-
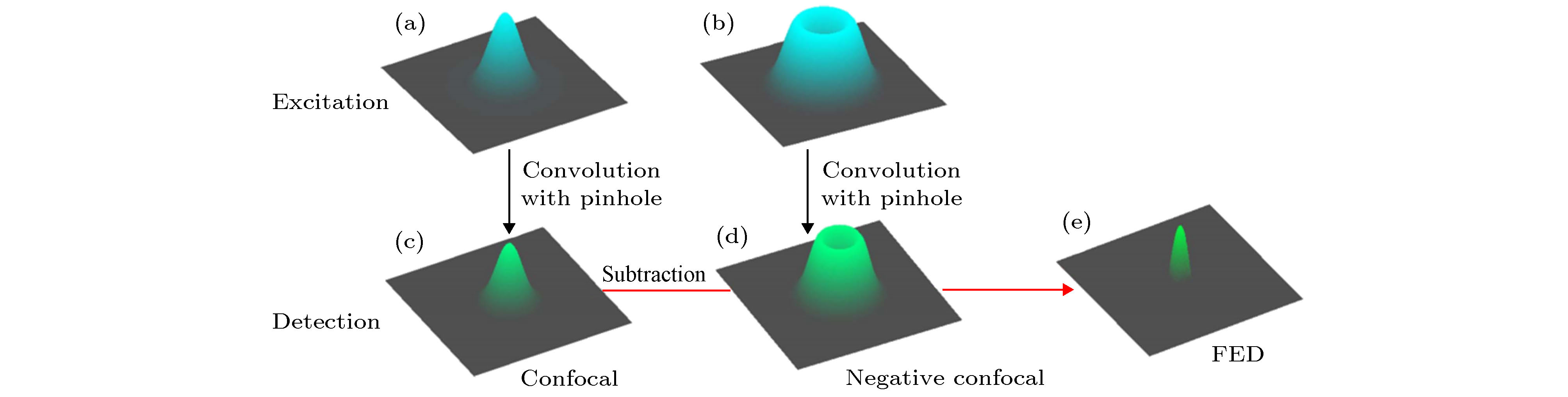
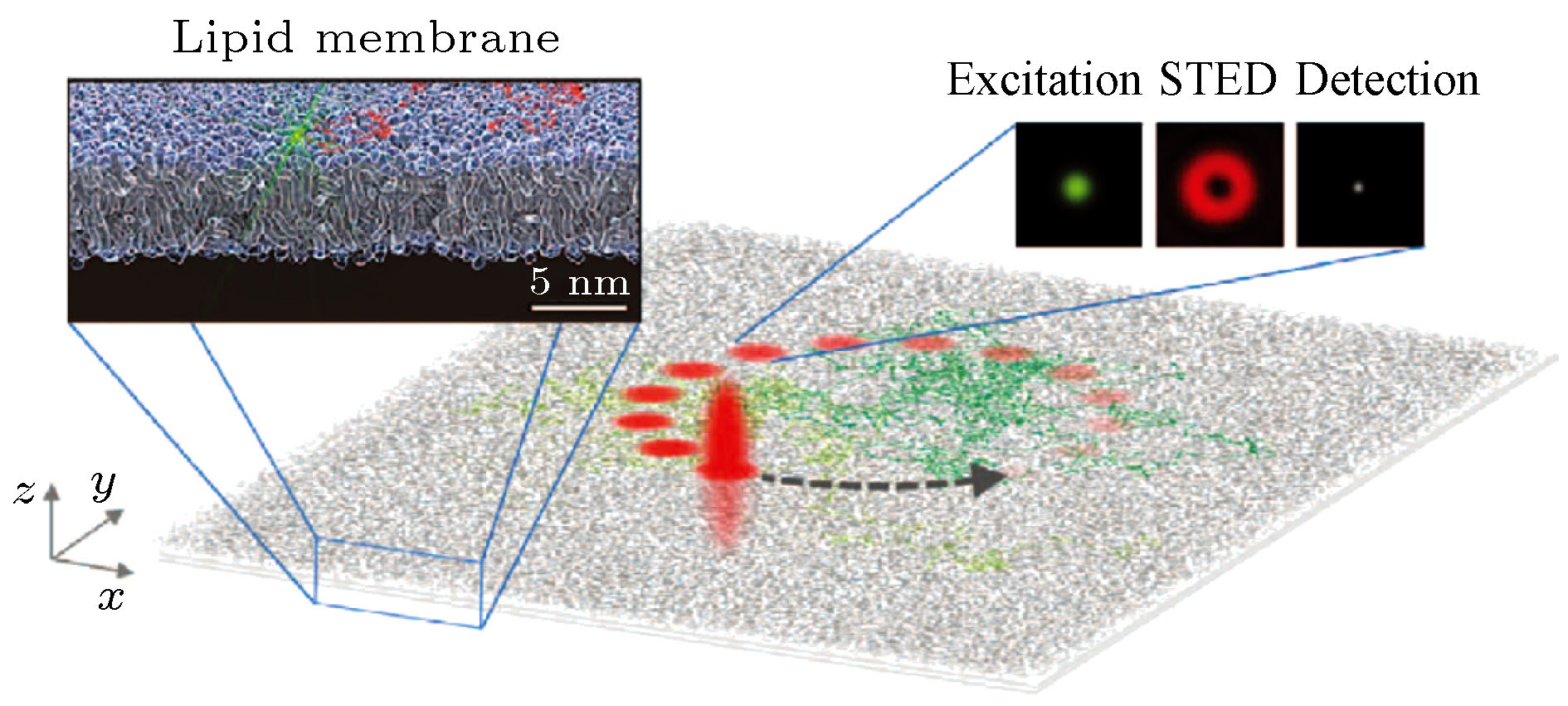
Due to the influence of the diffraction limit, the lateral spatial resolution and axial spatial resolution of traditional optical microscopes are limited to ~200 nm and ~500 nm, respectively. In the past two decades, with the rapid development of high-intensity lasers, high-sensitivity detectors and other optoelectronic devices, there have been reported many super-resolution imaging techniques that bypass the optical diffraction limit with different methods. Among these techniques, stimulated emission depletion microscopy (STED) technology has the advantages of high imaging resolution and fast imaging speed. This technology uses two lasers for imaging, one of which is used to excite fluorescence, and the other donut-shaped depletion laser is used to suppress the emission of fluorescent molecules around the fluorescent spot, in order to reduce the fluorescence point spread function and achieve super resolution Imaging. After recent years of development, the STED system has got great progress no matter from the generation, calibration and scanning of the beam, and the final imaging. In terms of laser source, new laser sources such as continuous wave beams, supercontinuum laser, stimulated Raman scattered laser, and higher-order Bessel beams have appeared; in scanning and calibration, new efficiency technology such as parallel scanning and automatic calibration have also appeared; In imaging, new methods such as time gating and phasor analysis have emerged to improve imaging quality. These new technologies and methods are of great significance to improve the efficiency of STED system construction and imaging. In addition, this paper also focuses on the ways to expand the imaging functions of the STED system. First, for three-dimensional STED imaging, this paper mainly introduces three methods to realize three-dimensional STED imaging by wavefront non-coherent adjustment, 4Pi and structured light illumination methods. Second, for multi-color imaging, this paper introduces several dual-color and multi-color imaging techniques for special dyes. Third, this paper introduces the combination of STED technology with fluorescence correlation spectroscopy technology, cell expansion technology, scanning ion-conductance microscope, photo-activated localization microscopy/stochastic optical reconstruction microscopy and other technologies. Finally, this paper systematically discusses the new research progress of STED technology in recent years, and discusses the future development trend of STED technology.
-
Keywords:
- stimulated emission depletionmicroscopy /
- three-dimensional imaging /
- multi-color imaging /
- super-resolution
[1] Hell SW, Wichmann J 1994 Opt. Lett. 19 780
 Google Scholar
Google Scholar
[2] Wildanger D, Patton B R, Schill H, et al. 2012 Adv. Mater. 24 309
[3] Willig K I, Harke B, Medda R, Hell S W 2007 Nat. Methods 4 915
 Google Scholar
Google Scholar
[4] Wildanger D, Rittweger E, Kastrup L, Hell S W 2008 Opt. Express 16 9614
 Google Scholar
Google Scholar
[5] Rankin B R, Kellner R R, Hell SW 2008 Opt. Lett. 33 2491
 Google Scholar
Google Scholar
[6] Török P, Munro P 2004 Opt. Express 12 3605
 Google Scholar
Google Scholar
[7] Yu W T, Ji Z H, Dong D S, Yang X S, Xiao Y F, Gong Q H, Xi P, Shi K B 2016 LaserPhotonicsRev. 10 147
[8] Zhang P, Goodwin P M, Werner J H 2014 Opt. Express 22 12398
 Google Scholar
Google Scholar
[9] Gould T J, Kromann E B, Burke D, et al. 2013 Opt. Lett. 38 1860
 Google Scholar
Google Scholar
[10] Reuss M, Engelhardt J, Hell S W 2010 Opt. Express 18 1049
 Google Scholar
Google Scholar
[11] Görlitz F, Guldbrand S, Runcorn T H, et al. 2018 J. Biophotonics. 11 e201800087
 Google Scholar
Google Scholar
[12] Yan L, Gregg P, Karimi E, et al. 2015 Optica 2 900
 Google Scholar
Google Scholar
[13] Gael M, Rebecca M, Birka H, Arnold G, Volker W, Hell SW 2010 Opt. Express 18 1302
 Google Scholar
Google Scholar
[14] MurJ, Kavčič B, PoberajI 2013 Appl. Opt. 52 6506
 Google Scholar
Google Scholar
[15] Wu Y, Wu X D, Toro L, Stefani E 2015 Methods 88 48
 Google Scholar
Google Scholar
[16] Wu X D, Toro L, Stefani E, Wu Y 2015 J. Microsc-Oxford 25 731
[17] Wagner O, Cheshnovsky O, Roichman Y 2013 Novel Techniques in Microscopy Waikoloa Beach, Hawaii, April 14–18, 2013 NM4 B.3
[18] Bingen P, Reuss M, Engelhardt J, Hell S W 2011 Opt. Express 19 23716
 Google Scholar
Google Scholar
[19] Lee S H, Grier D G 2005 Opt. Express 13 7458
 Google Scholar
Google Scholar
[20] Guerrieri F, Bellisai S, Tosi A, Padovini G, Tisa S 2010 23 rd Annual Meeting of the IEEEDenver, CO, USA November 7—11, 2010 p355
[21] Chang L K, Wang G C, Dolinsky S 2009 IEEETrans. Nucl. Sci. 56 2580
 Google Scholar
Google Scholar
[22] Diaspro A, Chirico G 2003 Cell. Tech. 126 195
[23] Helmchen F, Denk W 2005 Nat. Methods 2 932
 Google Scholar
Google Scholar
[24] Gael M, Hell SW 2009 Opt. Express 17 14567
 Google Scholar
Google Scholar
[25] Bianchini P, Harke B, Galiani S, Vicidomini G, Diaspro A 2012 Proc. Natl. Acad. Sci. 109 6390
 Google Scholar
Google Scholar
[26] Wang W S, Zhao G Y, Kuang C F, et al. 2018 Opt. Commun. 423 167
 Google Scholar
Google Scholar
[27] Göttfert F, Wurm C A, Mueller V, Berning S, Cordes V C, Honigmann A, Hell S W 2013 Biophys. J. 105 L01
 Google Scholar
Google Scholar
[28] Tønnesen J, Nadrigny F, Willig K, Wedlich-Söldner R, Nägerl U V 2011 Biophys. J. 101 2545
 Google Scholar
Google Scholar
[29] Bückers J, Wildanger D, Vicidomini G, KastrupL, Hell S W 2011 Opt. Express 19 3130
 Google Scholar
Google Scholar
[30] Wildanger D, Medda R, Kastrup L, Hell S W 2009 J. Microsc. 236 35
 Google Scholar
Google Scholar
[31] Hell S W, Stelzer E H K 1992 J. Opt. Soc. Am. A 9 2159
[32] Schmidt R, Wurm C A, Jakobs S, Engelhardt J, Egner A, Hell S W 2008 Nat. Methods 5 539
 Google Scholar
Google Scholar
[33] Yang X S, Xie H, Alonas E, Liu J, Chen X Z, Sangangelo P J, Ren Q, Xi Peng, Jin D Y 2016 Light Sci. Appl. 5 e16134
 Google Scholar
Google Scholar
[34] Gustafsson M G 2010 J. Microsc-Oxford 198 82
[35] Gustafsson M G, Lin S, Carlton P M, et al. 2008 Biophys. J. 94 4957
 Google Scholar
Google Scholar
[36] Xue Y, So P T C 2018 Opt. Express 26 20920
 Google Scholar
Google Scholar
[37] Xue Y, Kuang C F, Xiang H, Gu Z T, Xu L 2011 J. Opt. -UK 13 125704
 Google Scholar
Google Scholar
[38] Yan W, Yang Y L, Tan Y, Chen X, Li Y, Qu J L, Tong Y 2017 Photonics. Res. 5 176
 Google Scholar
Google Scholar
[39] Hovhannisyan V A, Su P J, Dong C Y 2008 J. Biomed. Opt. 13 44023
 Google Scholar
Google Scholar
[40] Wang L W, Yan W, Li R Z, Weng X Y, Zhang J, Yang Z G, Liu L W, Ye T, Qu J L 2018 Nanophotonics-Berlin 7 1971
 Google Scholar
Google Scholar
[41] Diaspro A, Tosi A, Boso G, Vicidomini G, Hernández I C, Buttafava M 2015 Biomed. Opt. Express 6 2258
 Google Scholar
Google Scholar
[42] Giuseppe V, Andreas S, Haisen T, Kyu Y H, Gael M, Christian E, Hell S W 2013 PlosOne 8 e54421
 Google Scholar
Google Scholar
[43] Castello M, Diaspro A, Vicidomini G 2015 Appl. Phys. Lett. 105 234106
[44] Wang Y F, Kuang C F, Gu Z T, Xu Y K, Li S, Hao X, Liu X 2013 Opt. Eng. 52 93107
 Google Scholar
Google Scholar
[45] Wang L W, Chen B L, Yan W, Yang Z G, Qu J L 2018 Nanoscale 10 1039
[46] Lanzanò L, Coto HI, Castello M, Gratton E, Diaspro A, Vicidomini G 2015 Nat. Commun. 66 701
[47] Tortarolo G, Sun Y, Teng KW, Ishitsuka Y, Vicidomini G 2019 Nanoscale 11 1754
 Google Scholar
Google Scholar
[48] Liu Y J, Lu Y Q, Yang X S, et al. 2017 Nature 543 229
 Google Scholar
Google Scholar
[49] Zhan Q Q, Liu H, Wang B, Wu Q, Pu R, Zhou C, Huang B, Peng X, He S 2017 Nat. Commun. 8 1058
 Google Scholar
Google Scholar
[50] Li D Y, Qin W, Xu B, Qian J, Tang B Z 2017 Adv. Mater. 29 1703643
 Google Scholar
Google Scholar
[51] Ye S, Yan W, Zhao M, Peng X, Song J, Qu J L 2018 Adv. Mater. 30 1800167
 Google Scholar
Google Scholar
[52] Li H, Ye S, Guo J, Wang H, Yan W, Song J, Qu J L 2019 Nano Res. 12 3075
 Google Scholar
Google Scholar
[53] Liang L, Yan W, Qin X, et al. 2020 Angew. Chem. 59 746
 Google Scholar
Google Scholar
[54] Kuang C F, Li S, Liu W, Hao X, Gu Z T, Wang Y F, Ge J H, Li H F, Liu X 2013 Sci. Rep. -UK 3 1441
 Google Scholar
Google Scholar
[55] Wang L W, Chen Y, Peng X, Zhang J, Wang J L, Liu L W, Yang Z G, Yan W, Qu J L 2020 Nanophotonics-BerlinDOI: 10.1515/nanoph-2019-0475
[56] Ries J, Schwille P 2012 BioEssays 34 361
 Google Scholar
Google Scholar
[57] Lars K, Hans B, Christian E, Hell SW 2005 Phys. Rev. Lett. 94 178104
 Google Scholar
Google Scholar
[58] Lanzanò L, Scipioni L, Bona MD, Bianchini P, Vicidomini G 2017 Nat. Commun. 8 65
 Google Scholar
Google Scholar
[59] Gould T J, Myers J R, Joerg B 2011 Opt. Express 19 13351
 Google Scholar
Google Scholar
[60] Marcel L, Christian R, Theo L, Hell S W, Christian E 2012 Opt. Express 20 5243
 Google Scholar
Google Scholar
[61] Honigmann A, Mueller V, Ta H, Schoenle A, Sezgin E, Hell S W, Eggeling C 2014 Nat. Commun. 5 5412
 Google Scholar
Google Scholar
[62] Chen F, Tillberg PW, Boyden ES 2015 Science 34 7543
[63] Gao M, Maraspini R, Beutel O, Zehtabian A, Ewers H 2018 ACSNano 12 4178
[64] Hansma P K, Drake B, Marti O, Gould S A, Prater C B 1989 Science 243 641
 Google Scholar
Google Scholar
[65] Gorelik J, Shevchuk A, Ramalho M, et al. 2002 Proc. Natl. Acad. Sci. U. S. A. 99 16018
 Google Scholar
Google Scholar
[66] Hagemann P, Gesper A, Happel P 2018 ACSNano 12 5807
[67] Eric B, Patterson G H, Rachid S, et al. 2006 Science 313 1642
 Google Scholar
Google Scholar
[68] Rust M J, Bates M, Zhuang X 2006 Nat. Methods 3 93
[69] Eilers Y, Ta H, Gwosch K C, Balzarotti F, Hell SW 2018 Proc. Natl. Acad. Sci. U. S. A. 115 6117
 Google Scholar
Google Scholar
[70] Göttfert F, Pleiner T, Heine J, Westphal V, Görlich D, Sahl SJ, Hell S W 2017 Proc. Natl. Acad. Sci. U. S. A. 114 201621495
[71] Bernhardt M J D N, Osterhoff M, Mittelst D H, et al. 2018 Nat. Commun. 9 3641
 Google Scholar
Google Scholar
[72] Harke B, Chacko J V, Haschke H, Canale C, Diaspro A 2012 Opt. Nanoscopy 1 3
 Google Scholar
Google Scholar
[73] Chacko J V, Canale C, Harke B, DiasproA 2013 PlosOne 8 e66608
 Google Scholar
Google Scholar
[74] Wegner W, Ilgen P, Gregor C, Dort J V, Mott A C, Steffens H, Willig K I 2017 Sci. Rep. 7 11781
 Google Scholar
Google Scholar
[75] Sebastian B, Willig K I, Heinz S, Payam D, Hell S W 2012 Science 335 551
 Google Scholar
Google Scholar
[76] Wijetunge LS, Julie A, Andreas F, Kind P C, Nägerl U V 2014 J. Neurosci. 34 6405
 Google Scholar
Google Scholar
-
图 6 共聚焦、传统STED与贝塞尔STED成像的分辨率随成像深度的变化曲线[7] (a) 40 nm荧光珠在固体琼脂糖样品不同深度成像的分辨率; (b) 40 nm荧光珠在类脑组织灰质不同深度成像的分辨率
Figure 6. Imaging curves of Confocal、traditional STED and Bessel-STED[7]: (a) Resolution of 40 nm fluorescent beads at different depths of solid agarose samples; (b) resolution of 40 nm fluorescent beads at different depths of like-gray matter in brain tissue.
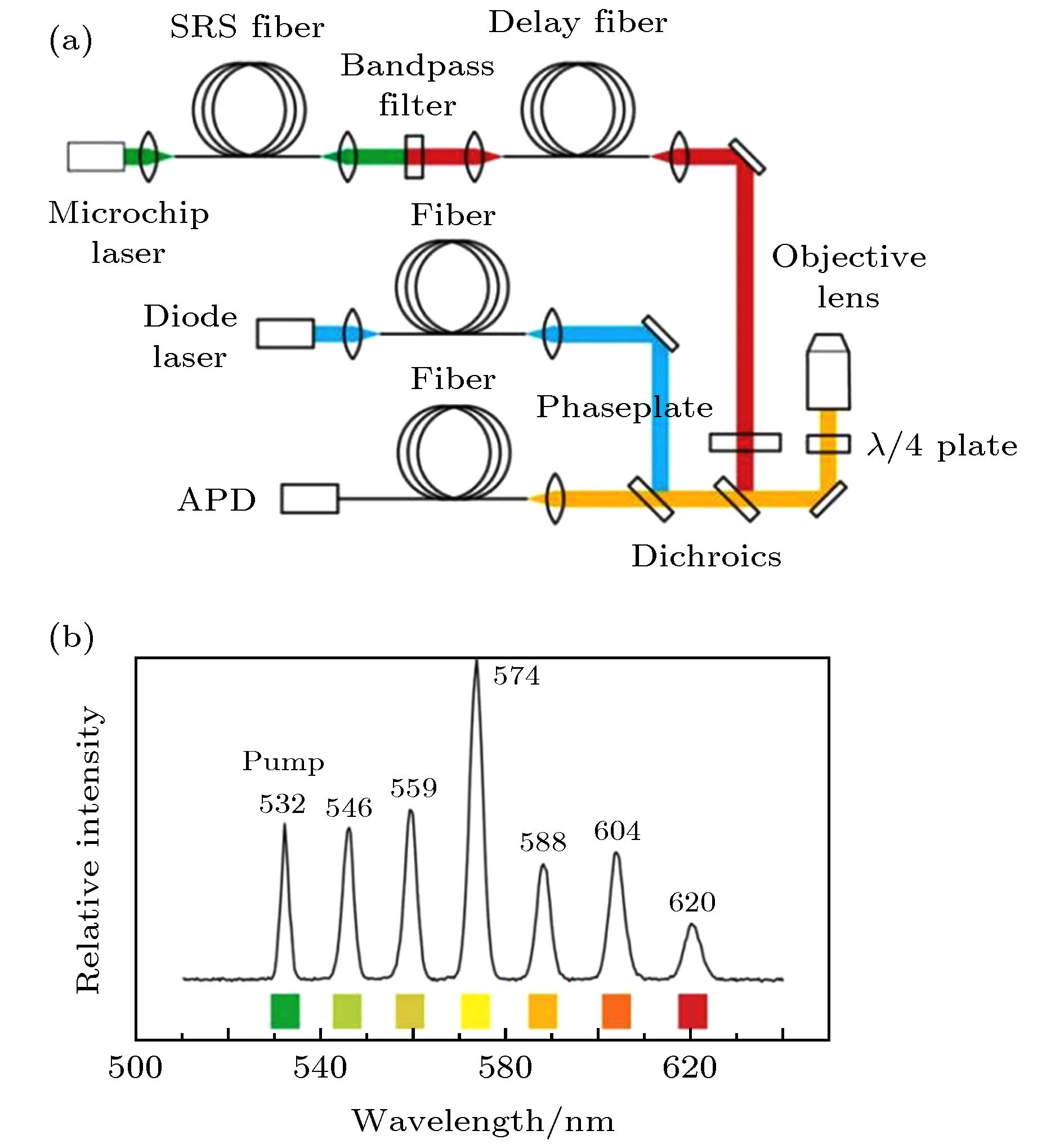
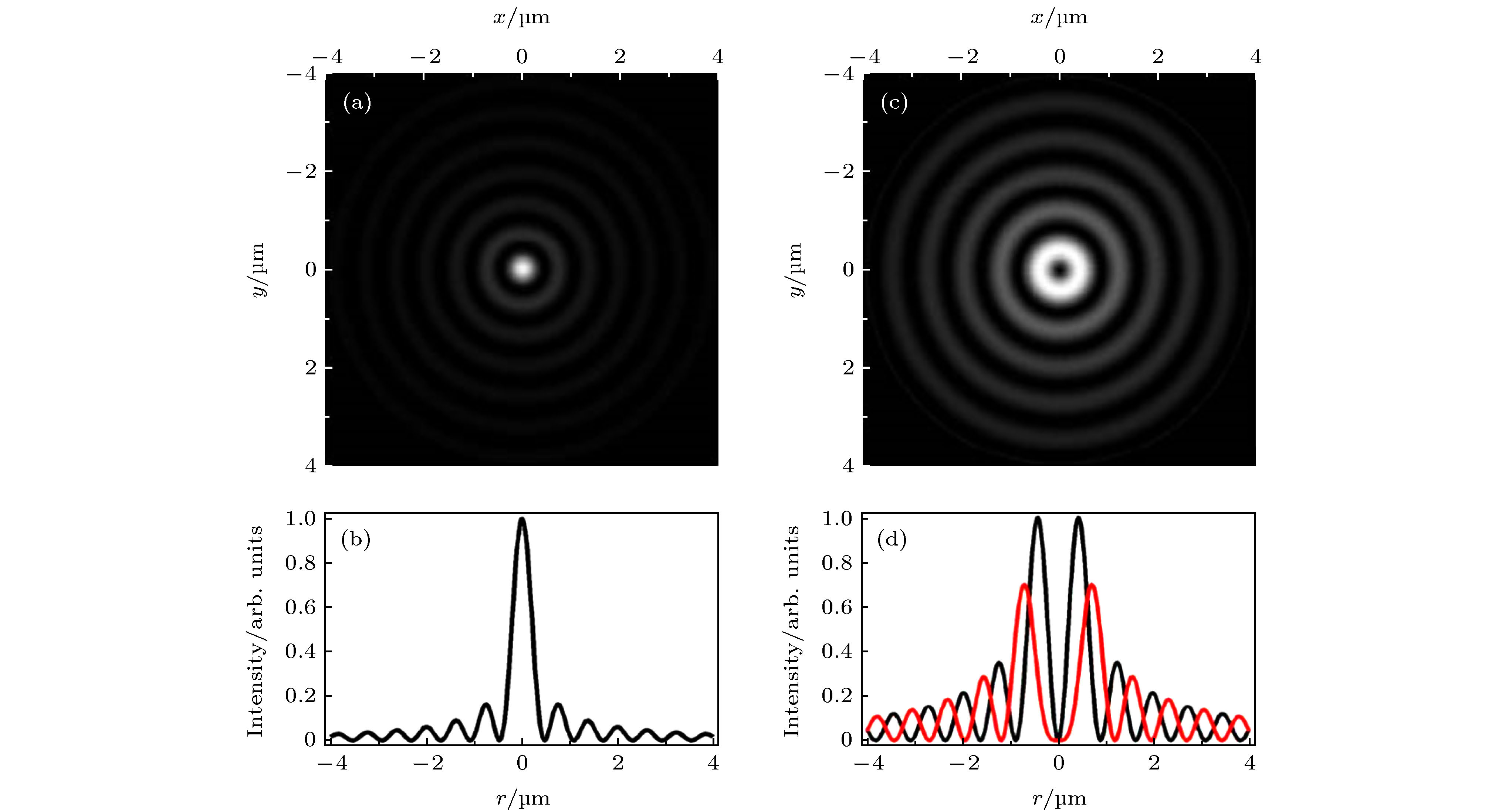
图 7 零阶贝塞尔激发光与高阶贝塞尔STED光[8] (a)零阶贝塞尔激发光在XY平面的光强分布; (b)零阶贝塞尔激发光的光强曲线; (c)一阶贝塞尔STED光在XY平面的光强分布; (d)一阶(黑色)与二阶(红色)贝塞尔STED光的光强曲线
Figure 7. The zero-order Bessel beam for excitation and the higher-order Bessel beam for depletion[8]: (a) The intensity distribution of zero-order Bessel excitation beam in the XY plane.; (b) the intensity curve of zero-order Bessel excitation beam; (c) the intensity distribution of first-order Bessel depletion beam in the XY plane; (d) the intensity curve of first-order (black) and second-order (red)Bessel depletion beam.
图 8 STED光束与激发光束的对准结果[9] (a)激发光与STED光未对准时的金纳米颗粒图像; (b) (a)中激发光与STED光未对准时所得到的荧光珠的共聚焦(绿色)与STED(红色)图像的合并; (c)沿图(b)中所示虚线的信号强度曲线: 共聚焦(绿色)和STED(红色); (d)激发光与STED光对准情况下的金纳米颗粒图像; (e) (d)中激发光与STED光对准时所得到的荧光珠的共聚焦(绿色)与STED(红色)图像的合并; (f)沿图(e)中所示直线和点划线的信号强度曲线: 共聚焦(绿色)和STED(红色)
Figure 8. Alignment results of depletion and excitation beams[9]: (a) Merged result of the excitation focus (green) and a poorly aligned depletion focus(red) using gold nanoparticles; (b) merged result of corresponding confocal (green) and STED(red) images of fluorescent beads imaged with the focus shown in (a); (c) line profiles across the dotted line in (b): confocal (green) and STED (red); (d) merged image of the excitation focus (green) and a well aligned depletion focus (red); (e) merged result of corresponding confocal (green) and STED (red) images of fluorescent beads imaged with the focus shown in (d); (f) line profiles across the solid and dashed lines in (e): confocal (green) and STED (red).
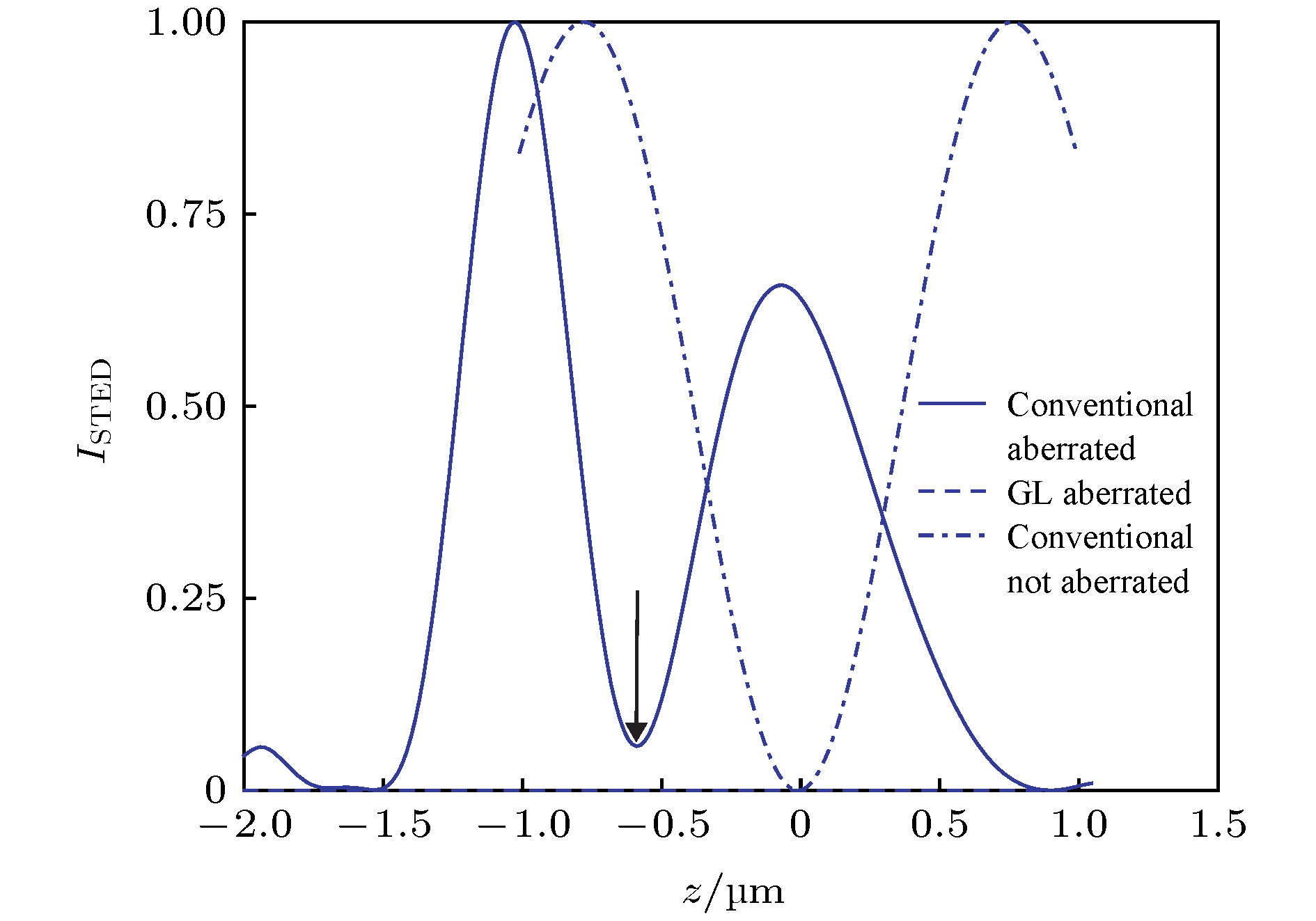
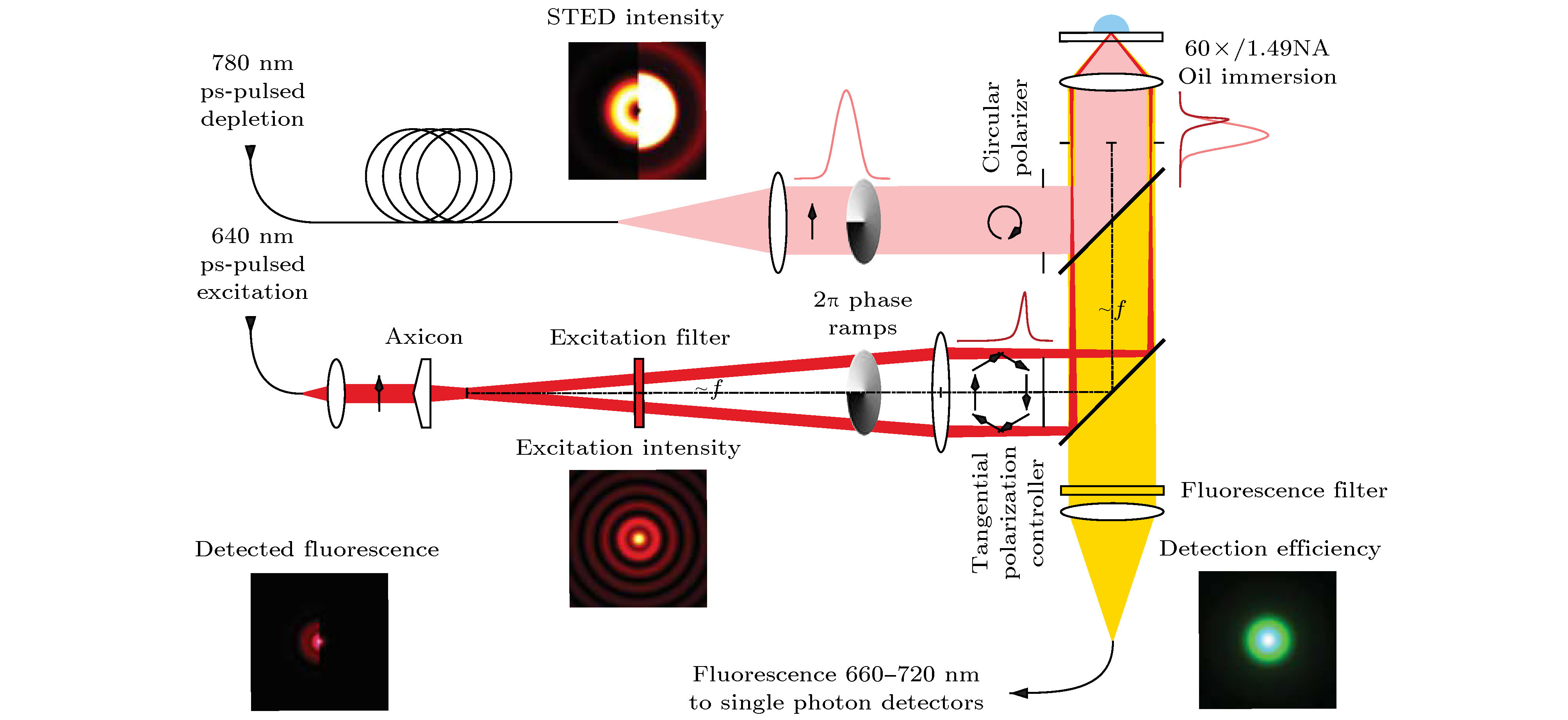
图 15 基于非相干波前调制的3D-STED成像系统示意图[30]. 插图: 用于实现横向超分辨(HR)或3D超分辨率(3D)的相位板及对应的STED PSF
Figure 15. Schematic diagram of 3D-STED system with non-coherent wavefront modulation[30]. Inset: combinations of phase plates and resulting STED PSFs used to achieve either ultimate lateral resolution (HR) or 3D superresolution.
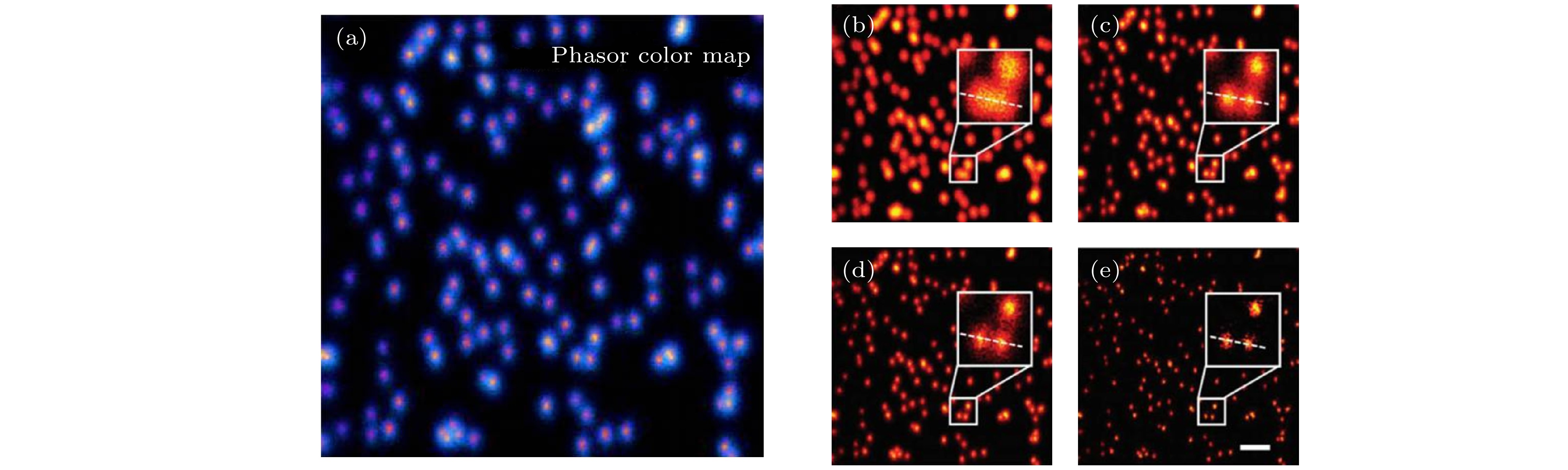
图 21 区域分割法与pSTED-SPLIT方法所得到的荧光珠的成像结果[47] (a)区域分割法示意图; (b) pSTED-SPLIT方法示意图; (c)从左到右依次为荧光珠的共聚焦、常规STED、区域分割法STED和pSTED-SPLIT的成像结果
Figure 21. Imaging results of fluorescent beads using segmentation and pSTED-SPLIT methods[47]: (a) Schematic diagram of segmentation method; (b) schematic diagram of pSTED-SPLIT method; (c) from left to right: Confocal, conventional STED, segmentation STED, and pSTED-SPLIT imaging results of fluorescence beads.
-
[1] Hell SW, Wichmann J 1994 Opt. Lett. 19 780
 Google Scholar
Google Scholar
[2] Wildanger D, Patton B R, Schill H, et al. 2012 Adv. Mater. 24 309
[3] Willig K I, Harke B, Medda R, Hell S W 2007 Nat. Methods 4 915
 Google Scholar
Google Scholar
[4] Wildanger D, Rittweger E, Kastrup L, Hell S W 2008 Opt. Express 16 9614
 Google Scholar
Google Scholar
[5] Rankin B R, Kellner R R, Hell SW 2008 Opt. Lett. 33 2491
 Google Scholar
Google Scholar
[6] Török P, Munro P 2004 Opt. Express 12 3605
 Google Scholar
Google Scholar
[7] Yu W T, Ji Z H, Dong D S, Yang X S, Xiao Y F, Gong Q H, Xi P, Shi K B 2016 LaserPhotonicsRev. 10 147
[8] Zhang P, Goodwin P M, Werner J H 2014 Opt. Express 22 12398
 Google Scholar
Google Scholar
[9] Gould T J, Kromann E B, Burke D, et al. 2013 Opt. Lett. 38 1860
 Google Scholar
Google Scholar
[10] Reuss M, Engelhardt J, Hell S W 2010 Opt. Express 18 1049
 Google Scholar
Google Scholar
[11] Görlitz F, Guldbrand S, Runcorn T H, et al. 2018 J. Biophotonics. 11 e201800087
 Google Scholar
Google Scholar
[12] Yan L, Gregg P, Karimi E, et al. 2015 Optica 2 900
 Google Scholar
Google Scholar
[13] Gael M, Rebecca M, Birka H, Arnold G, Volker W, Hell SW 2010 Opt. Express 18 1302
 Google Scholar
Google Scholar
[14] MurJ, Kavčič B, PoberajI 2013 Appl. Opt. 52 6506
 Google Scholar
Google Scholar
[15] Wu Y, Wu X D, Toro L, Stefani E 2015 Methods 88 48
 Google Scholar
Google Scholar
[16] Wu X D, Toro L, Stefani E, Wu Y 2015 J. Microsc-Oxford 25 731
[17] Wagner O, Cheshnovsky O, Roichman Y 2013 Novel Techniques in Microscopy Waikoloa Beach, Hawaii, April 14–18, 2013 NM4 B.3
[18] Bingen P, Reuss M, Engelhardt J, Hell S W 2011 Opt. Express 19 23716
 Google Scholar
Google Scholar
[19] Lee S H, Grier D G 2005 Opt. Express 13 7458
 Google Scholar
Google Scholar
[20] Guerrieri F, Bellisai S, Tosi A, Padovini G, Tisa S 2010 23 rd Annual Meeting of the IEEEDenver, CO, USA November 7—11, 2010 p355
[21] Chang L K, Wang G C, Dolinsky S 2009 IEEETrans. Nucl. Sci. 56 2580
 Google Scholar
Google Scholar
[22] Diaspro A, Chirico G 2003 Cell. Tech. 126 195
[23] Helmchen F, Denk W 2005 Nat. Methods 2 932
 Google Scholar
Google Scholar
[24] Gael M, Hell SW 2009 Opt. Express 17 14567
 Google Scholar
Google Scholar
[25] Bianchini P, Harke B, Galiani S, Vicidomini G, Diaspro A 2012 Proc. Natl. Acad. Sci. 109 6390
 Google Scholar
Google Scholar
[26] Wang W S, Zhao G Y, Kuang C F, et al. 2018 Opt. Commun. 423 167
 Google Scholar
Google Scholar
[27] Göttfert F, Wurm C A, Mueller V, Berning S, Cordes V C, Honigmann A, Hell S W 2013 Biophys. J. 105 L01
 Google Scholar
Google Scholar
[28] Tønnesen J, Nadrigny F, Willig K, Wedlich-Söldner R, Nägerl U V 2011 Biophys. J. 101 2545
 Google Scholar
Google Scholar
[29] Bückers J, Wildanger D, Vicidomini G, KastrupL, Hell S W 2011 Opt. Express 19 3130
 Google Scholar
Google Scholar
[30] Wildanger D, Medda R, Kastrup L, Hell S W 2009 J. Microsc. 236 35
 Google Scholar
Google Scholar
[31] Hell S W, Stelzer E H K 1992 J. Opt. Soc. Am. A 9 2159
[32] Schmidt R, Wurm C A, Jakobs S, Engelhardt J, Egner A, Hell S W 2008 Nat. Methods 5 539
 Google Scholar
Google Scholar
[33] Yang X S, Xie H, Alonas E, Liu J, Chen X Z, Sangangelo P J, Ren Q, Xi Peng, Jin D Y 2016 Light Sci. Appl. 5 e16134
 Google Scholar
Google Scholar
[34] Gustafsson M G 2010 J. Microsc-Oxford 198 82
[35] Gustafsson M G, Lin S, Carlton P M, et al. 2008 Biophys. J. 94 4957
 Google Scholar
Google Scholar
[36] Xue Y, So P T C 2018 Opt. Express 26 20920
 Google Scholar
Google Scholar
[37] Xue Y, Kuang C F, Xiang H, Gu Z T, Xu L 2011 J. Opt. -UK 13 125704
 Google Scholar
Google Scholar
[38] Yan W, Yang Y L, Tan Y, Chen X, Li Y, Qu J L, Tong Y 2017 Photonics. Res. 5 176
 Google Scholar
Google Scholar
[39] Hovhannisyan V A, Su P J, Dong C Y 2008 J. Biomed. Opt. 13 44023
 Google Scholar
Google Scholar
[40] Wang L W, Yan W, Li R Z, Weng X Y, Zhang J, Yang Z G, Liu L W, Ye T, Qu J L 2018 Nanophotonics-Berlin 7 1971
 Google Scholar
Google Scholar
[41] Diaspro A, Tosi A, Boso G, Vicidomini G, Hernández I C, Buttafava M 2015 Biomed. Opt. Express 6 2258
 Google Scholar
Google Scholar
[42] Giuseppe V, Andreas S, Haisen T, Kyu Y H, Gael M, Christian E, Hell S W 2013 PlosOne 8 e54421
 Google Scholar
Google Scholar
[43] Castello M, Diaspro A, Vicidomini G 2015 Appl. Phys. Lett. 105 234106
[44] Wang Y F, Kuang C F, Gu Z T, Xu Y K, Li S, Hao X, Liu X 2013 Opt. Eng. 52 93107
 Google Scholar
Google Scholar
[45] Wang L W, Chen B L, Yan W, Yang Z G, Qu J L 2018 Nanoscale 10 1039
[46] Lanzanò L, Coto HI, Castello M, Gratton E, Diaspro A, Vicidomini G 2015 Nat. Commun. 66 701
[47] Tortarolo G, Sun Y, Teng KW, Ishitsuka Y, Vicidomini G 2019 Nanoscale 11 1754
 Google Scholar
Google Scholar
[48] Liu Y J, Lu Y Q, Yang X S, et al. 2017 Nature 543 229
 Google Scholar
Google Scholar
[49] Zhan Q Q, Liu H, Wang B, Wu Q, Pu R, Zhou C, Huang B, Peng X, He S 2017 Nat. Commun. 8 1058
 Google Scholar
Google Scholar
[50] Li D Y, Qin W, Xu B, Qian J, Tang B Z 2017 Adv. Mater. 29 1703643
 Google Scholar
Google Scholar
[51] Ye S, Yan W, Zhao M, Peng X, Song J, Qu J L 2018 Adv. Mater. 30 1800167
 Google Scholar
Google Scholar
[52] Li H, Ye S, Guo J, Wang H, Yan W, Song J, Qu J L 2019 Nano Res. 12 3075
 Google Scholar
Google Scholar
[53] Liang L, Yan W, Qin X, et al. 2020 Angew. Chem. 59 746
 Google Scholar
Google Scholar
[54] Kuang C F, Li S, Liu W, Hao X, Gu Z T, Wang Y F, Ge J H, Li H F, Liu X 2013 Sci. Rep. -UK 3 1441
 Google Scholar
Google Scholar
[55] Wang L W, Chen Y, Peng X, Zhang J, Wang J L, Liu L W, Yang Z G, Yan W, Qu J L 2020 Nanophotonics-BerlinDOI: 10.1515/nanoph-2019-0475
[56] Ries J, Schwille P 2012 BioEssays 34 361
 Google Scholar
Google Scholar
[57] Lars K, Hans B, Christian E, Hell SW 2005 Phys. Rev. Lett. 94 178104
 Google Scholar
Google Scholar
[58] Lanzanò L, Scipioni L, Bona MD, Bianchini P, Vicidomini G 2017 Nat. Commun. 8 65
 Google Scholar
Google Scholar
[59] Gould T J, Myers J R, Joerg B 2011 Opt. Express 19 13351
 Google Scholar
Google Scholar
[60] Marcel L, Christian R, Theo L, Hell S W, Christian E 2012 Opt. Express 20 5243
 Google Scholar
Google Scholar
[61] Honigmann A, Mueller V, Ta H, Schoenle A, Sezgin E, Hell S W, Eggeling C 2014 Nat. Commun. 5 5412
 Google Scholar
Google Scholar
[62] Chen F, Tillberg PW, Boyden ES 2015 Science 34 7543
[63] Gao M, Maraspini R, Beutel O, Zehtabian A, Ewers H 2018 ACSNano 12 4178
[64] Hansma P K, Drake B, Marti O, Gould S A, Prater C B 1989 Science 243 641
 Google Scholar
Google Scholar
[65] Gorelik J, Shevchuk A, Ramalho M, et al. 2002 Proc. Natl. Acad. Sci. U. S. A. 99 16018
 Google Scholar
Google Scholar
[66] Hagemann P, Gesper A, Happel P 2018 ACSNano 12 5807
[67] Eric B, Patterson G H, Rachid S, et al. 2006 Science 313 1642
 Google Scholar
Google Scholar
[68] Rust M J, Bates M, Zhuang X 2006 Nat. Methods 3 93
[69] Eilers Y, Ta H, Gwosch K C, Balzarotti F, Hell SW 2018 Proc. Natl. Acad. Sci. U. S. A. 115 6117
 Google Scholar
Google Scholar
[70] Göttfert F, Pleiner T, Heine J, Westphal V, Görlich D, Sahl SJ, Hell S W 2017 Proc. Natl. Acad. Sci. U. S. A. 114 201621495
[71] Bernhardt M J D N, Osterhoff M, Mittelst D H, et al. 2018 Nat. Commun. 9 3641
 Google Scholar
Google Scholar
[72] Harke B, Chacko J V, Haschke H, Canale C, Diaspro A 2012 Opt. Nanoscopy 1 3
 Google Scholar
Google Scholar
[73] Chacko J V, Canale C, Harke B, DiasproA 2013 PlosOne 8 e66608
 Google Scholar
Google Scholar
[74] Wegner W, Ilgen P, Gregor C, Dort J V, Mott A C, Steffens H, Willig K I 2017 Sci. Rep. 7 11781
 Google Scholar
Google Scholar
[75] Sebastian B, Willig K I, Heinz S, Payam D, Hell S W 2012 Science 335 551
 Google Scholar
Google Scholar
[76] Wijetunge LS, Julie A, Andreas F, Kind P C, Nägerl U V 2014 J. Neurosci. 34 6405
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 21496
- PDF Downloads: 607
- Cited By: 0















 DownLoad:
DownLoad: