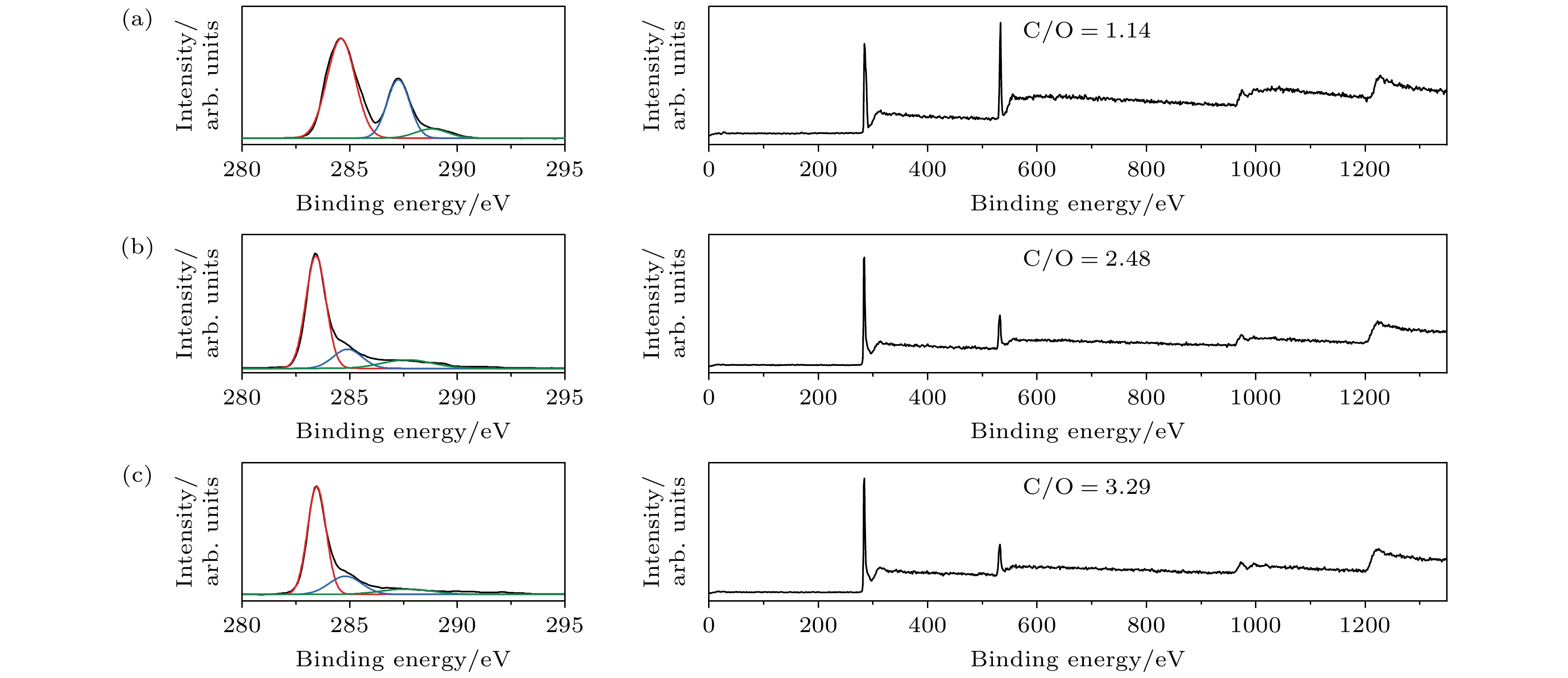
图 2 初始GO以及rGO薄膜的XPS谱 (a) 初始GO薄膜C 1s的XPS谱(左)以及XPS全谱(右); (b) 300 ℃-2 h-rGO薄膜C 1s的XPS谱(左)以及XPS全谱(右); (c) 400 ℃-2 h-rGO薄膜C 1s的XPS谱(左)以及XPS全谱
Figure 2. XPS spectra of original GO and rGO thin films: (a) C 1s XPS spectra (left) and XPS survey spectra (right) for original GO thin films; (b) C 1s XPS spectra (left) and XPS survey spectra (right) for 300 ℃-2 h-rGO thin films; (c) C 1s XPS spectra (left) and XPS survey spectra (right) for the 400 ℃-2 h-rGO thin films.
图 3 荧光标记DNA测量rGO的d0 (a) DNA成像实验示意图; (b) 在300 ℃-2 h-rGO (上)以及400 ℃-2 h-rGO (下)样品腔内观察DNA; Cy3标记在1 bp (c), 9 bp (d)和21 bp (e) 处的DNA在玻璃以及rGO上成像时的单分子光强
Figure 3. Determination of d0 of rGO by fluorescence labeled DNA: (a) Schematic representation of DNA imaging; (b) DNA imaging on 300 ℃-2 h-rGO (upper) and 400 ℃-2 h-rGO (lower); intensities of Cy3 labeled at 1 bp (c), 9 bp (d) and 21 bp (e) of DNA on glass and rGO.
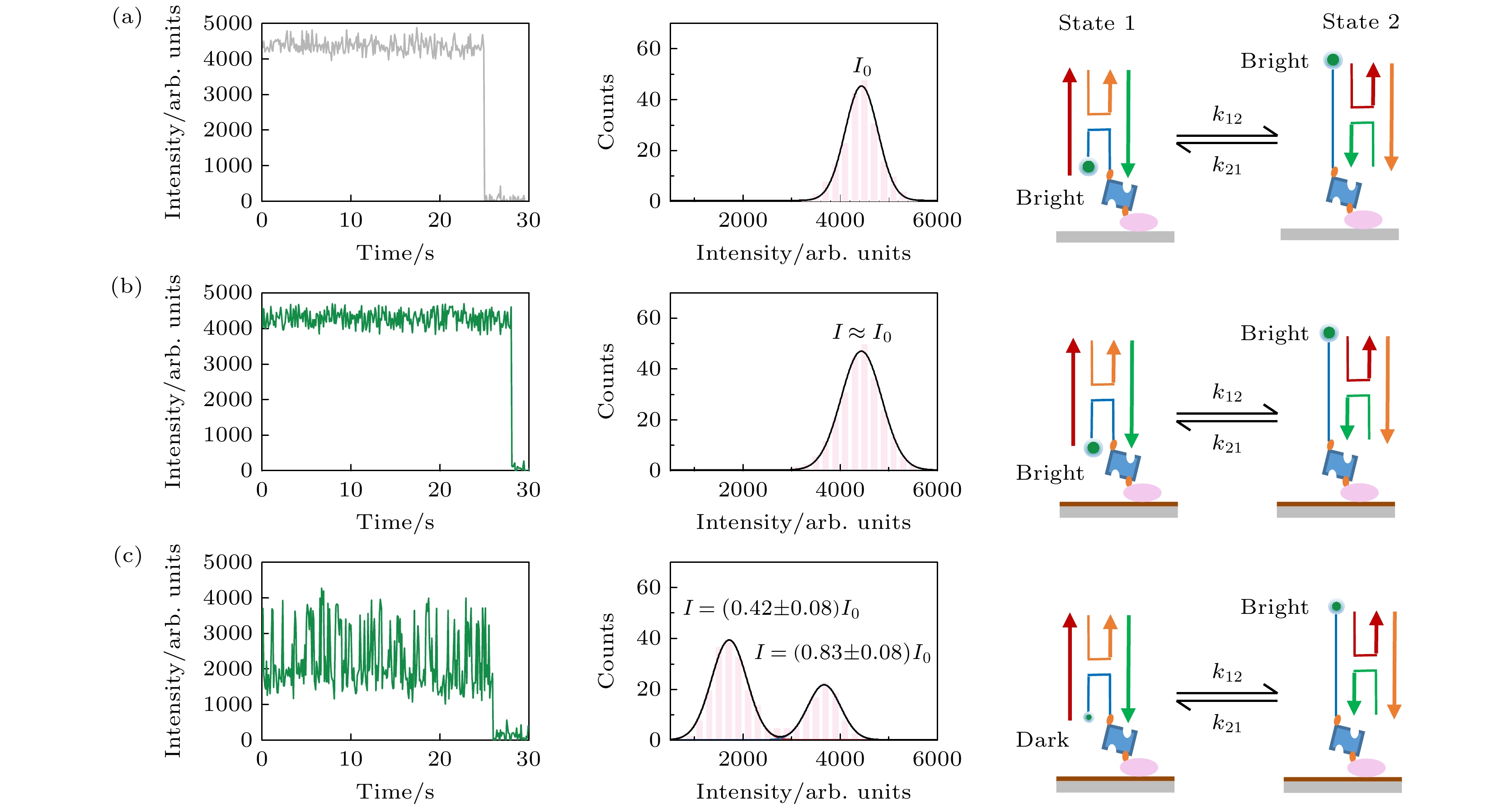
图 4 SIFA观察Holliday junction的构象变换, 在玻璃 (a), GO (b)和400 ℃-2 h-rGO (c)上观察Cy3标记的Holliday junction, 左列为单个Cy3的光强时间曲线, 中间为Cy3的光强统计图, 右列为Holliday junction构象变换导致Cy3光强变化的示意图
Figure 4. Observing conformational transformation of Holliday junction by SIFA, observing the Cy3 labeled Holliday junction on glass (a), GO (b) and 400 ℃-2 h-rGO (c), left columns show intensity-time curves of a single Cy3, middle columns show distribution of intensities of Cy3, right columns show schematic representation of the change of Cy3 light intensity caused by the conformational transformation of Holiday junction.
表 2 DNA Holliday junction的核苷酸序列
Table 2. Nucleotide sequence of DNA Holliday junction.
名称 核苷酸序列 X CCC AGT TGA GAG CTT GAT AGG G B CCC TAT CAA GCC GCT GTT ACG G R CCC ACC GCT CTT CTC AAC TGG G H biotin-CCG TAA CAG CGA GAG CGG TGG G(Cy3) 表 1 荧光标记DNA测量rGO的d0
Table 1. Determination of d0 of rGO by fluorescence labeled DNA.
样品 1 bp (7.5 nm) 9 bp (8.9 nm) 21 bp (10.9 nm) 300 ℃-2h-rGO I = (0.66 ± 0.08)I0
d0 = (6.4 ± 0.7) nmI = (0.79 ± 0.07)I0
d0 = (6.2 ± 0.6) nmI≈ I0 400 ℃-2h-rGO I = (0.45 ± 0.09)I0
d0 = (7.9 ± 0.7) nmI = (0.60 ± 0.10)I0
d0 = (8.1± 0.8) nmI = (0.80 ± 0.09)I0
d0 = (7.8 ± 0.9) nm -
[1] Lerner E, Barth A, Hendrix J, et al. 2021 Elife 10 e60416
 Google Scholar
Google Scholar
[2] Keller A M, DeVore M S, Stich D G, Vu D M, Causgrove T, Werner J H 2018 Anal. Chem. 90 6109
 Google Scholar
Google Scholar
[3] Ishikawa-Ankerhold H C, Ankerhold R, Drummen G P 2012 Molecules 17 4047
 Google Scholar
Google Scholar
[4] 贾棋, 樊秦凯, 侯文清, 杨晨光, 王利邦, 王浩, 徐春华, 李明, 陆颍 2021 70 158701
 Google Scholar
Google Scholar
Jia Q, Fan Q K, Hou W Q, Yang C G, Wang L B, Wang H, Xu C H, Li M, Lu Y 2021 Acta Phys. Sin. 70 158701
 Google Scholar
Google Scholar
[5] Almen M S, Nordstrom K J V, Fredriksson R, Schioth H B 2009 Bmc. Biology. 7 50
 Google Scholar
Google Scholar
[6] White S H, Wimley W C 1999 Annu. Rev. Bioph. Biom. 28 319
 Google Scholar
Google Scholar
[7] 马东飞, 侯文清, 徐春华, 赵春雨, 马建兵, 黄星榞, 贾棋, 马璐, 刘聪, 李明, 陆颖 2020 69 038701
 Google Scholar
Google Scholar
Ma D F, Hou W Q, Xu C H, Zhao C Y, Ma J B, Huang X Y, Jia Q, Ma L, Liu C, Li M, Lu Y 2020 Acta Phys. Sin. 69 038701
 Google Scholar
Google Scholar
[8] Ponmalar, II, Cheerla R, Ayappa K G, Basu J K 2019 Proc. Natl. Acad. Sci. USA 116 12839
 Google Scholar
Google Scholar
[9] King C, Raicu V, Hristova K 2017 J. Biol. Chem. 292 5291
 Google Scholar
Google Scholar
[10] King C, Sarabipour S, Byrne P, Leahy D J, Hristova K 2014 Biophys. J. 106 1309
 Google Scholar
Google Scholar
[11] Li Y, Qian Z, Ma L, Hu S, Nong D, Xu C, Ye F, Lu Y, Wei G, Li M 2016 Nat. Commun. 7 12906
 Google Scholar
Google Scholar
[12] Ma L, Li Y, Ma J B, Hu S X, Li M 2018 Biochemistry 57 4735
 Google Scholar
Google Scholar
[13] Jiang X, Yang C G, Qiu J, Ma D F, Xu C, Hu S X, Han W J, Yuan B, Lu Y 2022 Nanoscale 14 17654
 Google Scholar
Google Scholar
[14] Ma L, Hu S X, He X L, Yang N, Chen L C, Yang C G, Ye F F, Wei T T, Li M 2019 Nano. Lett. 19 6937
 Google Scholar
Google Scholar
[15] Kaminska I, Bohlen J, Yaadav R, Schuler P, Raab M, Schroder T, Zahringer J, Zielonka K, Krause S, Tinnefeld P 2021 Adv. Mater. 33 e2101099
 Google Scholar
Google Scholar
[16] Kaminska I, Bohlen J, Rocchetti S, Selbach F, Acuna G P, Tinnefeld P 2019 Nano. Lett. 19 4257
 Google Scholar
Google Scholar
[17] Federspiel F, Froehlicher G, Nasilowski M, Pedetti S, Mahmood A, Doudin B, Park S, Lee J O, Halley D, Dubertret B, Gilliot P, Berciaud S 2015 Nano. Lett. 15 1252
 Google Scholar
Google Scholar
[18] Gaudreau L, Tielrooij K J, Prawiroatmodjo G E D K, Osmond J, de Abajo F J G, Koppens F H L 2013 Nano. Lett. 13 2030
 Google Scholar
Google Scholar
[19] Li W, Wojcik M, Xu K 2019 Nano. Lett. 19 983
 Google Scholar
Google Scholar
[20] Eda G, Fanchini G, Chhowalla M 2008 Nat. Nanotechnol. 3 270
 Google Scholar
Google Scholar
[21] Pei S F, Cheng H M 2012 Carbon 50 3210
 Google Scholar
Google Scholar
[22] Stankovich S, Dikin D A, Piner R D, Kohlhaas K A, Kleinhammes A, Jia Y, Wu Y, Nguyen S T, Ruoff R S 2007 Carbon 45 1558
 Google Scholar
Google Scholar
[23] Sulowska K, Wiwatowski K, Szustakiewicz P, Grzelak J, Lewandowski W, Mackowski S 2018 Materials (Basel) 11 1567
 Google Scholar
Google Scholar
[24] Kim J, Cote L J, Kim F, Huang J X 2010 J. Am. Chem. Soc. 132 260
 Google Scholar
Google Scholar
[25] Kovtyukhova N I, Ollivier P J, Martin B R, Mallouk T E, Chizhik S A, Buzaneva E V, Gorchinskiy A D 1999 Chem. Mater. 11 771
 Google Scholar
Google Scholar
[26] Hummers W S, Offeman R E 1958 J. Am. Chem. Soc. 80 1339
 Google Scholar
Google Scholar
[27] Chen X, Meng D, Wang B, Li B W, Li W, Bielawski C W, Ruoff R S 2016 Carbon 101 71
 Google Scholar
Google Scholar
[28] Lazauskas A, Baltrusaitis J, Grigaliūnas V, Guobienė A, Prosyčevas I, Narmontas P, Abakevičienė B, Tamulevičius S 2014 Superlattices Microstruct. 75 461
 Google Scholar
Google Scholar
[29] Li J, Ma J, Kumar V, Fu H, Xu C, Wang S, Jia Q, Fan Q, Xi X, Li M, Liu H, Lu Y 2022 Nucleic. Acids. Res. 50 7002
 Google Scholar
Google Scholar
[30] Ma J B, Chen Z, Xu C H, Huang X Y, Jia Q, Zou Z Y, Mi C Y, Ma D F, Lu Y, Zhang H D, Li M 2020 Nucleic. Acids. Res. 48 3156
 Google Scholar
Google Scholar
[31] 陈 泽, 马建兵, 黄星榞, 贾棋, 徐春华, 张慧东, 陆颖 2018 67 118201
 Google Scholar
Google Scholar
Chen Z, Ma J B, Huang X Y, Jia Q, Xu C H, Zhang H D, Lu Y 2018 Acta Phys. Sin. 67 118201
 Google Scholar
Google Scholar
[32] Wei A, Wang J X, Long Q, Liu X M, Li X G, Dong X C, Huang W 2011 Mater. Res. Bull. 46 2131
 Google Scholar
Google Scholar
[33] Luo D, Zhang G, Liu J, Sun X 2011 J. Phys. Chem. C. 115 11327
 Google Scholar
Google Scholar
[34] Xu S T, Liu J K, Xue Y, Wu T Y, Zhang Z F 2017 Fuller. Nanotub. Car. N. 25 40
 Google Scholar
Google Scholar
[35] Zhen X J, Huang Y F, Yang S S, Feng Z Z, Wang Y, Li C H, Miao Y J, Yin H 2020 Mater. Lett. 260 126880
[36] Dessinges M N, Maier B, Zhang Y, Peliti M, Bensimon D, Croquette V 2002 Phys. Rev. Lett. 89 248102
 Google Scholar
Google Scholar
[37] Baumann C G, Smith S B, Bloomfield V A, Bustamante C 1997 Proc. Natl. Acad. Sci. U. S. A. 94 6185
 Google Scholar
Google Scholar
[38] Son S, Takatori S C, Belardi B, Podolski M, Bakalar M H, Fletcher D A 2020 Proc. Natl. Acad. Sci. U. S. A. 117 14209
 Google Scholar
Google Scholar
[39] Demirel G B, Caykara T 2009 Appl. Surf. Sci. 255 6571
 Google Scholar
Google Scholar
[40] Lu J R, Su T J, Thomas R K 1999 J. Colloid. Interf. Sci. 213 426
 Google Scholar
Google Scholar
[41] P. C. Weber J J W, f M. W. Pantoliano, and F. R. Salemme 1992 J. Am. Chem. Soc. 114 3197
 Google Scholar
Google Scholar
[42] Liu Y L, West S C 2004 Nat. Rev. Mol. Cell. Bio. 5 937
 Google Scholar
Google Scholar
[43] Clegg R M, Murchie A I, Lilley D M 1994 Biophysical. J. 66 99
 Google Scholar
Google Scholar
[44] McKinney S A, Tan E, Wilson T J, Nahas M K, Declais A C, Clegg R M, Lilley D M J, Ha T 2004 Biochem. Soc. T 32 41
 Google Scholar
Google Scholar
[45] McKinney S A, Declais A C, Lilley D M J, Ha T 2003 Nat. Struct. Biol. 10 93
 Google Scholar
Google Scholar
[46] Hohng S, Joo C, Ha T 2004 Biophys. J. 87 1328
 Google Scholar
Google Scholar
[47] Lee J, Lee S, Ragunathan K, Joo C, Ha T, Hohng S 2010 Angew. Chem. Int. Ed. Engl. 49 9922
 Google Scholar
Google Scholar
[48] Uphoff S, Holden S J, Le Reste L, Periz J, van de Linde S, Heilemann M, Kapanidis A N 2010 Nat. Methods. 7 831
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 6449
- PDF Downloads: 99
- Cited By: 0














 DownLoad:
DownLoad: