-
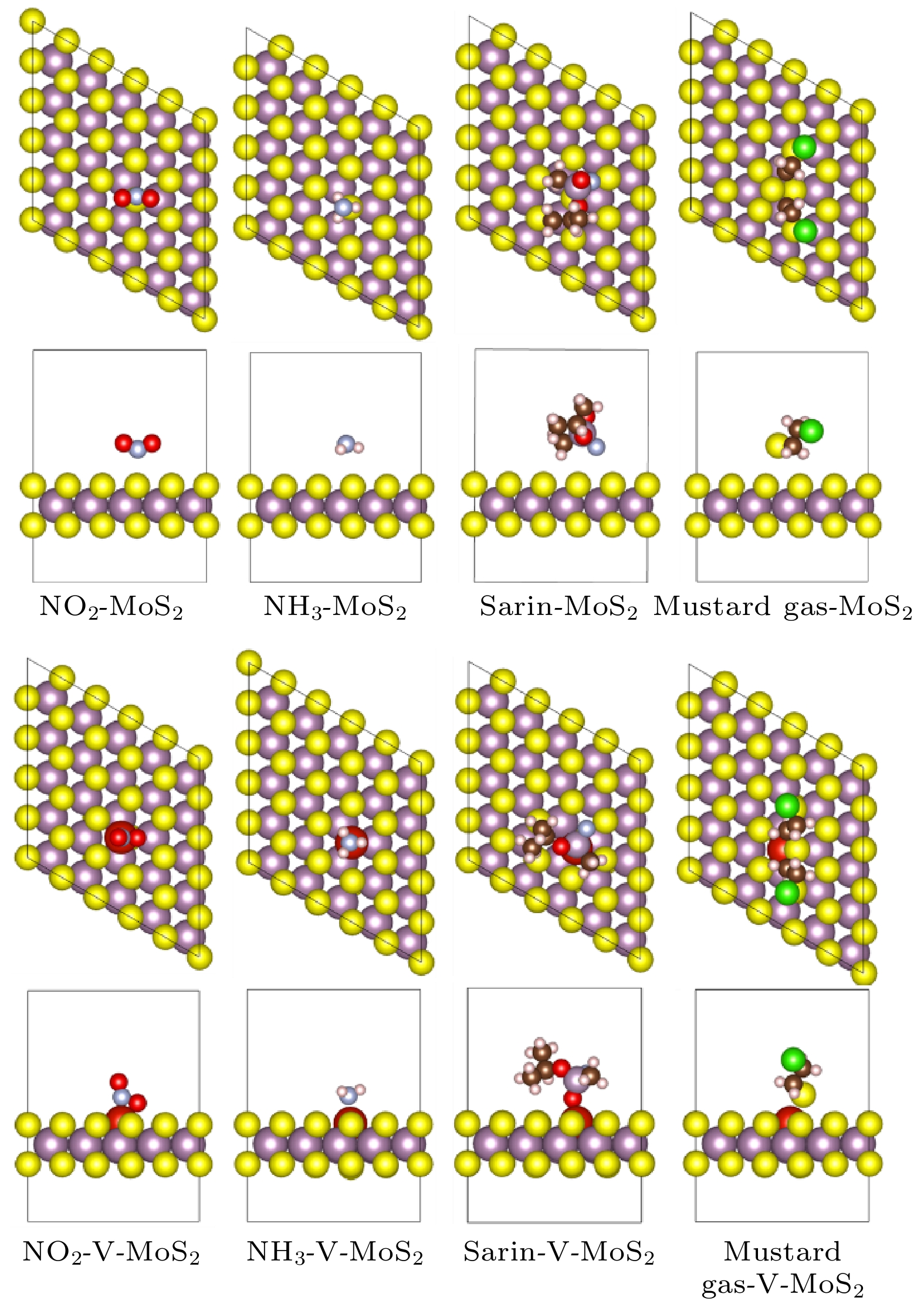
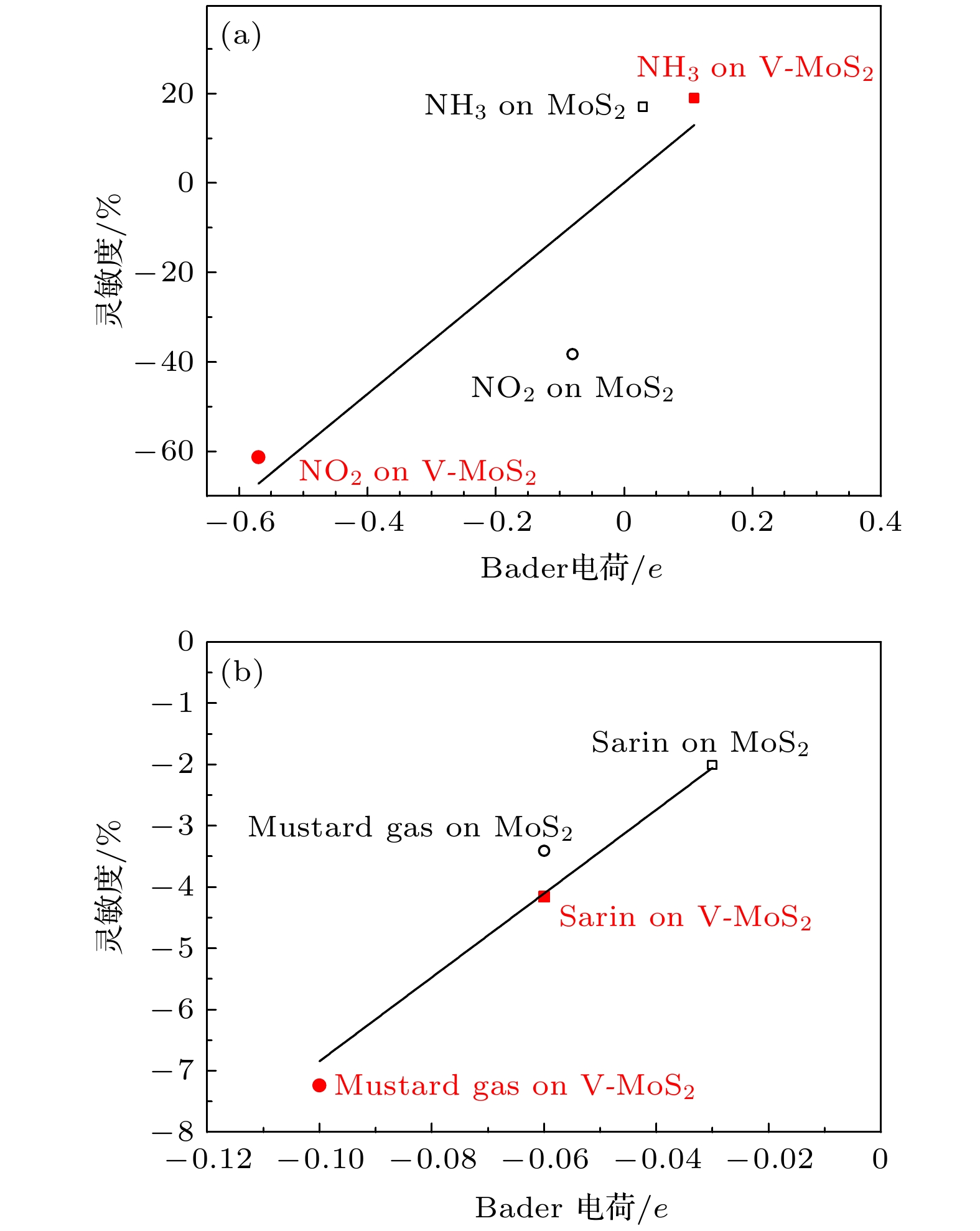
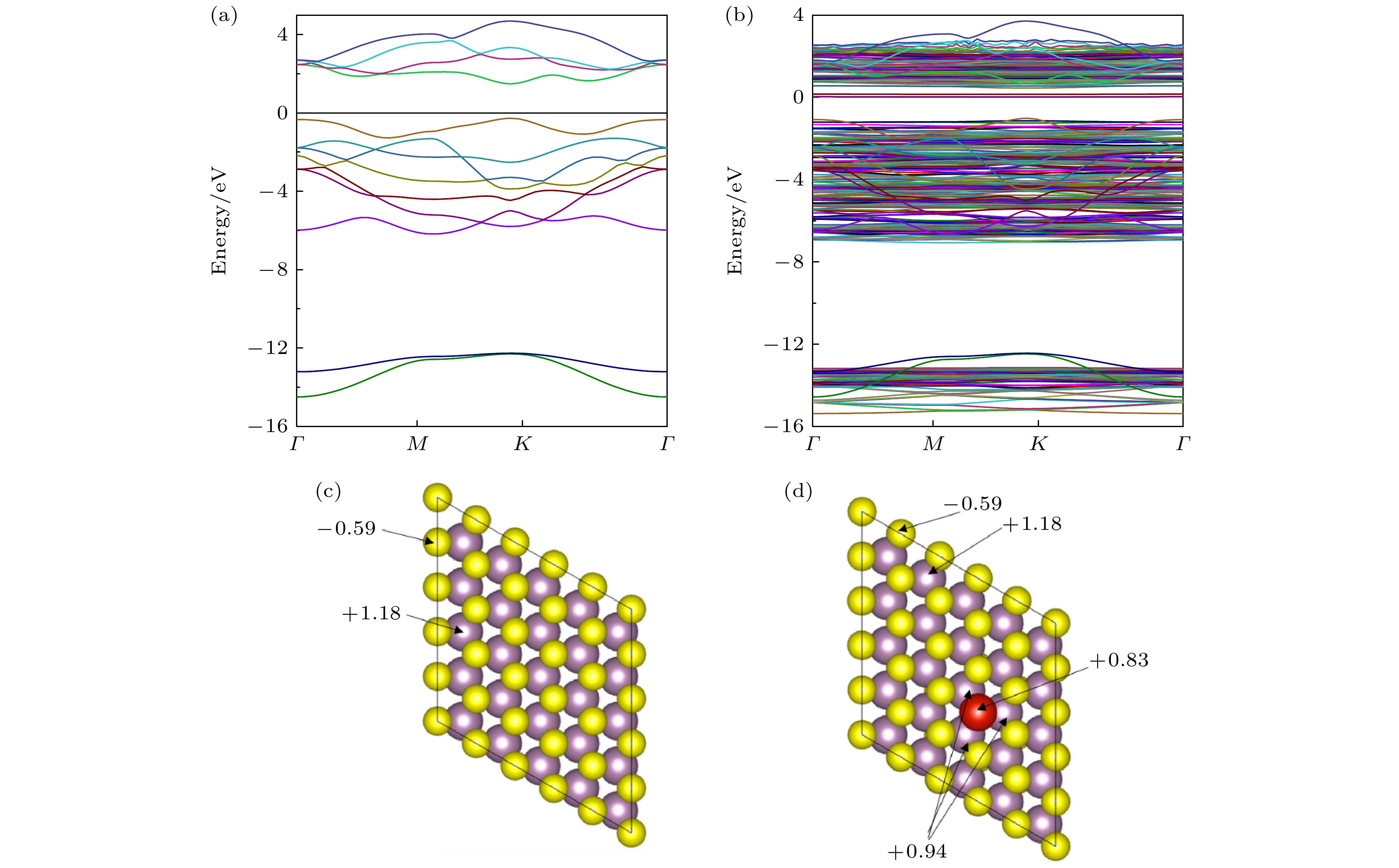
With their high toxicity and fast diffusion, toxic agents such as mustard gas and sarin are chemical warfare agents that are of high lethality and difficult to protect against. Therefore the high-sensitivity detection of toxic agents has become a focus in research on chemical detection in the world. Two-dimensional (2D) MoS2 is at the forefront of research because of its unique structure and promising sensing performance. In this study, theoretical calculations based on the first-principles method are carried out to investigate the structural stability, electronic properties, and gas adsorption of 2D MoS2 before and after V doping in order to explain the gas-sensing mechanism of V-doped 2D MoS2. The binding energy of V atom at the S-vacancy is –6.85 eV, indicating that the V atom can be stably doped into the S vacancy of the 2D MoS2 supercell structure at room temperature due to the strong interaction between the doped V atom and S vacancy of monolayer MoS2. The V atom doped into the 2D MoS2 system gives out electrons to surrounding Mo atoms as a donor center, thus enhancing the electric conductivity of the material. The calculation of adsorption energy indicates that the adsorption process of NO2, NH3, sarin, and mustard gas on the surface of 2D MoS2 are all spontaneous exothermic reactions. The doping of V increases the adsorption capacity of 2D MoS2 for the 4 aforesaid gases, and strengthens the interaction between the electrons of the absorbate molecules and those of substrate surface, thus effectively enhancing the gas-sensitive property of 2D MoS2. This effect occurs due to the strong overlap between the V 3d orbitals and gas molecule orbitals, which promotes the activation of the adsorbed gas molecules. The analysis of Bader charge shows that the charge transfer occurs from V-doped monolayer MoS2 to the oxidizing gas molecules (NO2, sarin, and mustard gas) acting as acceptors. Whereas the direction of charge transfers is reversed for the adsorption of the reducing gas (NH3) behaving as donors, in which 0.11e transfer from adsorbed gas to metal V-doped monolayer MoS2. Our results suggest that V-doped monolayer MoS2 is an ideal candidate for low-cost, highly active, and stable gas sensors, which provides an avenue to the design of high active 2D MoS2-based gas sensors.
-
Keywords:
- V-doping /
- 2 dimensional MoS2 /
- first principles calculation /
- gas-sensing mechanism
[1] 郑祖庆 1995 交通与港航 9 41
Zheng Z Q 1995 Public Util. 9 41
[2] 聂志勇, 吴弼东, 郭磊 2016 国际药学研究杂志 43 114
Nie Z Y, Wu B D, Guo L 2016 J. Int. Pharm. Res. 43 114
[3] Kim J, Cote L J, Kim F 2010 JACS 132 8180
 Google Scholar
Google Scholar
[4] Niu Z Q, Liu L L, Zhang L 2014 Adv. Mater. 26 3681
 Google Scholar
Google Scholar
[5] Shanmugasundaram A, Ramireddy B, Basak P 2014 J. Phys. Chem. C 118 6909
 Google Scholar
Google Scholar
[6] Yuan W J, Liu A R, Huang L 2013 Adv. Mater. 25 766
 Google Scholar
Google Scholar
[7] Shimizu T, Huang D Y, Yan F 2015 Chem. Rev. 115 6491
 Google Scholar
Google Scholar
[8] Choi S Y, Kim Y, Chung H S 2017 ACS Appl. Mater. Interfaces 9 3817
 Google Scholar
Google Scholar
[9] Baek J, Yin D, Liu N 2017 Nano Res. 10 1861
 Google Scholar
Google Scholar
[10] Ou J Z, Ge W Y, Carey B 2015 ACS Nano 9 10313
 Google Scholar
Google Scholar
[11] Late D J, Kanawade R V, Kannan P K 2016 Sens. Lett. 14 1249
 Google Scholar
Google Scholar
[12] Guo H Y, Lan C Y, Zhou Z F 2017 Nanoscale 9 6246
 Google Scholar
Google Scholar
[13] Li X G, Li X X, Li Z 2017 Sensor Actuat. B-Chem. 240 273
 Google Scholar
Google Scholar
[14] Abbasi A, Sardroodi J J 2018 Appl. Surf. Sci. 436 27
 Google Scholar
Google Scholar
[15] Huang Y X, Guo J H, Kang Y J 2015 Nanoscale 7 19358
 Google Scholar
Google Scholar
[16] Cui S M, Wen Z H, Huang X K 2015 Small 11 2305
 Google Scholar
Google Scholar
[17] Late D J, Huang Y K, Liu B 2013 ACS Nano 7 4879
 Google Scholar
Google Scholar
[18] Liu Y J, Hao L Z, Gao W 2015 Sensor Actuat. B-Chem. 211 537
 Google Scholar
Google Scholar
[19] Lee K, Gatensby R, Mcevoy N 2013 Adv. Mater. 25 6699
 Google Scholar
Google Scholar
[20] Huang H, Feng X, Du C C 2015 Chem. Commun. 51 7903
 Google Scholar
Google Scholar
[21] Si C D, Wu Y H, Sun Y F 2019 Electrochim. Acta 309 116
 Google Scholar
Google Scholar
[22] Zhang R F, Du Y B, Han G L 2019 J. Mater. Sci. 54 552
 Google Scholar
Google Scholar
[23] Zhao G, Li M 2018 Appl. Phys. A-Mater. 124 751
 Google Scholar
Google Scholar
[24] Koklioti M A, Bittencourt C, Noirfalise X 2018 ACS Appl. Mater. Interfaces 1 3625
-
表 1 四种探针分子在V掺杂前后二维MoS2表面的吸附能、吸附距离和Bader电荷
Table 1. Adsorption energy, adsorption distance, and Bader charge of four probe molecules on the surface of MoS2 before and after V doping.
吸附构型 吸附
能/eV吸附距
离/ÅBader电
荷ΔQ/eNH3-MoS2(0001) 5 × 5 × 1 –0.128 3.19 0.03 NO2-MoS2(0001) 5 × 5 × 1 –0.193 2.94 –0.08 Mustard gas-MoS2(0001)
5 × 5 × 1–0.535 2.86 –0.06 Sarin-MoS2(0001) 5 × 5 × 1 –0.420 2.82 –0.03 NH3-V-MoS2(0001) 5 × 5 × 1 –1.597 2.20 0.11 NO2-V-MoS2(0001) 5 × 5 × 1 –3.639 1.99 –0.57 Mustard gas-V-MoS2(0001)
5 × 5 × 1–1.641 2.45 –0.10 Sarin-V-MoS2(0001) 5 × 5 × 1 –2.198 2.10 –0.06 -
[1] 郑祖庆 1995 交通与港航 9 41
Zheng Z Q 1995 Public Util. 9 41
[2] 聂志勇, 吴弼东, 郭磊 2016 国际药学研究杂志 43 114
Nie Z Y, Wu B D, Guo L 2016 J. Int. Pharm. Res. 43 114
[3] Kim J, Cote L J, Kim F 2010 JACS 132 8180
 Google Scholar
Google Scholar
[4] Niu Z Q, Liu L L, Zhang L 2014 Adv. Mater. 26 3681
 Google Scholar
Google Scholar
[5] Shanmugasundaram A, Ramireddy B, Basak P 2014 J. Phys. Chem. C 118 6909
 Google Scholar
Google Scholar
[6] Yuan W J, Liu A R, Huang L 2013 Adv. Mater. 25 766
 Google Scholar
Google Scholar
[7] Shimizu T, Huang D Y, Yan F 2015 Chem. Rev. 115 6491
 Google Scholar
Google Scholar
[8] Choi S Y, Kim Y, Chung H S 2017 ACS Appl. Mater. Interfaces 9 3817
 Google Scholar
Google Scholar
[9] Baek J, Yin D, Liu N 2017 Nano Res. 10 1861
 Google Scholar
Google Scholar
[10] Ou J Z, Ge W Y, Carey B 2015 ACS Nano 9 10313
 Google Scholar
Google Scholar
[11] Late D J, Kanawade R V, Kannan P K 2016 Sens. Lett. 14 1249
 Google Scholar
Google Scholar
[12] Guo H Y, Lan C Y, Zhou Z F 2017 Nanoscale 9 6246
 Google Scholar
Google Scholar
[13] Li X G, Li X X, Li Z 2017 Sensor Actuat. B-Chem. 240 273
 Google Scholar
Google Scholar
[14] Abbasi A, Sardroodi J J 2018 Appl. Surf. Sci. 436 27
 Google Scholar
Google Scholar
[15] Huang Y X, Guo J H, Kang Y J 2015 Nanoscale 7 19358
 Google Scholar
Google Scholar
[16] Cui S M, Wen Z H, Huang X K 2015 Small 11 2305
 Google Scholar
Google Scholar
[17] Late D J, Huang Y K, Liu B 2013 ACS Nano 7 4879
 Google Scholar
Google Scholar
[18] Liu Y J, Hao L Z, Gao W 2015 Sensor Actuat. B-Chem. 211 537
 Google Scholar
Google Scholar
[19] Lee K, Gatensby R, Mcevoy N 2013 Adv. Mater. 25 6699
 Google Scholar
Google Scholar
[20] Huang H, Feng X, Du C C 2015 Chem. Commun. 51 7903
 Google Scholar
Google Scholar
[21] Si C D, Wu Y H, Sun Y F 2019 Electrochim. Acta 309 116
 Google Scholar
Google Scholar
[22] Zhang R F, Du Y B, Han G L 2019 J. Mater. Sci. 54 552
 Google Scholar
Google Scholar
[23] Zhao G, Li M 2018 Appl. Phys. A-Mater. 124 751
 Google Scholar
Google Scholar
[24] Koklioti M A, Bittencourt C, Noirfalise X 2018 ACS Appl. Mater. Interfaces 1 3625
Catalog
Metrics
- Abstract views: 11197
- PDF Downloads: 278
- Cited By: 0














 DownLoad:
DownLoad: