-
CH3NH3PbI3 is one of the most promising candidates for high-performance hybrid organic-inorganic perovskite solar cells. The CH3NH3PbI3 single crystal and polycrystalline thin film exhibit the unique features of long carrier lifetimes and diffusion lengths, however, their carrier mobilities are in fact rather modest in a range from 1 cm2·V–1·s–1 to 100 cm2·V–1·s–1. Experimentally, the temperature dependence of mobility is described as T–1.3 to T–1.6 due to the acoustic phonon scattering. To be sure, the rotating CH3NH
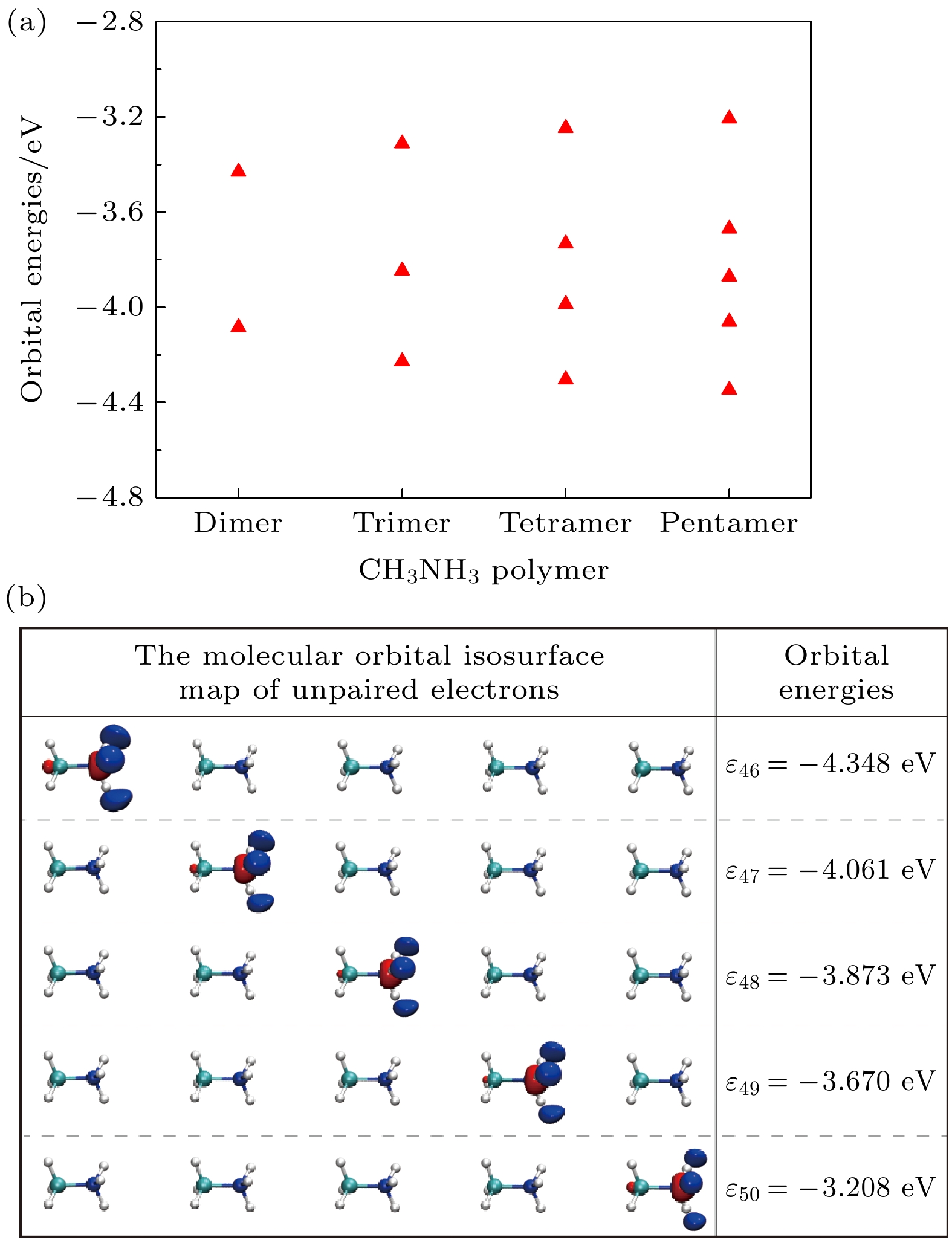
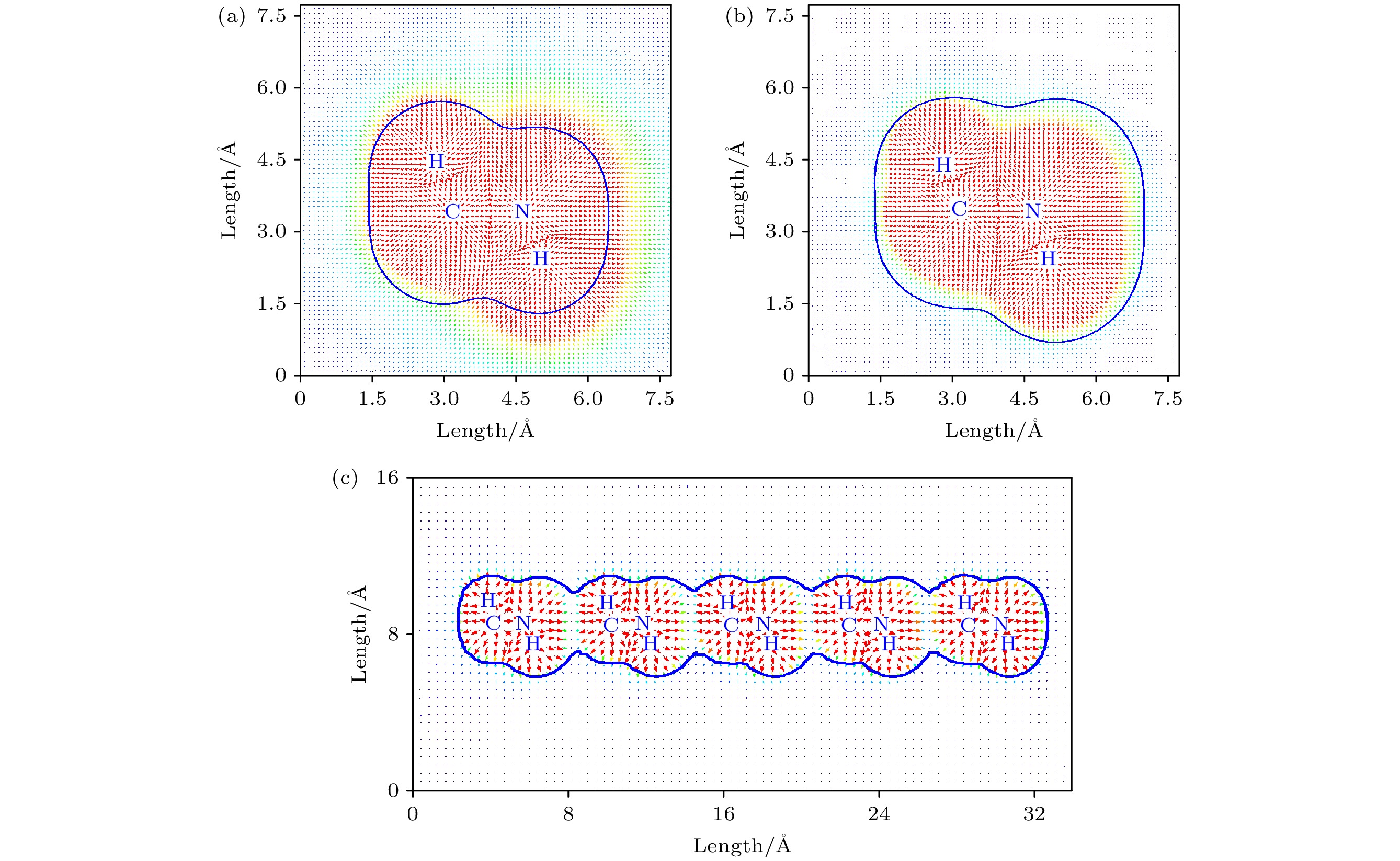
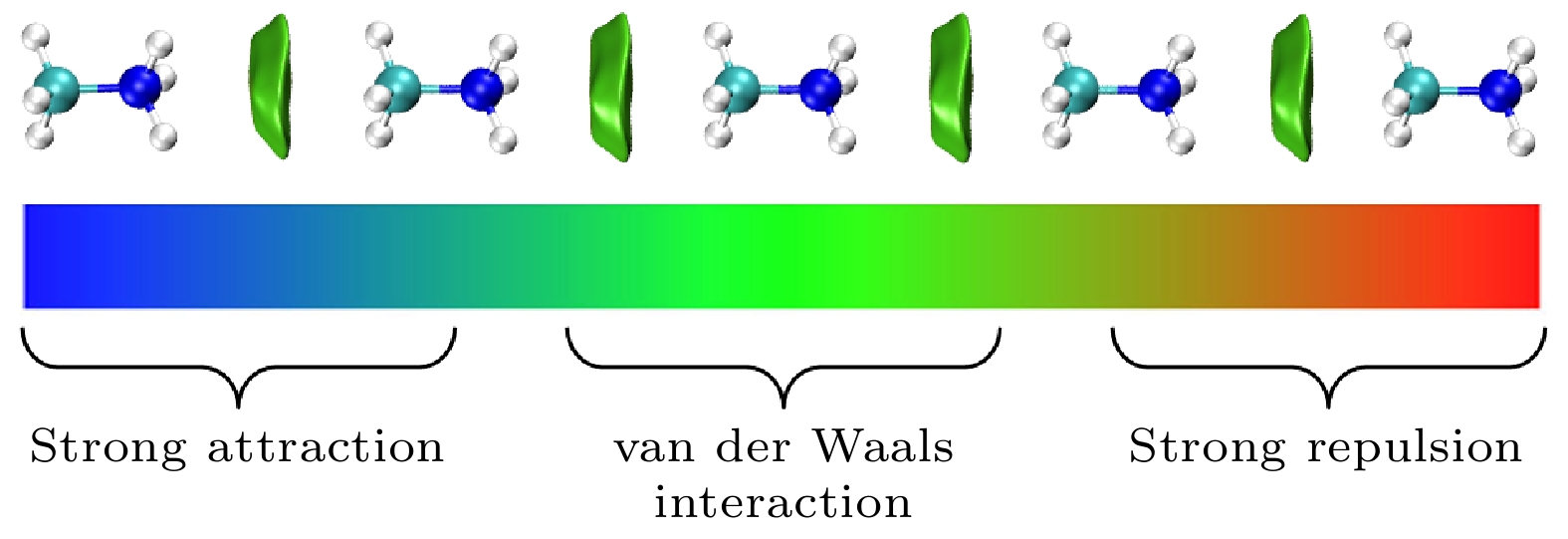
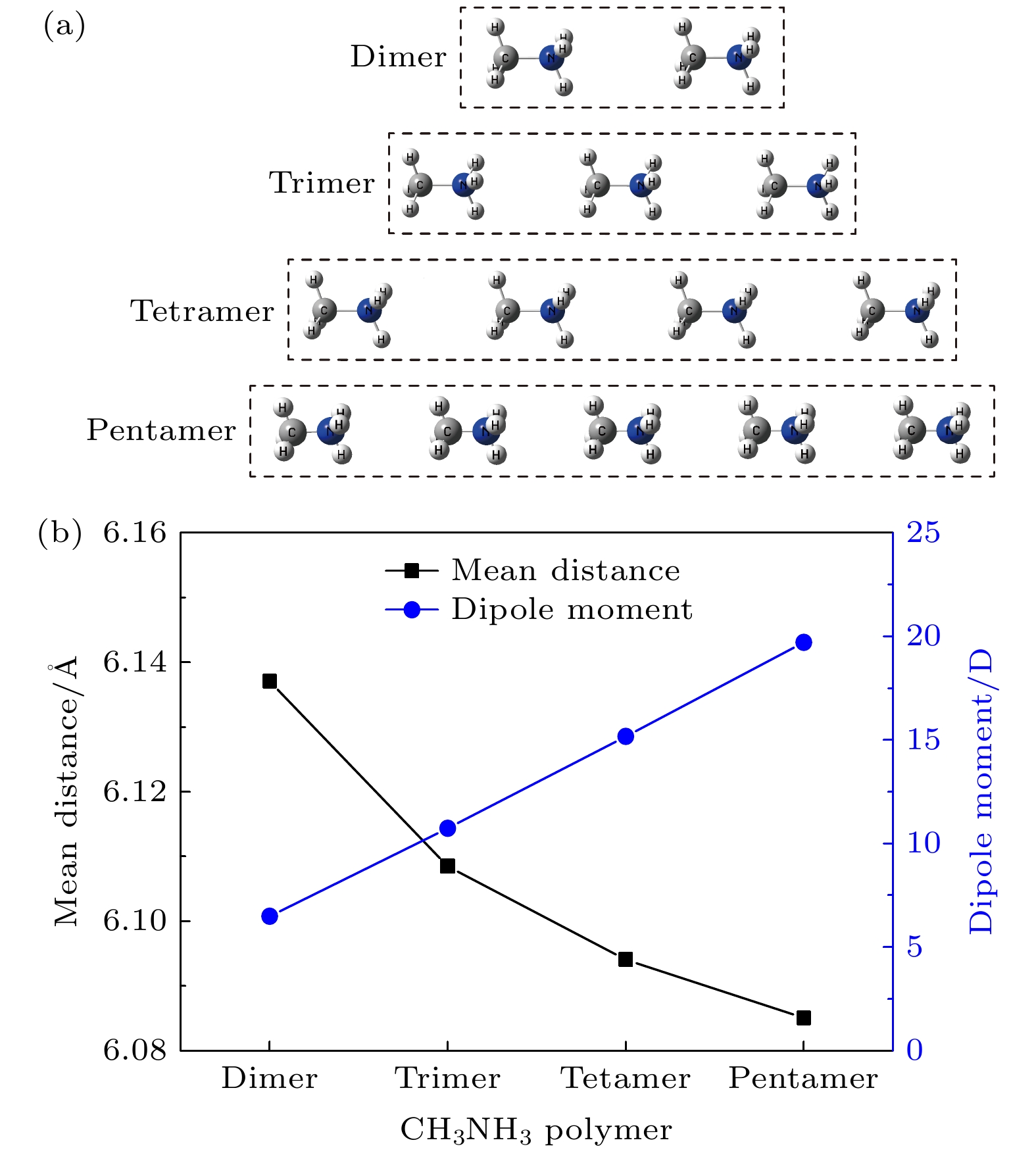
${}_3^+ $ cations are disadvantageous to the carrier transport and performance for CH3NH3PbI3 solar cells. The effect of the rotating CH3NH${}_3^+ $ cations on high-performance CH3NH3PbI3 solar cells remains an open question. The Gaussian 09 software has been utilized to optimize the geometrical structures of CH3NH3 dimer, trimer, tetramer, and pentamer in isolated state at the MP2 level with using the cc-PVTZ basis set. For CH3NH3 polymer, the mean distance between two centroids of neighboring CH3NH3 decreasing with the number of CH3NH3 is slightly smaller than the lattice constant 6.28 Å of tetragonal CH3NH3PbI3, which is advantageous to structural stability and higher structural order of inorganic [PbI3]– framework. It signifies that the long range order of electrically neutral CH3NH3 is easily formed for room-temperature CH3NH3PbI3. The total dipole moment linearly increases with the number of CH3NH3 for CH3NH3 polymer, and attains a large value 19.7 Debye for CH3NH3 pentamer, which may be the origin of strong polarization in CH3NH3PbI3 heterojunction. The molecular orbitals of five unpaired electrons for CH3NH3 pentamer are distributed around NH3-sides of five different CH3NH3 pentamers respectively, and these orbital energies are in a range from –4.4 eV to –3.2 eV. The unpaired electrons in CH3NH3 polymer have an electrostatic attraction on the CH3-side of neighboring CH3NH3, which is the key cause of forming the ordered CH3NH3 polymer. Hence it can be inferred that the orbital energies of unpaired electrons are getting closer when the longer range order of CH3NH3 are formed in room-temperature CH3NH3PbI3 through the interfacial electron injection. The vector field map of electrostatic potential (ESP) shows that CH3NH${}_3^+ $ has strong electrophilic character, and the NH3-side has a stronger electrophilic character than CH3-side, however, CH3NH3 monomer and polymer have weak electrophilic and nucleophilic character. Thus, the forming of CH3NH3 polymer at the CH3NH3PbI3 heterojunction leads the organic and inorganic portions to be decoupled, which can effectively reduce the anharmonic phonon modes. Under an applied electric field, the unpaired electrons in CH3NH3 pentamer can transfer along the C-N axis through the hopping mechanism. According to these results, we can draw three useful conclusions below. i) The electrons under an applied electric field are easily injected into the CH3NH3PbI3 material through the heterojunction, the CH3NH3 polymer is easily formed, and the unpaired electrons in polymer are transferred between two neighboring CH3NH3 through hopping mechanism. ii) The decoupling between organic CH3NH3 and inorganic [PbI3]– framework can effectively reduce the anharmonic phonon modes, which can lead the carrier scattering decrease and the efficiency of carrier separation and transport to improve; iii) The ordered CH3NH3 polymer at the CH3NH3PbI3 heterojunction can enhance the order of inorganic [PbI3]– framework. Our researches may help to further understand the origin of high power conversion efficiency (PCE) for hybrid organic-inorganic perovskite solar cells.[1] Zhang W, Eperon G E, Snaith H J 2016 Nat. Energy 1 16048
 Google Scholar
Google Scholar
[2] Kojima A, Teshima K, Shirai Y, Miyasaka T 2009 J. Am. Chem. Soc. 131 6050
 Google Scholar
Google Scholar
[3] Ding H, Li B, Zareen S, Li G, Tu Y, Zhang D, Cao X, Xu Q, Yang S, Tait S L, Zhu J 2020 ACS Appl. Mater. Interfaces 12 28861
 Google Scholar
Google Scholar
[4] Breternitz J, Lehmann F, Barnett S A, Nowell H, Schorr S 2020 Angew. Chem. Int. Ed. 59 424
 Google Scholar
Google Scholar
[5] Herz L M 2016 Annu. Rev. Phys. Chem. 67 65
 Google Scholar
Google Scholar
[6] Bi Y, Hutter E M, Fang Y, Dong Q, Huang J, Savenije T J 2016 J. Phys. Chem. Lett. 7 923
 Google Scholar
Google Scholar
[7] Ponseca C S, Savenije T J, Abdellah M, Zheng K, Yartsev A, Pascher T, Harlang T, Chabera P, Pullerits T, Stepanov A, Wolf J P, Sundströ V 2014 J. Am. Chem. Soc. 136 5189
 Google Scholar
Google Scholar
[8] Chen Y, Yi H T, Wu X, Haroldson R, Gartstein Y N, Rodionov Y I, Tikhonov K S, Zakhidov A, Zhu Z Y, Podzorov V 2016 Nat. Commun. 7 12253
 Google Scholar
Google Scholar
[9] He J L, Fang W H, Long R, Prezhdo O V 2020 J. Am. Chem. Soc. 142 14664
 Google Scholar
Google Scholar
[10] Li W W, Man Z Y, Zeng J T, Zheng L Y, Li G R, Kassiba A 2020 J. Phys. Chem. C 124 13348
 Google Scholar
Google Scholar
[11] Brenner T M, Egger D A, Rappe A M, Kronik L, Hodes G, Cahen D 2015 J. Phys. Chem. Lett. 6 4754
 Google Scholar
Google Scholar
[12] Dong Q, Fang Y, Shao Y, Mulligan P, Qiu J, Cao L, Huang J 2015 Science 347 967
 Google Scholar
Google Scholar
[13] Shi D, Adinolfi V, Comin R, Yuan M, Alarousu E, Buin A, Chen Y, Hoogland S, Rothenberger A, Katsiev K, Losovyj Y, Zhang X, Dowben P A, Mohammed O F, Sargent E H, Bakr O M 2015 Science 347 519
 Google Scholar
Google Scholar
[14] Mei Y, Zhang C, Vardeny Z V, Jurchescu O D 2015 MRS Commun. 5 297
 Google Scholar
Google Scholar
[15] Savenije T J, Ponseca C S, Kunneman L, Abdellah M, Zheng K, Tian Y, Zhu Q, Canton S E, Scheblykin I G, Pullerits T, Yartsev A, Sundstrom V 2014 J. Phys. Chem. Lett. 5 2189
 Google Scholar
Google Scholar
[16] Karakus M, Jensen S A, D’Angelo F, Turchinovich D, Bonn M, Canovas E 2015 J. Phys. Chem. Lett. 6 4991
 Google Scholar
Google Scholar
[17] Oga H, Saeki A, Ogomi Y, Hayase S, Seki S 2014 J. Am. Chem. Soc. 136 13818
 Google Scholar
Google Scholar
[18] He Y, Galli G 2014 Chem. Mater. 26 5394
 Google Scholar
Google Scholar
[19] Wang Y, Zhang Y, Zhang P, Zhang W 2015 Phys. Chem. Chem. Phys. 17 11516
 Google Scholar
Google Scholar
[20] Whalley L D, Skelton J M, Frost J M, Walsh A 2016 Phys. Rev. B 94 220301
 Google Scholar
Google Scholar
[21] Monahan D M, Guo L, Lin J, Dou L, Yang P, Fleming G R 2017 J. Phys. Chem. Lett. 8 83211
[22] Ma J, Wang L W 2017 Nano Lett. 17 3646
 Google Scholar
Google Scholar
[23] Frisch M J, Trucks G W, Schlegel H B, et al. 2009 Gaussian 09 (Revision C.01) (Wallingford: Gaussian, Inc.)
[24] Murray J S, Brinck T, Lane P, Paulsen K, Politzer P 1994 J. Mol. Struct. Theochem. 307 55
 Google Scholar
Google Scholar
[25] Lefebvre C, Rubez G, Khartabil H, Boisson J C, Contreras-García J, Hénon E 2017 Phys. Chem. Chem. Phys. 19 17928
 Google Scholar
Google Scholar
[26] Belpassi L, Infante I, Tarantelli F, Visscher L 2008 J. Am. Chem. Soc. 130 1048
 Google Scholar
Google Scholar
[27] Zhang A, Chen Y L, Zhang C X, Yan J 2019 Chin. Phys. Lett. 36 026701
 Google Scholar
Google Scholar
[28] 张翱, 陈云琳, 闫君, 张春秀 2018 67 106701
 Google Scholar
Google Scholar
Zhang A, Chen Y L, Yan J, Zhang C X 2018 Acta Phys. Sin. 67 106701
 Google Scholar
Google Scholar
[29] 张翱, 张春秀, 陈云琳, 张春梅, 孟涛 2020 69 118801
 Google Scholar
Google Scholar
Zhang A, Zhang C X, Chen Y L, Zhang C M, Meng T 2020 Acta Phys. Sin 69 118801
 Google Scholar
Google Scholar
-
图 2 在MP2/Aug-cc-PVTZ水平下 (a)优化的CH3NH3多聚体未配对电子的分子轨道能, (b) 优化的CH3NH3五聚体的分子轨道等值面图和轨道能量, 红色和蓝色分别表示正相和负相
Figure 2. (a) The molecular orbital energies of unpaired electrons for optimized CH3NH3 polymer, and (b) the molecular orbitals isosurface map and energies of unpaired electrons of optimized CH3NH3 pentamer at MP2/Aug-cc-PVTZ level. Red and blue colors correspond to positive and negative phases, respectively.
图 3 在MP2/Aug-cc-PVTZ水平下优化的 (a) CH3NH
${}_3^+ $ , (b) CH3NH3, (c) CH3NH3五聚体的静电势矢量场图, 蓝色的轮廓线表示范德瓦耳斯表面, 红色的箭头表示对应坐标处的电场Figure 3. Vector field map of ESP for optimized (a) CH3NH
${}_3^+ $ , (b) CH3NH3, and (c) CH3NH3 pentamer at MP2/Aug-cc-PVTZ level. The blue contour line and red arrow represent van der Waals surface and electric field at corresponding position, respectively. -
[1] Zhang W, Eperon G E, Snaith H J 2016 Nat. Energy 1 16048
 Google Scholar
Google Scholar
[2] Kojima A, Teshima K, Shirai Y, Miyasaka T 2009 J. Am. Chem. Soc. 131 6050
 Google Scholar
Google Scholar
[3] Ding H, Li B, Zareen S, Li G, Tu Y, Zhang D, Cao X, Xu Q, Yang S, Tait S L, Zhu J 2020 ACS Appl. Mater. Interfaces 12 28861
 Google Scholar
Google Scholar
[4] Breternitz J, Lehmann F, Barnett S A, Nowell H, Schorr S 2020 Angew. Chem. Int. Ed. 59 424
 Google Scholar
Google Scholar
[5] Herz L M 2016 Annu. Rev. Phys. Chem. 67 65
 Google Scholar
Google Scholar
[6] Bi Y, Hutter E M, Fang Y, Dong Q, Huang J, Savenije T J 2016 J. Phys. Chem. Lett. 7 923
 Google Scholar
Google Scholar
[7] Ponseca C S, Savenije T J, Abdellah M, Zheng K, Yartsev A, Pascher T, Harlang T, Chabera P, Pullerits T, Stepanov A, Wolf J P, Sundströ V 2014 J. Am. Chem. Soc. 136 5189
 Google Scholar
Google Scholar
[8] Chen Y, Yi H T, Wu X, Haroldson R, Gartstein Y N, Rodionov Y I, Tikhonov K S, Zakhidov A, Zhu Z Y, Podzorov V 2016 Nat. Commun. 7 12253
 Google Scholar
Google Scholar
[9] He J L, Fang W H, Long R, Prezhdo O V 2020 J. Am. Chem. Soc. 142 14664
 Google Scholar
Google Scholar
[10] Li W W, Man Z Y, Zeng J T, Zheng L Y, Li G R, Kassiba A 2020 J. Phys. Chem. C 124 13348
 Google Scholar
Google Scholar
[11] Brenner T M, Egger D A, Rappe A M, Kronik L, Hodes G, Cahen D 2015 J. Phys. Chem. Lett. 6 4754
 Google Scholar
Google Scholar
[12] Dong Q, Fang Y, Shao Y, Mulligan P, Qiu J, Cao L, Huang J 2015 Science 347 967
 Google Scholar
Google Scholar
[13] Shi D, Adinolfi V, Comin R, Yuan M, Alarousu E, Buin A, Chen Y, Hoogland S, Rothenberger A, Katsiev K, Losovyj Y, Zhang X, Dowben P A, Mohammed O F, Sargent E H, Bakr O M 2015 Science 347 519
 Google Scholar
Google Scholar
[14] Mei Y, Zhang C, Vardeny Z V, Jurchescu O D 2015 MRS Commun. 5 297
 Google Scholar
Google Scholar
[15] Savenije T J, Ponseca C S, Kunneman L, Abdellah M, Zheng K, Tian Y, Zhu Q, Canton S E, Scheblykin I G, Pullerits T, Yartsev A, Sundstrom V 2014 J. Phys. Chem. Lett. 5 2189
 Google Scholar
Google Scholar
[16] Karakus M, Jensen S A, D’Angelo F, Turchinovich D, Bonn M, Canovas E 2015 J. Phys. Chem. Lett. 6 4991
 Google Scholar
Google Scholar
[17] Oga H, Saeki A, Ogomi Y, Hayase S, Seki S 2014 J. Am. Chem. Soc. 136 13818
 Google Scholar
Google Scholar
[18] He Y, Galli G 2014 Chem. Mater. 26 5394
 Google Scholar
Google Scholar
[19] Wang Y, Zhang Y, Zhang P, Zhang W 2015 Phys. Chem. Chem. Phys. 17 11516
 Google Scholar
Google Scholar
[20] Whalley L D, Skelton J M, Frost J M, Walsh A 2016 Phys. Rev. B 94 220301
 Google Scholar
Google Scholar
[21] Monahan D M, Guo L, Lin J, Dou L, Yang P, Fleming G R 2017 J. Phys. Chem. Lett. 8 83211
[22] Ma J, Wang L W 2017 Nano Lett. 17 3646
 Google Scholar
Google Scholar
[23] Frisch M J, Trucks G W, Schlegel H B, et al. 2009 Gaussian 09 (Revision C.01) (Wallingford: Gaussian, Inc.)
[24] Murray J S, Brinck T, Lane P, Paulsen K, Politzer P 1994 J. Mol. Struct. Theochem. 307 55
 Google Scholar
Google Scholar
[25] Lefebvre C, Rubez G, Khartabil H, Boisson J C, Contreras-García J, Hénon E 2017 Phys. Chem. Chem. Phys. 19 17928
 Google Scholar
Google Scholar
[26] Belpassi L, Infante I, Tarantelli F, Visscher L 2008 J. Am. Chem. Soc. 130 1048
 Google Scholar
Google Scholar
[27] Zhang A, Chen Y L, Zhang C X, Yan J 2019 Chin. Phys. Lett. 36 026701
 Google Scholar
Google Scholar
[28] 张翱, 陈云琳, 闫君, 张春秀 2018 67 106701
 Google Scholar
Google Scholar
Zhang A, Chen Y L, Yan J, Zhang C X 2018 Acta Phys. Sin. 67 106701
 Google Scholar
Google Scholar
[29] 张翱, 张春秀, 陈云琳, 张春梅, 孟涛 2020 69 118801
 Google Scholar
Google Scholar
Zhang A, Zhang C X, Chen Y L, Zhang C M, Meng T 2020 Acta Phys. Sin 69 118801
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 6384
- PDF Downloads: 92
- Cited By: 0















 DownLoad:
DownLoad: