-
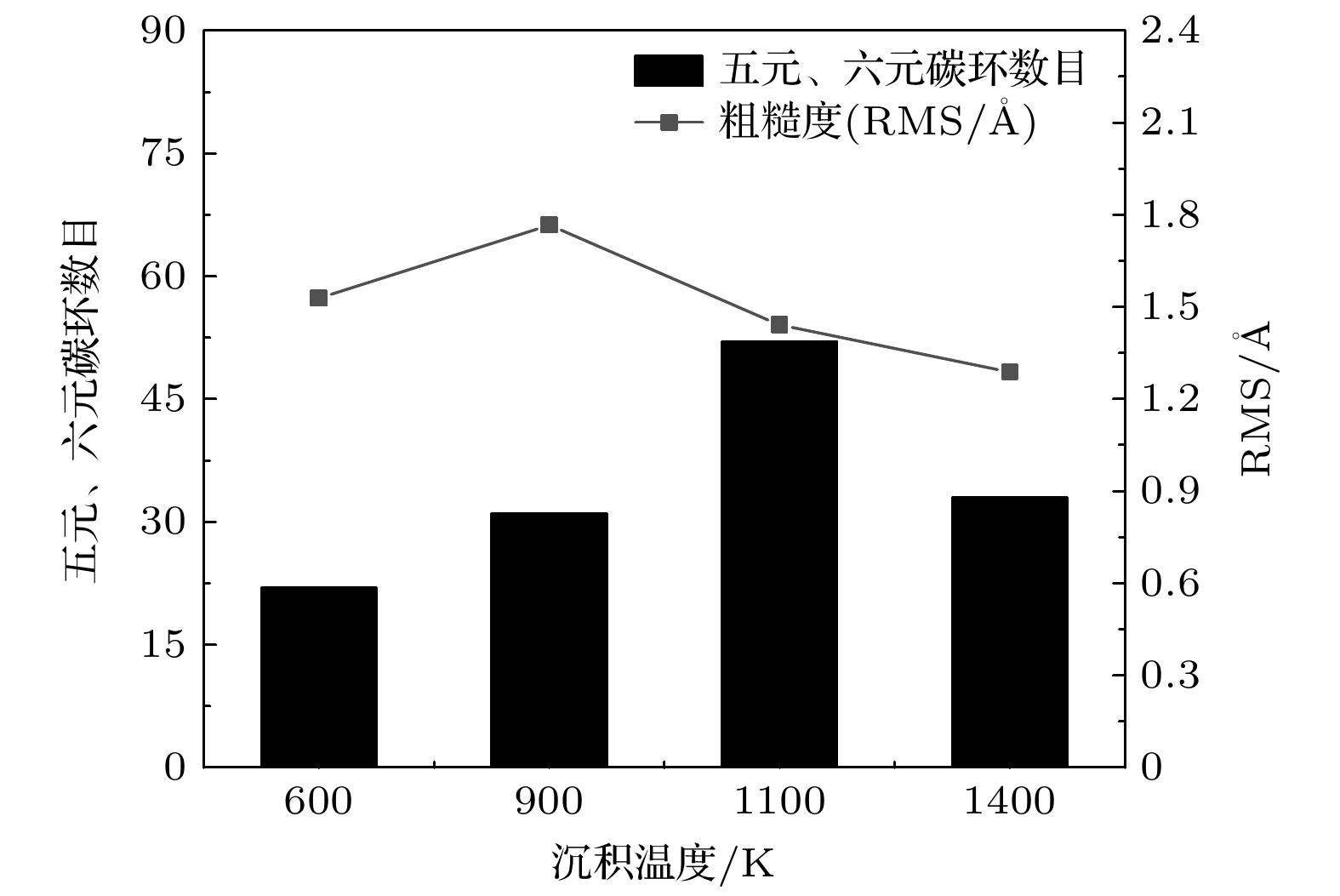
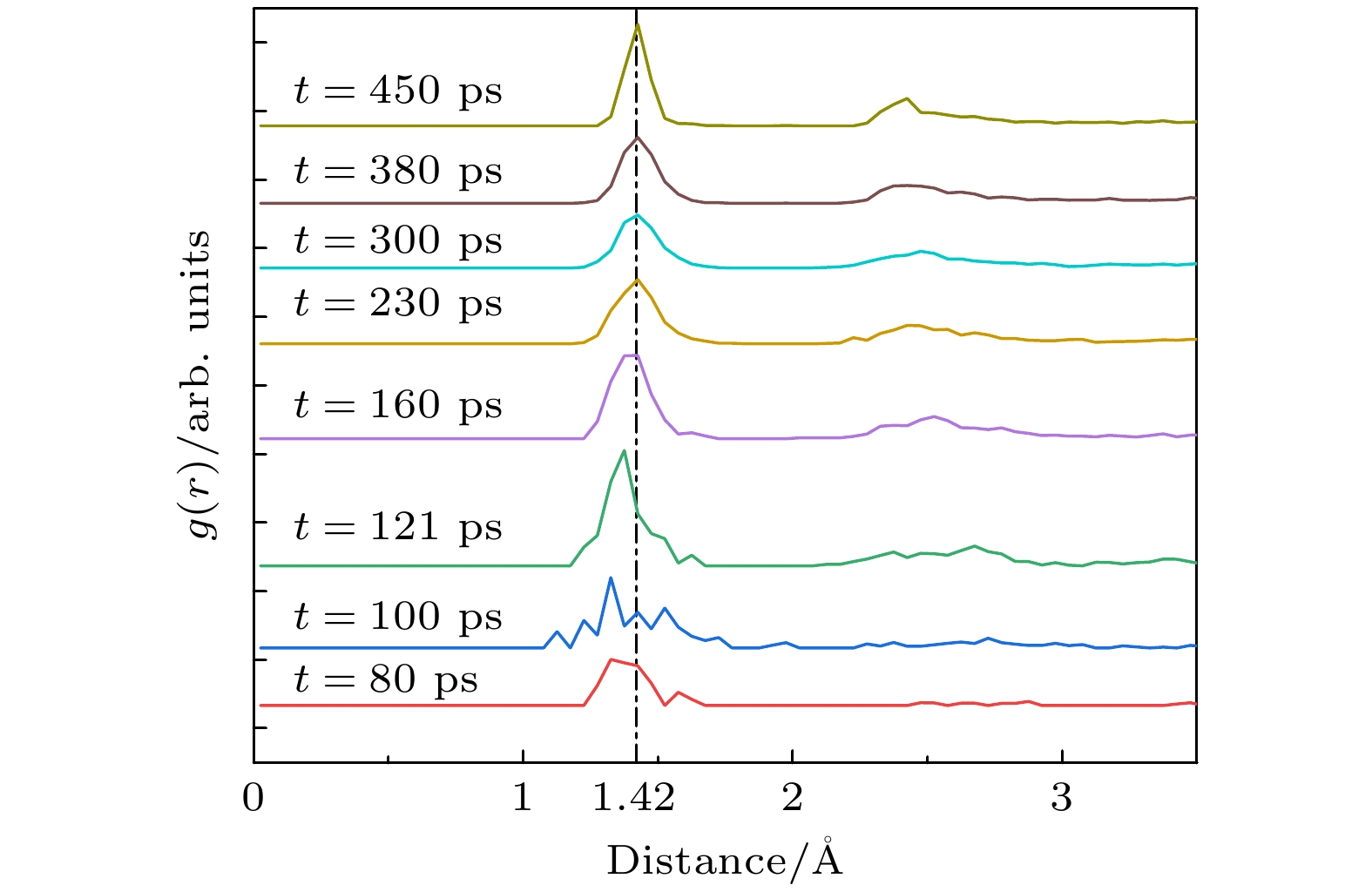
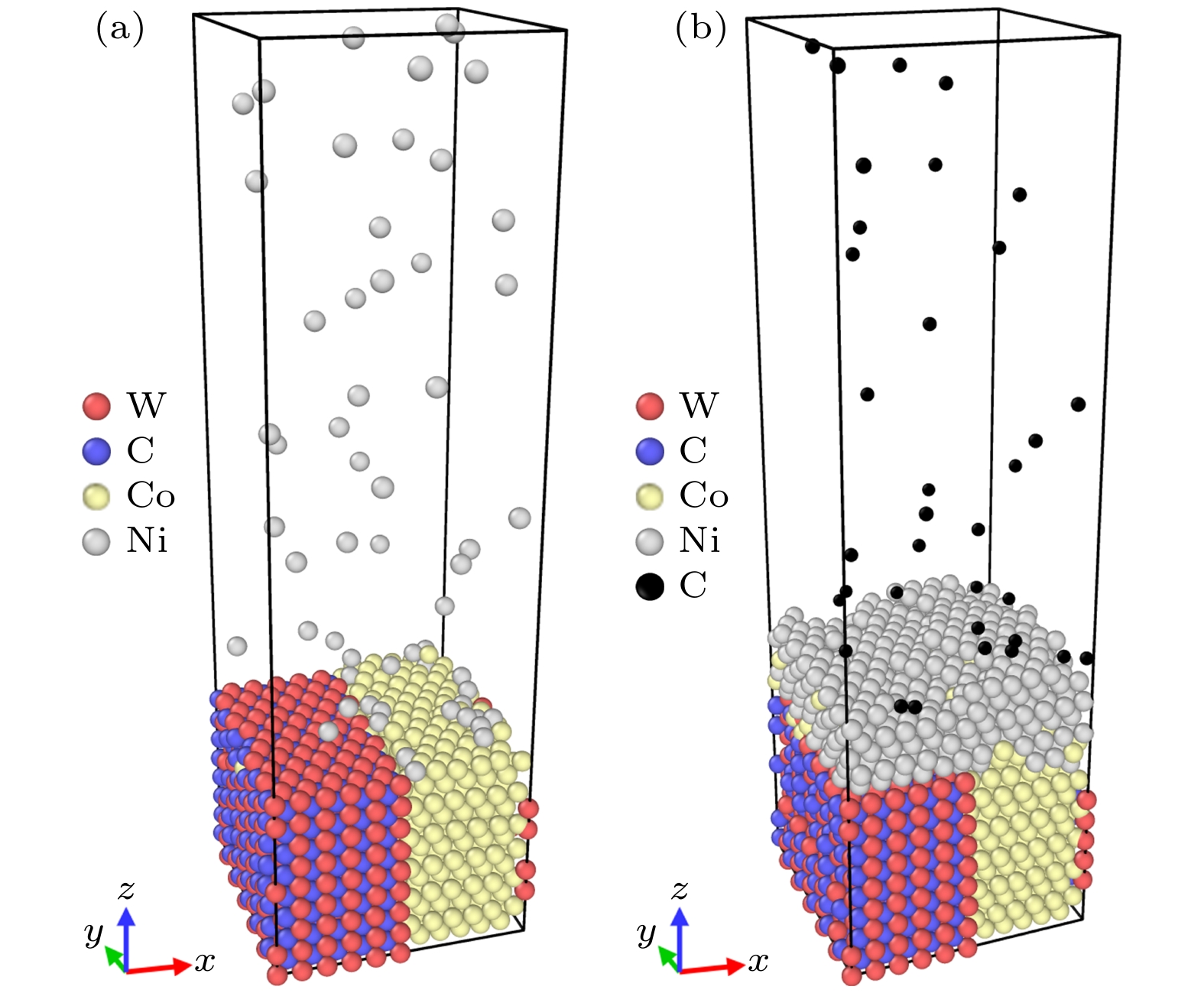
WC-Co cemented carbide has excellent cutting performance, which is a potential tool material. But when it is used as cutting ultra-high strength and high hardness materials, the machining accuracy and service life of the tool are significantly reduced. Graphene is a potential coating material for cemented carbide cutting tools due to its excellent mechanical properties. In this work, molecular dynamics (MD) is used to simulate the deposition of nickel transition layer and high-temperature catalytic growth of graphene in cemented carbide. The Ni and C atomic deposition process and the high temperature annealing process are simulated, and a combination of potential functions is adopted to continuously simulate these two deposition processes. The effect of deposition temperature and the effect of incident energy on the growth of graphene are analyzed. The healing mechanism of nickel-based catalytic defective graphene under high-temperature annealing is explored in detail. The simulation results show that at the deposition temperature of 1100 K, the coverage of graphene is higher and the microstructure is flat. The higher temperature helps to provide enough kinetic energy for carbon atoms to overcome the potential energy barrier of nucleation, thereby promoting the migration and rearrangement of carbon atoms and reducing graphene growth defects. Too high a temperature will lead to continuous accumulation of carbon atoms on the deposited carbon rings, forming a multilayered reticulation and disordered structure, which will cause a low coverage rate of graphene. The increase of incident energy helps to reduce the vacancy defects in the film, but excessive energy leads to poor continuity of the film, agglomeration, the more obvious stacking effect of carbon atoms and the tendency of epitaxial growth. When the incident energy is 1 eV, the surface roughness of the film is lower, and more monolayer graphene can be grown. During annealing at 1100 K, the carbon film dissolves and nucleates simultaneously in the Ni transition layer, and the nickel transition layer catalyzes the repair of defective graphene. The graphene film becomes more uniform, and the number of hexagonal carbon rings increases. Appropriate high-temperature annealing can help to repair and reconstruct defective carbon rings and rearrange carbon chains into rings. Therefore, when the deposition temperature is 1100 K and the incident energy is 1 eV, graphene can be deposited and annealed to grow a high-quality graphene coatings. The simulation results provide the reference for preparing the cemented carbide graphene coated tools. -
Keywords:
- molecular dynamics /
- graphene /
- physical vapour deposition /
- nickel transition layer /
- high temperature annealing
[1] 储开宇 2011 机床与液压 39 117
 Google Scholar
Google Scholar
Chu K Y 2011 Machine Tools Hydraul. 39 117
 Google Scholar
Google Scholar
[2] Tian Q Q, Huang N, Yang B, Zhuang H, Wang C, Zhai Z F, Li J H, Jia X Y, Liu L S, Jiang X 2017 J. Mater. Sci. Technol. 33 1097
 Google Scholar
Google Scholar
[3] Bouzakis K D, Michailidis N, Skordaris G, Bouzakis E, Biermann D, M'Saoubi R 2012 CIRP Ann. 61 703
 Google Scholar
Google Scholar
[4] Bobzin K, Brögelmann T, Kalscheuer C, Naderi M 2016 Surf. Coat. Technol. 308 349
 Google Scholar
Google Scholar
[5] Konstantiniuk F, Tkadletz M, Kainz C, Czettl C, Schalk N 2021 Surf. Coat. Technol. 410 126959
 Google Scholar
Google Scholar
[6] Wei Q P, Yu Z M, Ashfold M N, Chen Z, Wang L, Ma L 2010 Surf. Coat. Technol. 205 158
 Google Scholar
Google Scholar
[7] 吴张欣 2023 硕士学位论文 (上海: 华东理工大学)
Wu Z X 2023 M.S. Thesis (Shanghai: East China University of Science and Technology
[8] Kabir M S, Munroe P, Zhou Z, Xie Z 2017 Surf. Coat. Technol. 309 779
 Google Scholar
Google Scholar
[9] Contreras E, Galindez Y, Rodas M A, Bejarano G, Gómez M A 2017 Surf. Coat. Technol. 332 214
 Google Scholar
Google Scholar
[10] Suresh S 2001 Science 292 2447
 Google Scholar
Google Scholar
[11] Lu K 2014 Science 345 1455
 Google Scholar
Google Scholar
[12] Geim A K, Novoselov K S 2007 Nat. Mater. 6 183
 Google Scholar
Google Scholar
[13] 林奎鑫, 李多生, 叶寅, 江五贵, 叶志国, Qinghua Qin, 邹伟 2018 67 246802
 Google Scholar
Google Scholar
Lin K X, Li D S, Ye Y, Jiang W G, Ye Z G 2018 Acta Phys. Sin. 67 246802
 Google Scholar
Google Scholar
[14] Balandin A A, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F, Lau C N 2008 Nano Lett. 8 90
 Google Scholar
Google Scholar
[15] Lee C, Wei X, Kysar J W, Hone J 2008 Science 321 385
 Google Scholar
Google Scholar
[16] Bolotin K I, Sikes K J, Jiang Z, Klima M, Fudenberg G, Hone J, Stormer H L 2008 Solid State Commun. 146 351
 Google Scholar
Google Scholar
[17] Zhang Z, Du Y, Huang S, Meng F, Chen L, Xie W, Chang K, Zhang C, Lu Y, Lin C, Li S, Parkin I P, Guo D 2020 Adv. Sci. 7 1903239
 Google Scholar
Google Scholar
[18] Li S Z, Li Q Y, Carpick R W, Gumbsch P, Liu X Z, Ding X D, Li J 2016 Nature 539 541
 Google Scholar
Google Scholar
[19] Fan S Y, Chen Y N, Xiao S, Shi K J, Meng X Y, Lin S S, Su F H, Su Y F, Chu P K 2024 Carbon 216 118561
 Google Scholar
Google Scholar
[20] Min F L, Yu S B, Sheng W A N G, Yao Z H, Noudem J G, Liu S J, Zhang J F 2022 Trans. Nonferrous Met. Soc. China 32 1935
 Google Scholar
Google Scholar
[21] Garlow J A, Barrett L K, Wu L, Kisslinger K, Zhu Y, Pulecio J F 2016 Sci. Rep. 6 19804
 Google Scholar
Google Scholar
[22] Orofeo C M, Ago H, Hu B, Tsuji M 2011 Nano Res. 4 531
 Google Scholar
Google Scholar
[23] Reina A, Thiele S, Jia X, Bhaviripudi S, Dresselhaus M S, Schaefer J A, Kong J 2009 Nano Res. 2 509
 Google Scholar
Google Scholar
[24] Seo J H, Lee H W, Kim J K, Kim D G, Kang J W, Kang M S, Kim C S 2012 Curr. Appl. Phys. 12 S131
 Google Scholar
Google Scholar
[25] Li X S, Cai W W, Colombo L, Ruoff R S 2009 Nano Lett. 9 4268
 Google Scholar
Google Scholar
[26] Li X, Li H, Lee K R, Wang A 2020 Appl. Surf. Sci. 529 147042
 Google Scholar
Google Scholar
[27] 王泽, 李国禄, 王海斗, 徐滨士, 康嘉杰 材料导报 28 91
Wang Z, Li G L, Wang H D, Xu B S, Kang J J 2014 Mater. Rev. 28 91
[28] Kato T, Nagai T, Sasajima Y, Onuki J 2010 Mater. Trans. 51 664
 Google Scholar
Google Scholar
[29] 丁业章, 叶寅, 李多生, 徐锋, 朗文昌, 刘俊红, 温鑫 2023 72 068703
 Google Scholar
Google Scholar
Ding Y Z, Ye Y, Li D S, Xu F, Lang W C, Liu J H, Wen X 2023 Acta Phys. Sin. 72 068703
 Google Scholar
Google Scholar
[30] Backholm M, Foss M, Nordlund K 2013 Appl. Surf. Sci. 268 270
 Google Scholar
Google Scholar
[31] Shibuta Y, Elliott J A 2009 Chem. Phys. Lett. 472 200
 Google Scholar
Google Scholar
[32] Juslin N, Erhart P, Träskelin P, Nord J, Henriksson K O E, Nordlund K, Salonen E, Albe K 2005 J. Appl. Phys. 98 123520
 Google Scholar
Google Scholar
[33] Béland L K, Lu C, Osetskiy Y N, Samolyuk G D, Caro A, Wang L, Stoller R E 2016 J. Appl. Phys. 119 085901
 Google Scholar
Google Scholar
[34] Morse P M 1929 Phys. Rev. 34 57
 Google Scholar
Google Scholar
[35] 冯艳, 段海明 2011 原子与分子 28 251
 Google Scholar
Google Scholar
Feng Y, Duan H M 2011 J. At. Mol. Phys. 28 251
 Google Scholar
Google Scholar
[36] Stuart S J, Tutein A B, Harrison J A 2000 J. Chem. Phys. 112 6472
 Google Scholar
Google Scholar
[37] Jones J E 1924 Proc. Roy. Soc. A 106 463
[38] Cao Q, Chen Y J, Shao W, Ma X T, Zheng C, Cui Z, Liu Y, Yu B 2020 J. Mol. Liq. 319 114218
 Google Scholar
Google Scholar
[39] 王涛 2010 硕士学位论文 (湘潭: 湘潭大学)
Wang T 2010 M. S. Thesis ( Xiangtan: Xiangtan University
[40] Mueller J E, Van Duin A C, Goddard III W A 2010 J. Phys. Chem. C 114 4939
 Google Scholar
Google Scholar
[41] Loginova E, Bartelt N C, Feibelman P J, McCarty K F 2008 New J. Phys. 10 093026
 Google Scholar
Google Scholar
[42] Pagon A M, Partridge J G, Hubbard P, Taylor M B, McCulloch D G, Doyle E D, Li G 2010 Surf. Coat. Technol. 204 3552
 Google Scholar
Google Scholar
[43] 王璐, 高峻峰, 丁峰 2014 化学学报 72 345
 Google Scholar
Google Scholar
Wang L, Gao J F, Ding F 2014 Acta Chim. Sin. 72 345
 Google Scholar
Google Scholar
[44] 戴达煌 2008 薄膜与涂层现代表面技术(长沙: 中南大学出版社) 第411页
Dai D H 2008 Thin Films and Coatings Modern Surface Technology (Changsha: Central South University Press) p411
[45] Chen S D, Bai Q S, Wang H F, Dou Y H, Guo W 2022 Physics E 144 115465
 Google Scholar
Google Scholar
[46] Chen S, Xiong W, Zhou Y S, Lu Y F, Zeng X C 2016 Nanoscale 8 9746
 Google Scholar
Google Scholar
[47] Rut’kov E V, Gall N R 2011 Equilibrium Nucleation, Growth, and Thermal Stability of Graphene on Solids (Russia St. Petersburg: inTech) pp209–292
-
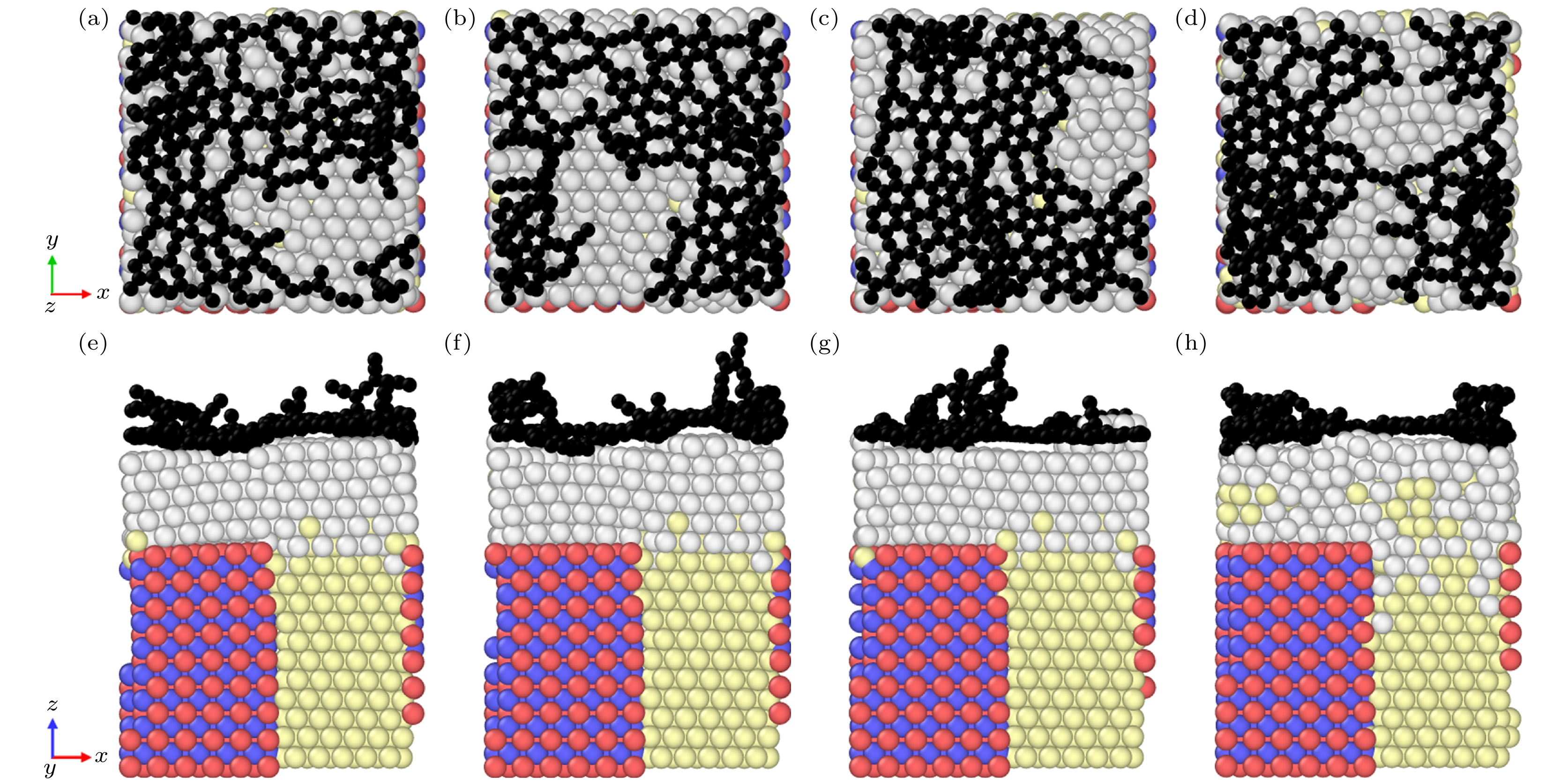
图 3 不同温度下石墨烯生长的俯视图和主视图 (a) 600 K时的俯视图; (b) 900 K时的俯视图; (c) 1100 K时的俯视图; (d) 1400 K时的俯视图; (e) 600 K时的主视图; (f) 900 K时的主视图; (g) 1100 K时的主视图; (i) 1400 K时的主视图
Figure 3. Top and main views of graphene growth at different temperatures: (a) Top view at 600 K; (b) top view at 900 K; (c) top view at 1100 K; (d) top view at 1400 K; (e) main view at 600 K; (f) main view at 900 K; (g) main view at 1100 K; (i) main view at 1400 K.
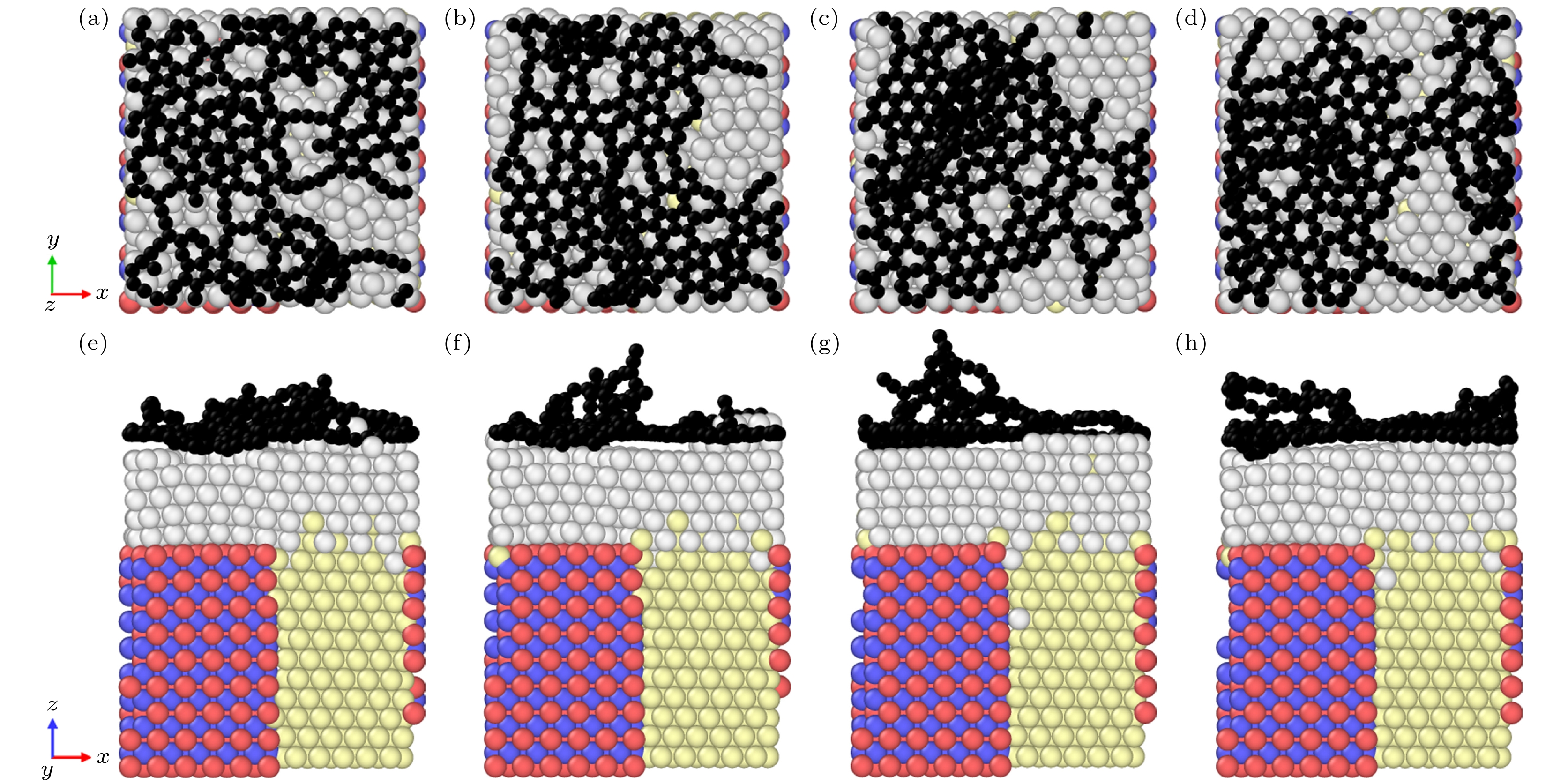
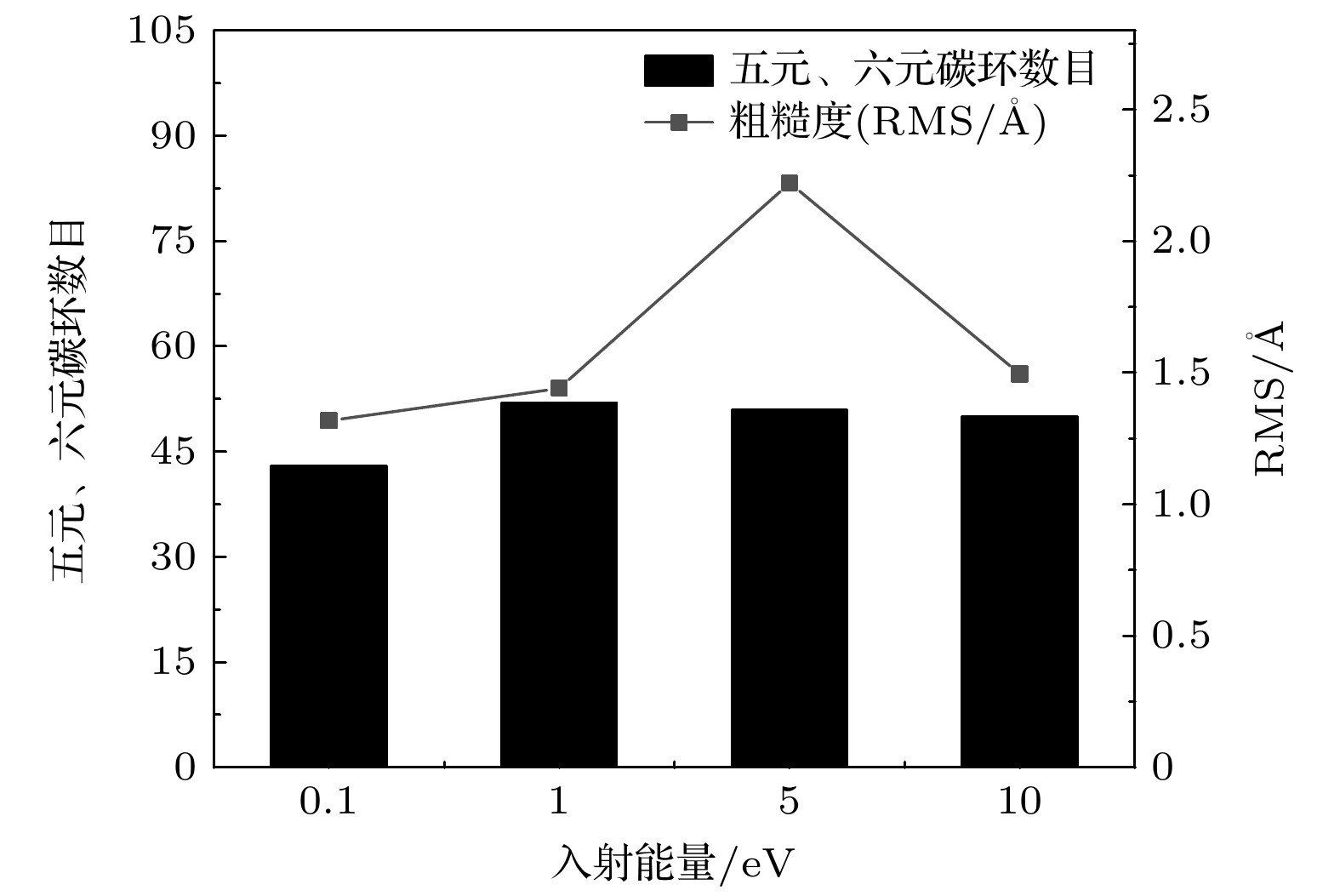
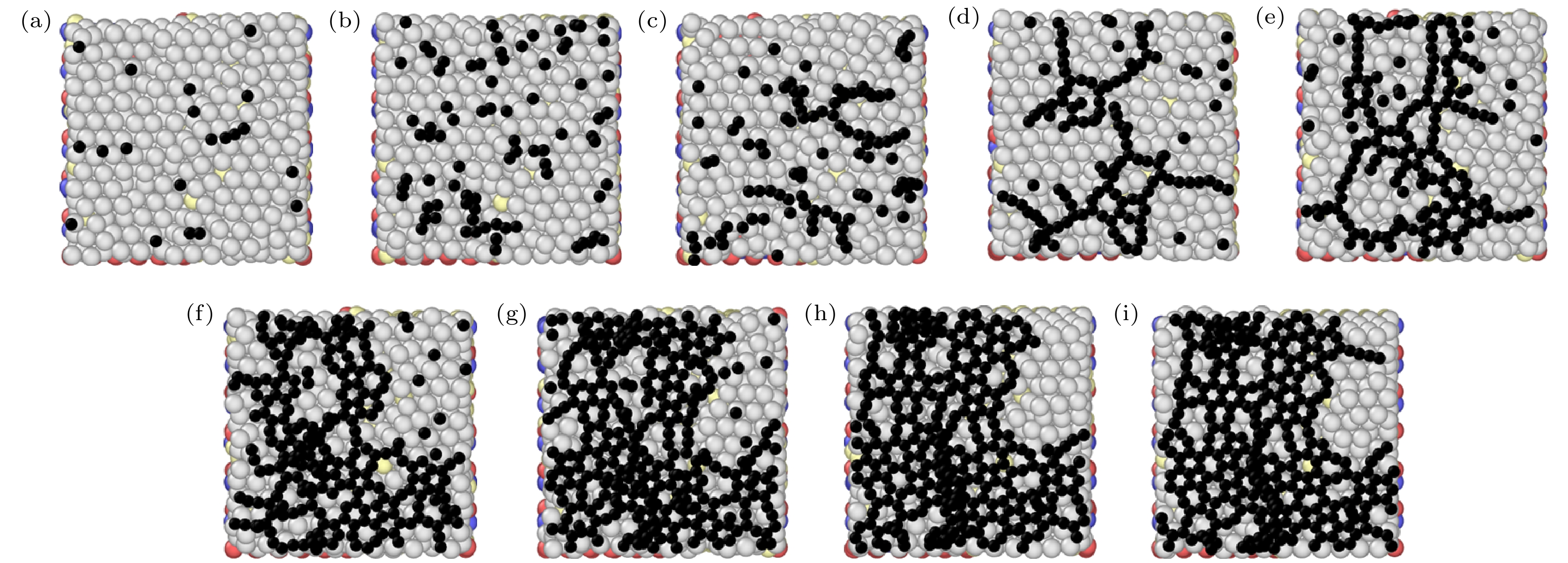
图 5 不同入射能量下石墨烯生长的俯视图和主视图 (a) 0.1 eV时的俯视图; (b) 1 eV时的俯视图; (c) 5 eV时的俯视图; (d) 10 eV时的俯视图; (e) 0.1 eV时的主视图; (f) 1 eV时的主视图; (g) 5 eV时的主视图; (h) 10 eV时的主视图
Figure 5. Top and main views of graphene growth at different incident energies: (a) Top view at 0.1 eV; (b) top view at 1 eV; (c) top view at 5 eV; (d) top view at 10 eV; (e) main view at 0.1 eV; (f) main view at 1 eV; (g) main view at 5 eV; (h) main view at 10 eV.
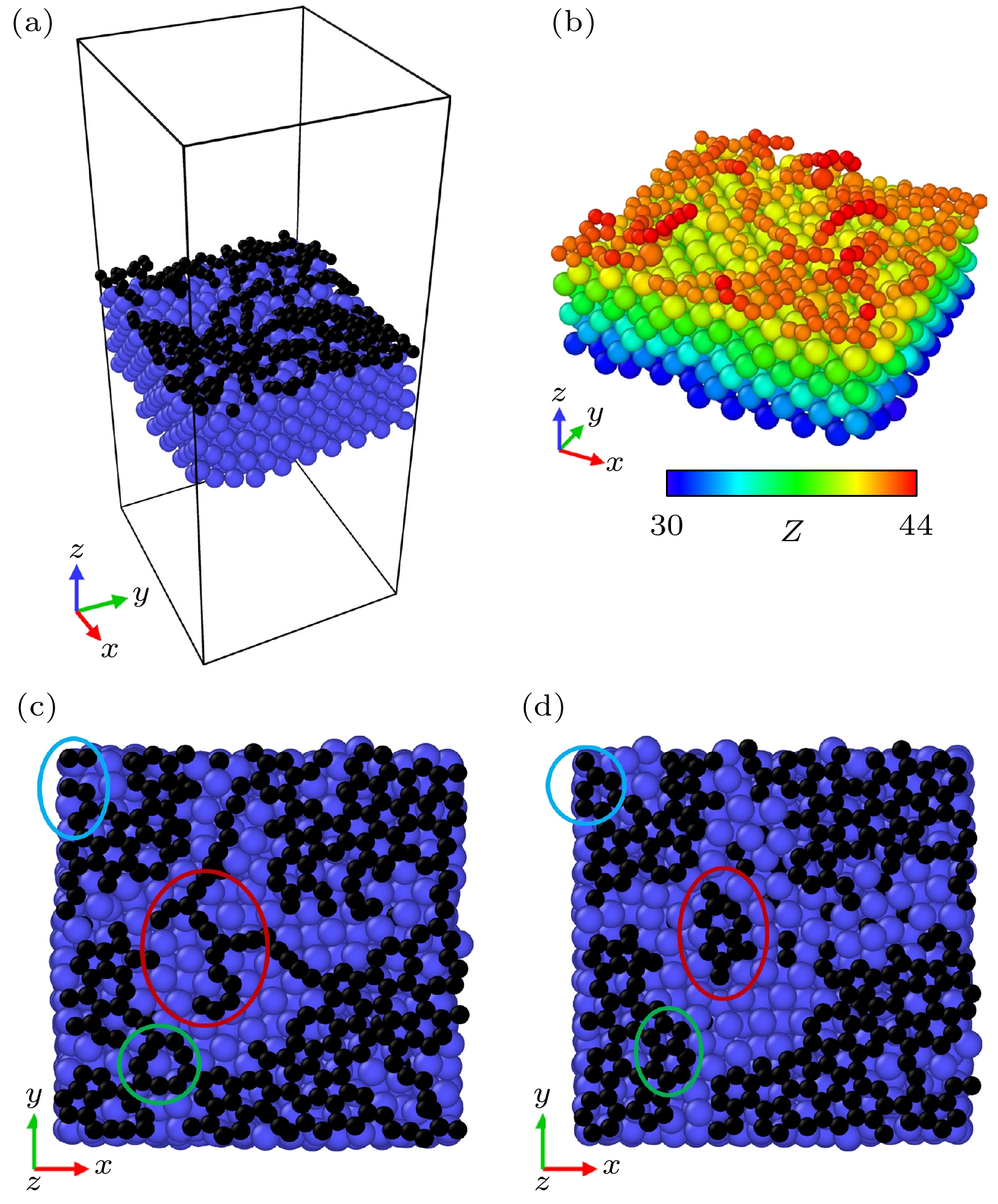
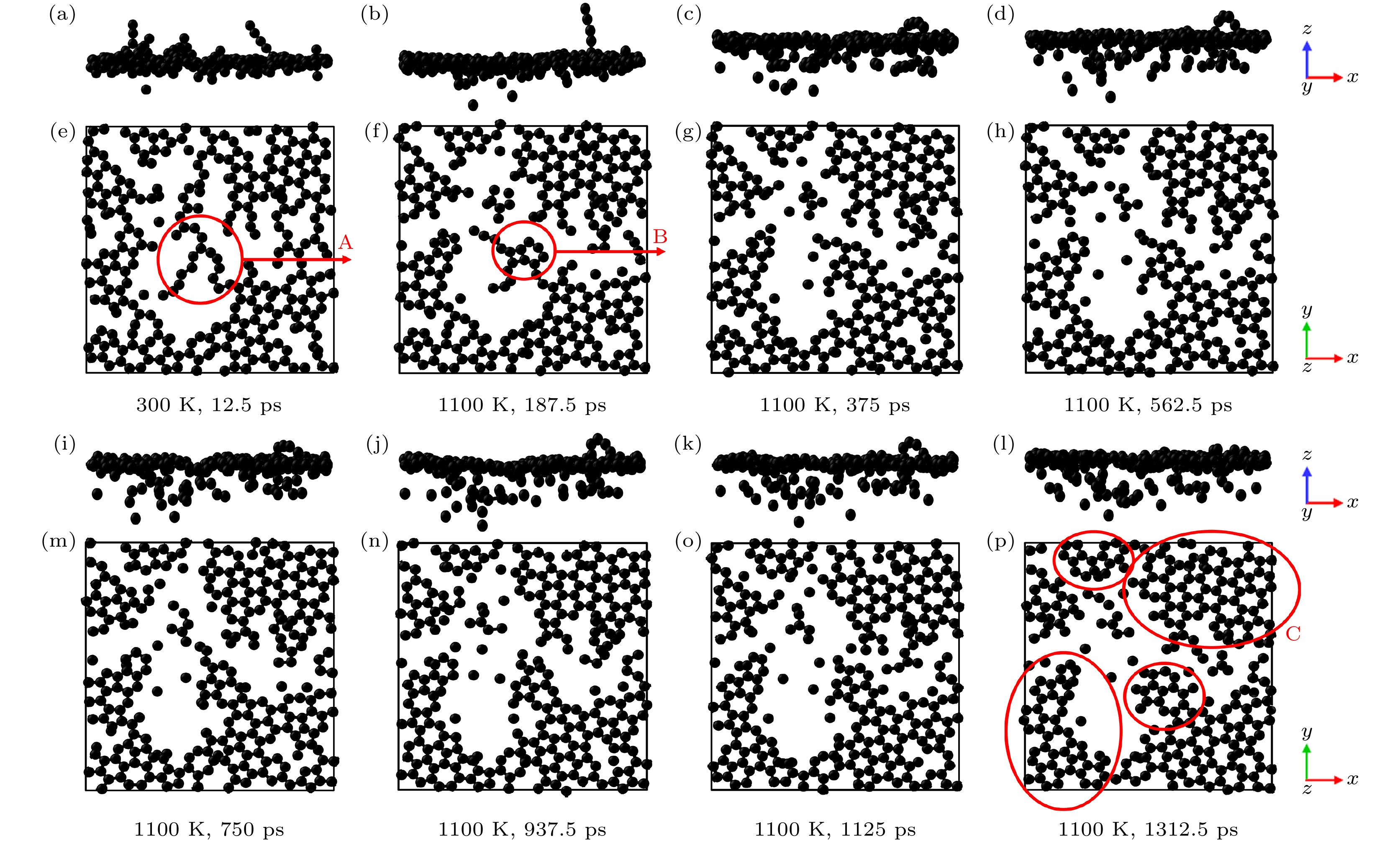
图 10 不同时间的石墨烯涂层高温退火模拟的主视图和俯视图 (a), (b), (c), (d), (i), (j), (k), (l) 主视图; (e), (f), (g), (h), (m), (n), (o), (p) 俯视图
Figure 10. Main and top views of high-temperature annealing simulations of graphene coatings at different times: (a), (b), (c), (d), (i), (j), (k), (l) Main view; (e), (f), (g), (h), (m), (n), (o), (p) top view.
表 1 WC与Co原子之间的Morse势函数参数[35]
Table 1. Parameters of Morse potential function between WC and Co atoms[35].
Atom pair $ {D}_{{\mathrm{e}}} $/eV $ \alpha $/Å–1 $ {r}_{0} $/Å W-Co 0.098 25.14 0.0872 C-Co 0.1114 19.725 0.1743 表 2 WC与过渡层Ni、过渡层Ni和基底硬质合金各元素及沉积碳原子之间的L-J参数
Table 2. L-J parameters between WC and transition layer Ni, transition layer Ni and base carbide and each element of deposited carbon atoms.
Atom pair $ \varepsilon $/eV $ \sigma $/Å W-Ni 0.07449 2.4180 C-Ni 0.0487 2.9645 W-C 0.0073 3.1411 C-C 0.0050 3.8510 Co-C 0.0017 3.3257 Ni-C 0.0487 2.9645 -
[1] 储开宇 2011 机床与液压 39 117
 Google Scholar
Google Scholar
Chu K Y 2011 Machine Tools Hydraul. 39 117
 Google Scholar
Google Scholar
[2] Tian Q Q, Huang N, Yang B, Zhuang H, Wang C, Zhai Z F, Li J H, Jia X Y, Liu L S, Jiang X 2017 J. Mater. Sci. Technol. 33 1097
 Google Scholar
Google Scholar
[3] Bouzakis K D, Michailidis N, Skordaris G, Bouzakis E, Biermann D, M'Saoubi R 2012 CIRP Ann. 61 703
 Google Scholar
Google Scholar
[4] Bobzin K, Brögelmann T, Kalscheuer C, Naderi M 2016 Surf. Coat. Technol. 308 349
 Google Scholar
Google Scholar
[5] Konstantiniuk F, Tkadletz M, Kainz C, Czettl C, Schalk N 2021 Surf. Coat. Technol. 410 126959
 Google Scholar
Google Scholar
[6] Wei Q P, Yu Z M, Ashfold M N, Chen Z, Wang L, Ma L 2010 Surf. Coat. Technol. 205 158
 Google Scholar
Google Scholar
[7] 吴张欣 2023 硕士学位论文 (上海: 华东理工大学)
Wu Z X 2023 M.S. Thesis (Shanghai: East China University of Science and Technology
[8] Kabir M S, Munroe P, Zhou Z, Xie Z 2017 Surf. Coat. Technol. 309 779
 Google Scholar
Google Scholar
[9] Contreras E, Galindez Y, Rodas M A, Bejarano G, Gómez M A 2017 Surf. Coat. Technol. 332 214
 Google Scholar
Google Scholar
[10] Suresh S 2001 Science 292 2447
 Google Scholar
Google Scholar
[11] Lu K 2014 Science 345 1455
 Google Scholar
Google Scholar
[12] Geim A K, Novoselov K S 2007 Nat. Mater. 6 183
 Google Scholar
Google Scholar
[13] 林奎鑫, 李多生, 叶寅, 江五贵, 叶志国, Qinghua Qin, 邹伟 2018 67 246802
 Google Scholar
Google Scholar
Lin K X, Li D S, Ye Y, Jiang W G, Ye Z G 2018 Acta Phys. Sin. 67 246802
 Google Scholar
Google Scholar
[14] Balandin A A, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F, Lau C N 2008 Nano Lett. 8 90
 Google Scholar
Google Scholar
[15] Lee C, Wei X, Kysar J W, Hone J 2008 Science 321 385
 Google Scholar
Google Scholar
[16] Bolotin K I, Sikes K J, Jiang Z, Klima M, Fudenberg G, Hone J, Stormer H L 2008 Solid State Commun. 146 351
 Google Scholar
Google Scholar
[17] Zhang Z, Du Y, Huang S, Meng F, Chen L, Xie W, Chang K, Zhang C, Lu Y, Lin C, Li S, Parkin I P, Guo D 2020 Adv. Sci. 7 1903239
 Google Scholar
Google Scholar
[18] Li S Z, Li Q Y, Carpick R W, Gumbsch P, Liu X Z, Ding X D, Li J 2016 Nature 539 541
 Google Scholar
Google Scholar
[19] Fan S Y, Chen Y N, Xiao S, Shi K J, Meng X Y, Lin S S, Su F H, Su Y F, Chu P K 2024 Carbon 216 118561
 Google Scholar
Google Scholar
[20] Min F L, Yu S B, Sheng W A N G, Yao Z H, Noudem J G, Liu S J, Zhang J F 2022 Trans. Nonferrous Met. Soc. China 32 1935
 Google Scholar
Google Scholar
[21] Garlow J A, Barrett L K, Wu L, Kisslinger K, Zhu Y, Pulecio J F 2016 Sci. Rep. 6 19804
 Google Scholar
Google Scholar
[22] Orofeo C M, Ago H, Hu B, Tsuji M 2011 Nano Res. 4 531
 Google Scholar
Google Scholar
[23] Reina A, Thiele S, Jia X, Bhaviripudi S, Dresselhaus M S, Schaefer J A, Kong J 2009 Nano Res. 2 509
 Google Scholar
Google Scholar
[24] Seo J H, Lee H W, Kim J K, Kim D G, Kang J W, Kang M S, Kim C S 2012 Curr. Appl. Phys. 12 S131
 Google Scholar
Google Scholar
[25] Li X S, Cai W W, Colombo L, Ruoff R S 2009 Nano Lett. 9 4268
 Google Scholar
Google Scholar
[26] Li X, Li H, Lee K R, Wang A 2020 Appl. Surf. Sci. 529 147042
 Google Scholar
Google Scholar
[27] 王泽, 李国禄, 王海斗, 徐滨士, 康嘉杰 材料导报 28 91
Wang Z, Li G L, Wang H D, Xu B S, Kang J J 2014 Mater. Rev. 28 91
[28] Kato T, Nagai T, Sasajima Y, Onuki J 2010 Mater. Trans. 51 664
 Google Scholar
Google Scholar
[29] 丁业章, 叶寅, 李多生, 徐锋, 朗文昌, 刘俊红, 温鑫 2023 72 068703
 Google Scholar
Google Scholar
Ding Y Z, Ye Y, Li D S, Xu F, Lang W C, Liu J H, Wen X 2023 Acta Phys. Sin. 72 068703
 Google Scholar
Google Scholar
[30] Backholm M, Foss M, Nordlund K 2013 Appl. Surf. Sci. 268 270
 Google Scholar
Google Scholar
[31] Shibuta Y, Elliott J A 2009 Chem. Phys. Lett. 472 200
 Google Scholar
Google Scholar
[32] Juslin N, Erhart P, Träskelin P, Nord J, Henriksson K O E, Nordlund K, Salonen E, Albe K 2005 J. Appl. Phys. 98 123520
 Google Scholar
Google Scholar
[33] Béland L K, Lu C, Osetskiy Y N, Samolyuk G D, Caro A, Wang L, Stoller R E 2016 J. Appl. Phys. 119 085901
 Google Scholar
Google Scholar
[34] Morse P M 1929 Phys. Rev. 34 57
 Google Scholar
Google Scholar
[35] 冯艳, 段海明 2011 原子与分子 28 251
 Google Scholar
Google Scholar
Feng Y, Duan H M 2011 J. At. Mol. Phys. 28 251
 Google Scholar
Google Scholar
[36] Stuart S J, Tutein A B, Harrison J A 2000 J. Chem. Phys. 112 6472
 Google Scholar
Google Scholar
[37] Jones J E 1924 Proc. Roy. Soc. A 106 463
[38] Cao Q, Chen Y J, Shao W, Ma X T, Zheng C, Cui Z, Liu Y, Yu B 2020 J. Mol. Liq. 319 114218
 Google Scholar
Google Scholar
[39] 王涛 2010 硕士学位论文 (湘潭: 湘潭大学)
Wang T 2010 M. S. Thesis ( Xiangtan: Xiangtan University
[40] Mueller J E, Van Duin A C, Goddard III W A 2010 J. Phys. Chem. C 114 4939
 Google Scholar
Google Scholar
[41] Loginova E, Bartelt N C, Feibelman P J, McCarty K F 2008 New J. Phys. 10 093026
 Google Scholar
Google Scholar
[42] Pagon A M, Partridge J G, Hubbard P, Taylor M B, McCulloch D G, Doyle E D, Li G 2010 Surf. Coat. Technol. 204 3552
 Google Scholar
Google Scholar
[43] 王璐, 高峻峰, 丁峰 2014 化学学报 72 345
 Google Scholar
Google Scholar
Wang L, Gao J F, Ding F 2014 Acta Chim. Sin. 72 345
 Google Scholar
Google Scholar
[44] 戴达煌 2008 薄膜与涂层现代表面技术(长沙: 中南大学出版社) 第411页
Dai D H 2008 Thin Films and Coatings Modern Surface Technology (Changsha: Central South University Press) p411
[45] Chen S D, Bai Q S, Wang H F, Dou Y H, Guo W 2022 Physics E 144 115465
 Google Scholar
Google Scholar
[46] Chen S, Xiong W, Zhou Y S, Lu Y F, Zeng X C 2016 Nanoscale 8 9746
 Google Scholar
Google Scholar
[47] Rut’kov E V, Gall N R 2011 Equilibrium Nucleation, Growth, and Thermal Stability of Graphene on Solids (Russia St. Petersburg: inTech) pp209–292
Catalog
Metrics
- Abstract views: 1864
- PDF Downloads: 171
- Cited By: 0














 DownLoad:
DownLoad: