-
Chemical vapor deposition (CVD) is an essential method of depositing and fabricating large-area and high-quality graphene. In this work, molecular dynamics (MD) simulation technology is adopted to simulate the fabrication of graphene on the copper (111) crystal surface by chemical vapor deposition method. In order to eliminate the adverse effects of traditional MD method, an adapted potential system between carbon and copper atoms is introduced into the modeling of deposition and growth simulation of graphene. The results reveal the microscale growth mechanism of the graphene depositing on Cu(111) crystal surfaces, and the influence of temperature and carbon deposition rate (CDR) on the quality of graphene. The simulation results indicate that the deposition and growth of graphene consists of two stages. The first stage is to form binary carbons, trinary carbons and carbon chains. The second stage is to form carbon rings and the defects healing. The research results also reveal that high temperature can provide the carbon atoms with sufficient energy, which can help the carbon atoms to skip the energetic barrier between the two stages, and then achieve the deposition and growth of graphene. Moreover, the influence of temperature and carbon deposition rate are investigated in detail. The temperature mainly affects the defects and the flatness of graphene. The defects of graphene are the least and the surface can become the flattest at a deposition temperature of 1300 K. Higher temperature can cause the carbon atoms to irregularly move, and lower temperature can suppress the catalysis of the copper substrate. Both the higher and lower temperature can degrade the quality of the graphene surface. The CDR can influence the defects of graphene in growth. The lower value of CDR can lead to local growth on the graphene surface because of the lower nucleation density while the higher CDR is also able to cause the defects to form because of the uneven free energy distribution on the copper surface that has thermal fluctuation. It is shown that graphene can present the flattest surface when the value of CDR is set to be 5 ps–1. According to the simulation process of deposition, it validates that the bi-layer and multi-layer graphene may grow based on the deposition of original single layer of graphene. As to the deposition and growth practice, it is suggested that the temperature 1300K should be suitable for the graphene CVD process of Cu(111) surface. The results in this work can provide a reference for understanding and implementing the fabrication of graphene on the Cu substrate by CVD methods.
-
Keywords:
- graphene /
- molecular dynamics /
- deposition and growth /
- mechanism
[1] Lee C, Wei X, Kysar J W, Hone J 2008 Science 321 385
 Google Scholar
Google Scholar
[2] He X, Bai Q S, Shen R Q 2018 Carbon 130 672
 Google Scholar
Google Scholar
[3] Zhu L, Wang J, Zhang T, Ma L, Chee Wah Lim, Ding F, Zeng X 2010 Nano Lett. 10 494
 Google Scholar
Google Scholar
[4] Balandin A A, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F, Lau C N 2008 Nano Lett. 8 902
 Google Scholar
Google Scholar
[5] Novoselov K S, Jiang D, Schedin F, Booth T J, Khotkevich V V, Morozov S V, Geim A K 2005 Proc. Natl. Acad. Sci. 102 10451
 Google Scholar
Google Scholar
[6] Sutter P 2009 Nat. Mater. 8 171
 Google Scholar
Google Scholar
[7] Choucair M, Thordarson P, Stride J A 2009 Nat. Nanotechnol. 4 30
 Google Scholar
Google Scholar
[8] Liao C D, Lu Y Y, Tamalampudi S R, Cheng H C, Chen Y T 2013 J. Phys. Chem. A 117 9454
 Google Scholar
Google Scholar
[9] Wang Y, Page A J, Nishimoto Y, Qian H J, Morokuma K, Irle S 2011 J. Am. Chem. Soc. 133 18837
 Google Scholar
Google Scholar
[10] Elliott J A, Shibuta Y, Amara H, Bichara C, Neyts E C 2013 Nanoscale 5 6662
 Google Scholar
Google Scholar
[11] Karoui S, Amara H, Bichara C, Ducastelle F 2010 ACS Nano 4 6114
 Google Scholar
Google Scholar
[12] Meng L, Sun Q, Wang J, Ding F 2012 J. Phys. Chem. C 116 6097
 Google Scholar
Google Scholar
[13] He Y Y, Wang H, Jiang S J, Mo Y J 2019 Comput. Mater. Sci. 168 17
 Google Scholar
Google Scholar
[14] 王浪, 冯伟, 杨连乔, 张建华 2014 63 176801
 Google Scholar
Google Scholar
Wang L, Feng W, Yang L Q, Zhang J H 2014 Acta Phys. Sin. 63 176801
 Google Scholar
Google Scholar
[15] Rasuli R, Mostafavi K, Davoodi J 2014 J. Appl. Phys. 115 185503
 Google Scholar
Google Scholar
[16] Syuhada I, Rosikhin A, Fikri A, Noor F A, Winata T. 2016 AIP Conf. Proc. 1710 185503
 Google Scholar
Google Scholar
[17] Xu Z, Yan T, Liu G, Qiao G, Ding F 2015 Nanoscale 8 921
 Google Scholar
Google Scholar
[18] Stuart S J, Tutein A B, Harrison J A 2000 J. Chem. Phys. 112 6472
 Google Scholar
Google Scholar
[19] Brenner D W, Shenderova O A, Harrison J, Stuart S J, Ni B, Sinnott S B 2012 J. Phys. Condens. Matter 14 783
 Google Scholar
Google Scholar
[20] Daw M S, Baskes M I 1984 Phys. Rev. B 29 8486
 Google Scholar
Google Scholar
[21] Jones J E 1924 Proc R. Soc. London 106 463
 Google Scholar
Google Scholar
[22] Girifalco L A, Weizer V G 1959 Phys. Rev. B 114 687
 Google Scholar
Google Scholar
[23] Zhang J, Liu C, Shu Y, Fan J 2012 Appl. Surf. Sci. 261 690
 Google Scholar
Google Scholar
[24] Wu Y, Chou H, Ji H, Wu Q, Chen S, Jiang Wei, Hao Y, Kang J, Ren Y, Richard D P, Rodney S R 2012 ACS Nano 6 7731
 Google Scholar
Google Scholar
[25] Didar B R, Khosravian H, Balbuena P B 2018 RSC Adv. 8 27825
 Google Scholar
Google Scholar
[26] 李浩, 付志兵, 王红斌, 易勇, 黄维, 张继成 2017 66 058101
 Google Scholar
Google Scholar
Li H, Fu Z B, Wang H B, Yi Y, Huang W, Zhang J C 2017 Acta Phys. Sin. 66 058101
 Google Scholar
Google Scholar
-
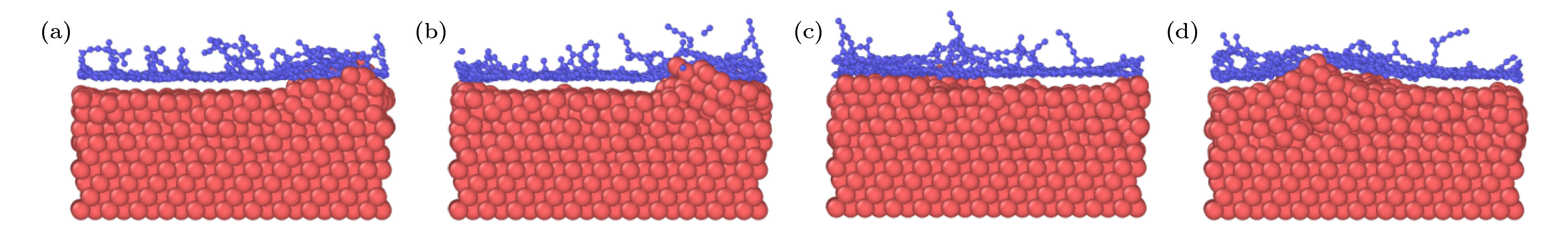
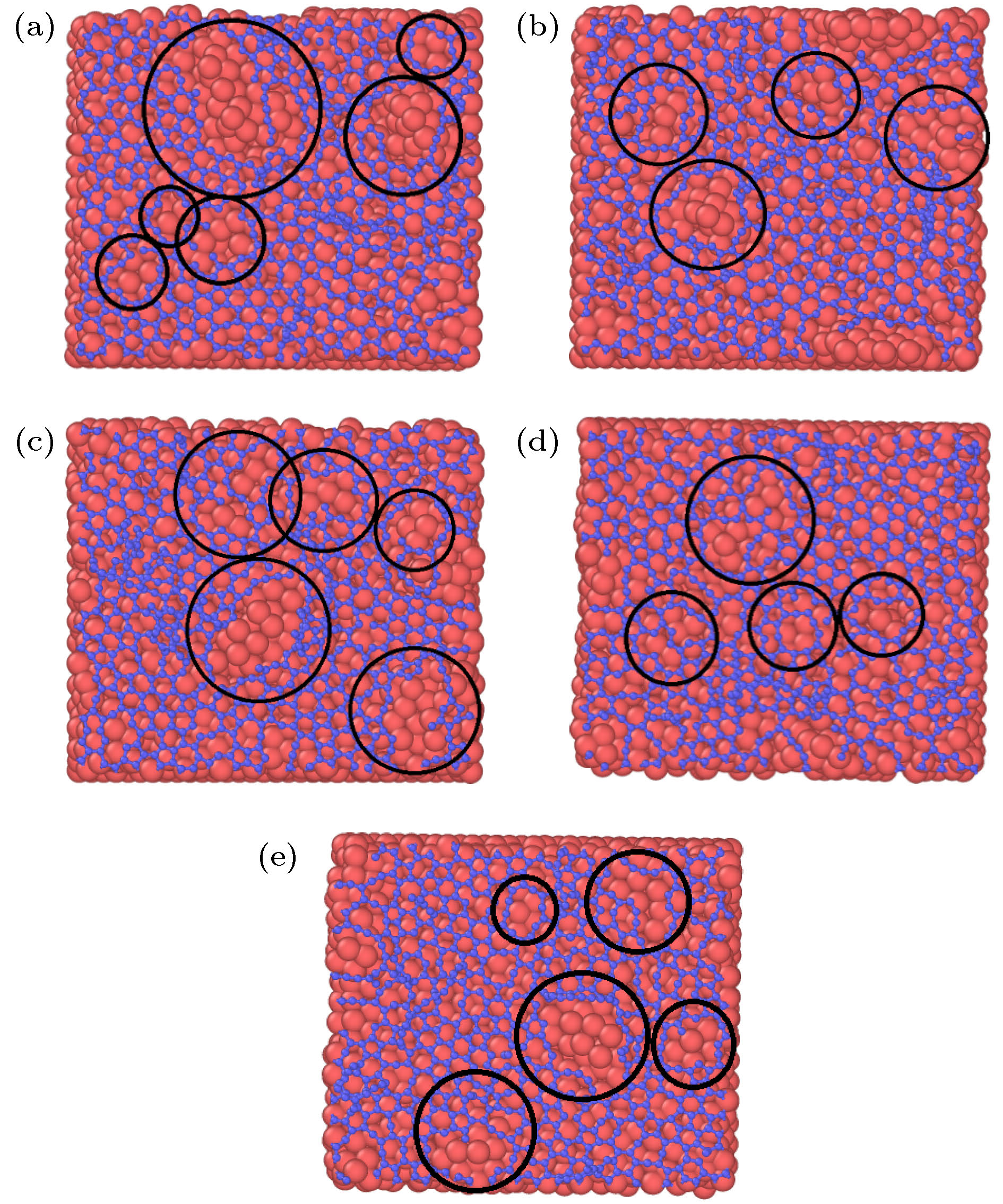
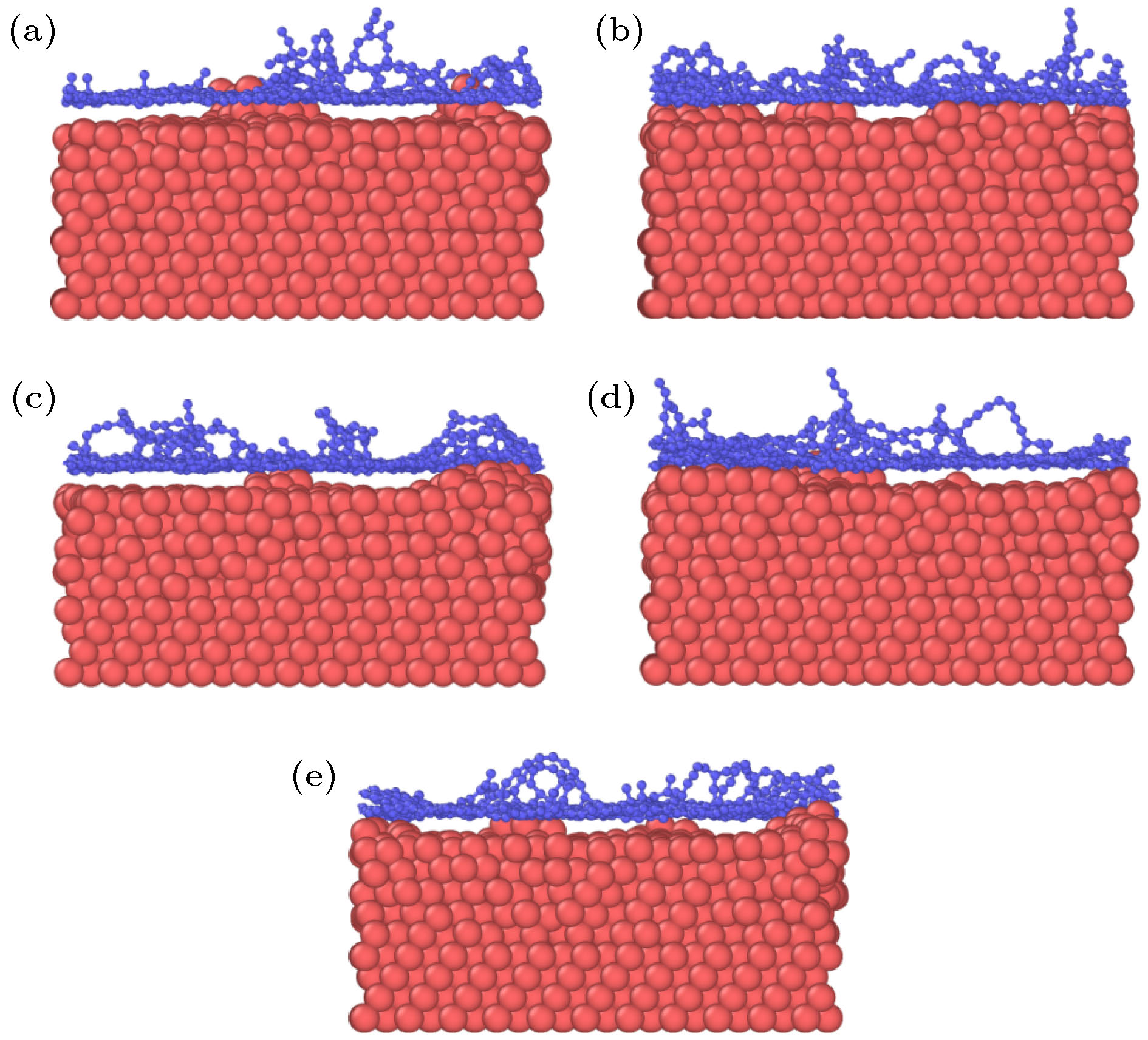
图 2 石墨烯沉积生长的原子分布图 (a) 28.3 ps; (b) 36.1 ps; (c) 39.7 ps; (d) 42.4 ps; (e) 52.2 ps; (f) 100 ps; (g) 116 ps; (h) 118 ps; (i) 200 ps
Figure 2. Atomic distributation of graphene deposition and growth: (a) 28.3 ps; (b) 36.1 ps; (c) 39.7 ps; (d) 42.4 ps; (e) 52.2 ps; (f) 100 ps; (g) 116 ps; (h) 118 ps; (i) 200 ps.
-
[1] Lee C, Wei X, Kysar J W, Hone J 2008 Science 321 385
 Google Scholar
Google Scholar
[2] He X, Bai Q S, Shen R Q 2018 Carbon 130 672
 Google Scholar
Google Scholar
[3] Zhu L, Wang J, Zhang T, Ma L, Chee Wah Lim, Ding F, Zeng X 2010 Nano Lett. 10 494
 Google Scholar
Google Scholar
[4] Balandin A A, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F, Lau C N 2008 Nano Lett. 8 902
 Google Scholar
Google Scholar
[5] Novoselov K S, Jiang D, Schedin F, Booth T J, Khotkevich V V, Morozov S V, Geim A K 2005 Proc. Natl. Acad. Sci. 102 10451
 Google Scholar
Google Scholar
[6] Sutter P 2009 Nat. Mater. 8 171
 Google Scholar
Google Scholar
[7] Choucair M, Thordarson P, Stride J A 2009 Nat. Nanotechnol. 4 30
 Google Scholar
Google Scholar
[8] Liao C D, Lu Y Y, Tamalampudi S R, Cheng H C, Chen Y T 2013 J. Phys. Chem. A 117 9454
 Google Scholar
Google Scholar
[9] Wang Y, Page A J, Nishimoto Y, Qian H J, Morokuma K, Irle S 2011 J. Am. Chem. Soc. 133 18837
 Google Scholar
Google Scholar
[10] Elliott J A, Shibuta Y, Amara H, Bichara C, Neyts E C 2013 Nanoscale 5 6662
 Google Scholar
Google Scholar
[11] Karoui S, Amara H, Bichara C, Ducastelle F 2010 ACS Nano 4 6114
 Google Scholar
Google Scholar
[12] Meng L, Sun Q, Wang J, Ding F 2012 J. Phys. Chem. C 116 6097
 Google Scholar
Google Scholar
[13] He Y Y, Wang H, Jiang S J, Mo Y J 2019 Comput. Mater. Sci. 168 17
 Google Scholar
Google Scholar
[14] 王浪, 冯伟, 杨连乔, 张建华 2014 63 176801
 Google Scholar
Google Scholar
Wang L, Feng W, Yang L Q, Zhang J H 2014 Acta Phys. Sin. 63 176801
 Google Scholar
Google Scholar
[15] Rasuli R, Mostafavi K, Davoodi J 2014 J. Appl. Phys. 115 185503
 Google Scholar
Google Scholar
[16] Syuhada I, Rosikhin A, Fikri A, Noor F A, Winata T. 2016 AIP Conf. Proc. 1710 185503
 Google Scholar
Google Scholar
[17] Xu Z, Yan T, Liu G, Qiao G, Ding F 2015 Nanoscale 8 921
 Google Scholar
Google Scholar
[18] Stuart S J, Tutein A B, Harrison J A 2000 J. Chem. Phys. 112 6472
 Google Scholar
Google Scholar
[19] Brenner D W, Shenderova O A, Harrison J, Stuart S J, Ni B, Sinnott S B 2012 J. Phys. Condens. Matter 14 783
 Google Scholar
Google Scholar
[20] Daw M S, Baskes M I 1984 Phys. Rev. B 29 8486
 Google Scholar
Google Scholar
[21] Jones J E 1924 Proc R. Soc. London 106 463
 Google Scholar
Google Scholar
[22] Girifalco L A, Weizer V G 1959 Phys. Rev. B 114 687
 Google Scholar
Google Scholar
[23] Zhang J, Liu C, Shu Y, Fan J 2012 Appl. Surf. Sci. 261 690
 Google Scholar
Google Scholar
[24] Wu Y, Chou H, Ji H, Wu Q, Chen S, Jiang Wei, Hao Y, Kang J, Ren Y, Richard D P, Rodney S R 2012 ACS Nano 6 7731
 Google Scholar
Google Scholar
[25] Didar B R, Khosravian H, Balbuena P B 2018 RSC Adv. 8 27825
 Google Scholar
Google Scholar
[26] 李浩, 付志兵, 王红斌, 易勇, 黄维, 张继成 2017 66 058101
 Google Scholar
Google Scholar
Li H, Fu Z B, Wang H B, Yi Y, Huang W, Zhang J C 2017 Acta Phys. Sin. 66 058101
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 13362
- PDF Downloads: 440
- Cited By: 0














 DownLoad:
DownLoad: