-
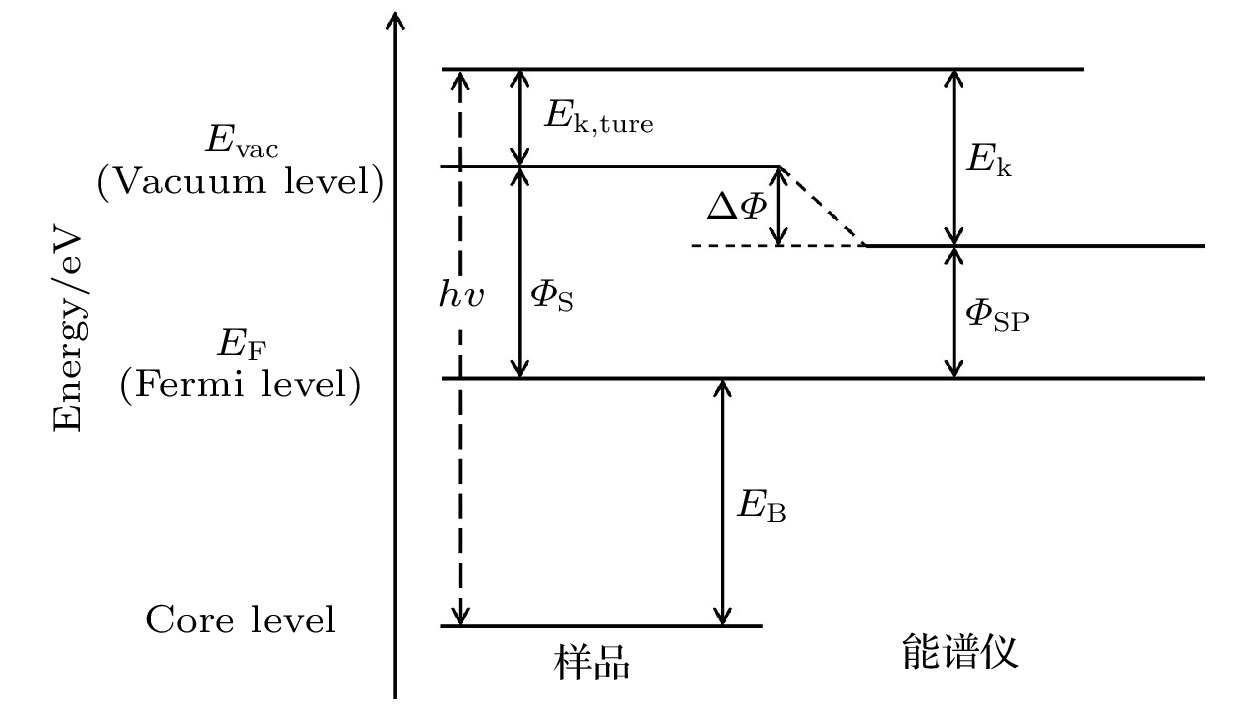
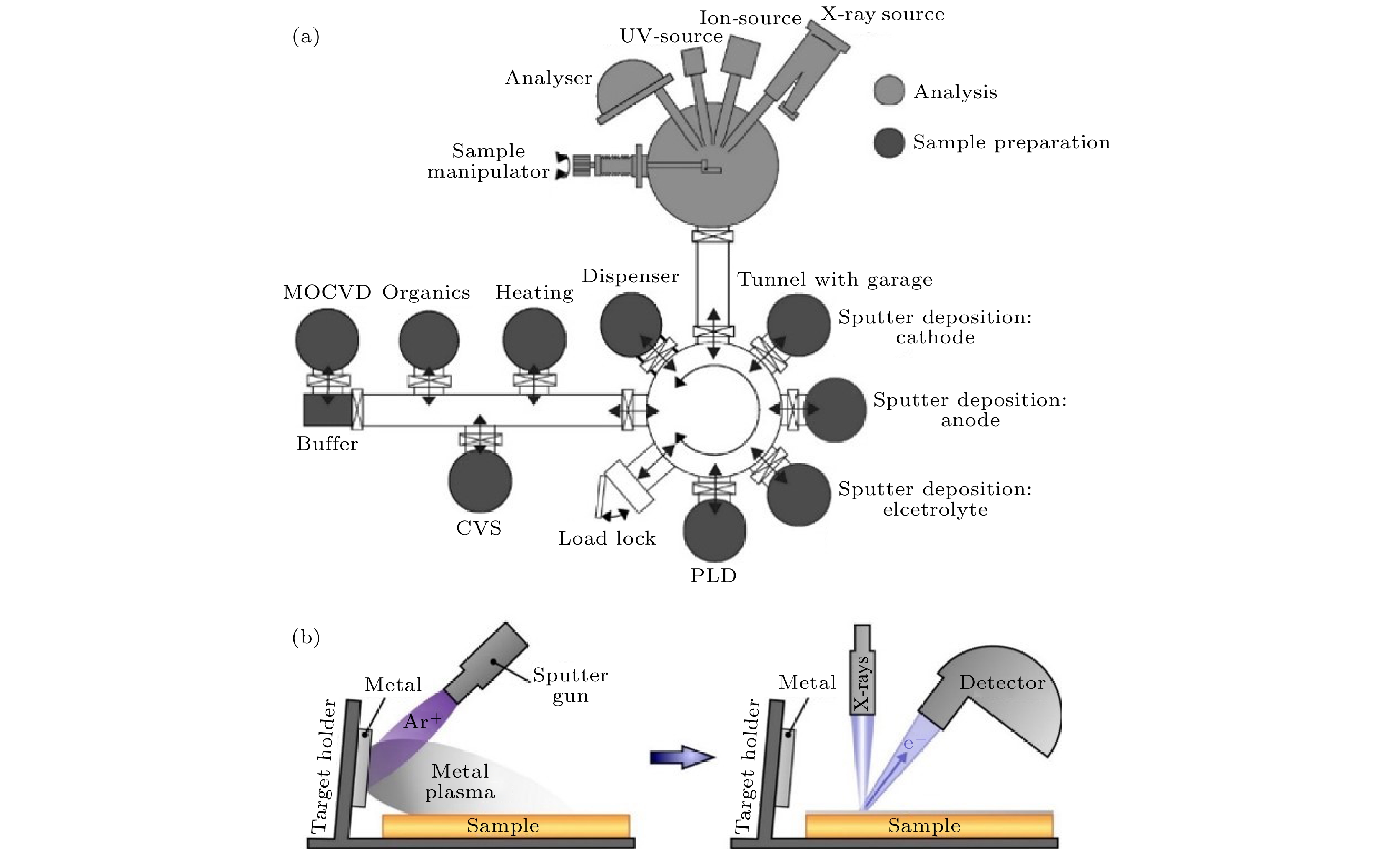
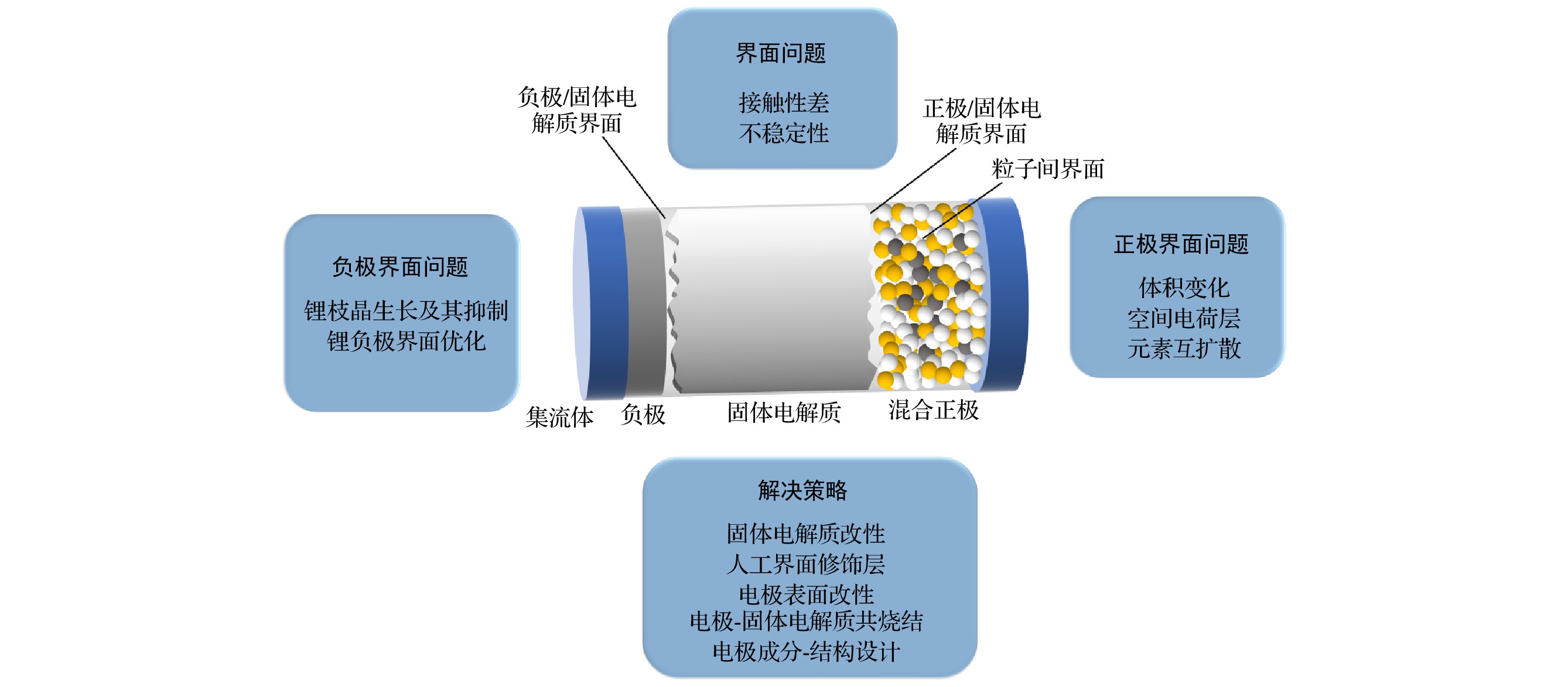
Solid-state lithium-ion batteries have attracted much attention due to their high safety, high energy densities and other advantages. However, solid-state lithium-ion batteries cannot realize large-scale commercial use. There are key scientific and technical issues that have not been resolved, especially interface issues, such as high resistance and instability of the interface. The X-ray photoelectron spectroscopy (XPS), as an important surface analysis method, can perform qualitative and semi-quantitative chemical analysis of the interface, which makes XPS can be widely used to study the solid-state lithium-ion battery interfaces. In this paper, we review the recent research progress of solid-state lithium-ion battery interfaces by using XPS, and summarize and review the XPS experimental principle, experimental method, experimental results and their effects on interface performance. The XPS analysis methods for solid-state lithium-ion batteries include ex-situ XPS, in-situ XPS reflecting the real-time changes of the battery interface, and operando XPS based on the actual working conditions of the battery. The ex-situ XPS can study oxide solid electrolyte interfaces, sulfide solid electrolyte interfaces and artificial solid electrolyte interface (SEI) layers to access information about the chemical composition of the interface, predict the performance of the interface, obtain the chemical distribution in space, and evaluate the chemical structure and irregularity of the interface. With ultraviolet photoemission spectroscopy (UPS) the interface work function, energy band bending and energy structure of the full battery can be obtained. In-situ XPS can effectively study the process of chemical reactions between the electrolyte and the electrode. The key prerequisite is the controllable in-situ construction of the electrolyte/electrode interface. In-situ XPS research can directly study the electrochemical changes of the interface. In-situ XPS/UPS can study the energy level alignment of solid-state lithium-ion batteries, indicating that a space charge layer is formed at the solid electrolyte interface, and the energy band bending occurs. The degree of energy band bending is reflected in the binding energy shifts of the related elements at the interface. The change of the energy structure in the deposition process can be determined by the binding energy shifts of the related elements at the interface and the change of the interface work function. Operando XPS performs XPS characterization at the same time under the working condition of the battery. Operando XPS can be combined with electrochemical characterization to observe the effects of interface reaction and solid electrolyte decomposition products on electrochemical performance, thereby determining the main components that affect electrochemical performance. It can also be combined with the ex-situ XPS to study the interface reaction mechanism and influencing factors. The information obtained includes the chemical states of elements after the interface reaction has occurred, the evolution of interface elements in the process of real-time interface reaction, the energy structure change and interface component overpotential, thus having a better understanding of interface composition, interfacial structure change, kinetics of interface reaction, and interfacial ion migration of the solid-state lithium ion batteries. -
Keywords:
- solid-state lithium-ion battery (SSLIB) /
- X-ray photoelectron spectroscopy (XPS) /
- solid electrolyte interface
[1] Scrosati B, Garche J 2010 J. Power Sources 195 2419
 Google Scholar
Google Scholar
[2] Wen J, Yu Y, Chen C 2012 Mater. Express 2 197
 Google Scholar
Google Scholar
[3] Xiang Y, Li X, Cheng Y, Sun X, Yang Y 2020 Mater. Today 36 139
 Google Scholar
Google Scholar
[4] Wang H, Liu Y, Li Y, Cui Y 2019 Electrochem. Energy Rev. 2 509
 Google Scholar
Google Scholar
[5] Kim J G, Son B, Mukherjee S, Schuppert N, Bates A, Kwon O, Choi M J, Chung H Y, Park S 2015 J. Power Sources 282 299
 Google Scholar
Google Scholar
[6] Wang P, Qu W, Song W L, Chen H, Chen R, Fang D 2019 Adv. Funct. Mater. 29 1900950
[7] Shen Z, Zhang W, Zhu G, Huang Y, Feng Q, Lu Y 2020 Small Methods 4 1900592
 Google Scholar
Google Scholar
[8] Xu L, Tang S, Cheng Y, Wang K, Liang J, Liu C, Cao Y, Wei F, Mai L 2018 Joule 2 1991
 Google Scholar
Google Scholar
[9] Colclasure A M, Smith K A, Kee R J 2011 Electrochim. Acta 58 33
 Google Scholar
Google Scholar
[10] Chen W, Ou Z, Tang H, Wang H, Yang Y 2008 Electrochim. Acta 53 4414
 Google Scholar
Google Scholar
[11] Wang A, Kadam S, Li H, Shi S, Qi Y 2018 NPJ Comput. Mater. 4 15
 Google Scholar
Google Scholar
[12] Schwöbel A, Jaegermann W, Hausbrand R 2016 Solid State Ionics 288 224
 Google Scholar
Google Scholar
[13] Brundle C R 1974 J. Vac. Sci. Technol. 11 212
 Google Scholar
Google Scholar
[14] Watts J F, Wolstenholme J 2003 An Introduction to Surface Analysis by XPS and AES (Chichester: John Wiley & Sons) pp1–14
[15] Watts J F, Wolstenholme J 2003 An Introduction to Surface Analysis by XPS and AES (Chichester: John Wiley & Sons) pp59–76
[16] Meunier G, Dormoy R, Levasseur A 1989 Mater. Sci. Eng., B 3 19
 Google Scholar
Google Scholar
[17] Ueda S 2013 J. Electron Spectrosc. Relat. Phenom. 190 235
 Google Scholar
Google Scholar
[18] Zhao W, Yi J, He P, Zhou H 2019 Electrochem. Energy Rev. 2 574
 Google Scholar
Google Scholar
[19] Reddy M V, Julien C M, Mauger A, Zaghib K 2020 Nanomaterials 10 1606
 Google Scholar
Google Scholar
[20] Manthiram A, Yu X, Wang S 2017 Nat. Rev. Mater. 2 16103
 Google Scholar
Google Scholar
[21] Murugan R, Thangadurai V, Weppner W 2007 Angew. Chem. Int. Ed. 46 7778
 Google Scholar
Google Scholar
[22] Sudo R, Nakata Y, Ishiguro K, Matsui M, Hirano A, Takeda Y, Yamamoto O, Imanishi N 2014 Solid State Ionics 262 151
 Google Scholar
Google Scholar
[23] Luo W, Gong Y, Zhu Y, Fu K K, Dai J, Lacey S D, Wang C, Liu B, Han X, Mo Y, Wachsman E D, Hu L 2016 J. Am. Chem. Soc. 138 12258
 Google Scholar
Google Scholar
[24] Meng J, Zhang Y, Zhou X, Lei M, Li C 2020 Nat. Commun. 11 3716
 Google Scholar
Google Scholar
[25] Sharafi A, Yu S, Naguib M, Lee M, Ma C, Meyer H M, Nanda J, Chi M, Siegel D J, Sakamoto J 2017 J. Mater. Chem. A 5 13475
 Google Scholar
Google Scholar
[26] Wu J F, Pu B W, Wang D, Shi S Q, Zhao N, Guo X, Guo X 2019 ACS Appl. Mater. Interfaces 11 898
 Google Scholar
Google Scholar
[27] Samson A J, Hofstetter K, Bag S, Thangadurai V 2019 Energy Environ. Sci. 12 2957
 Google Scholar
Google Scholar
[28] Li Y, Wang Z, Cao Y, Du F, Chen C, Cui Z, Guo X 2015 Electrochim. Acta 180 37
 Google Scholar
Google Scholar
[29] Ohta S, Kobayashi T, Asaoka T 2011 J. Power Sources 196 3342
 Google Scholar
Google Scholar
[30] Zhu Y, He X, Mo Y 2016 J. Mater. Chem. A 4 3253
 Google Scholar
Google Scholar
[31] Han F, Zhu Y, He X, Mo Y, Wang C 2016 Adv. Energy Mater. 6 1501590
 Google Scholar
Google Scholar
[32] Wolfenstine J, Allen J L, Read J, Sakamoto J 2013 J. Mater. Sci. 48 5846
 Google Scholar
Google Scholar
[33] Tan D H S, Banerjee A, Chen Z, Meng Y S 2020 Nat. Nanotechnol. 15 170
 Google Scholar
Google Scholar
[34] Connell J G, Zhu Y, Zapol P, Tepavcevic S, Sharafi A, Sakamoto J, Curtiss L A, Fong D D, Freeland J W, Markovic N M 2018 ACS Appl. Mater. Interfaces 10 17471
 Google Scholar
Google Scholar
[35] Zhu J, Zhao J, Xiang Y, Lin M, Wang H, Zheng B, He H, Wu Q, Huang J Y, Yang Y 2020 Chem. Mater. 32 4998
 Google Scholar
Google Scholar
[36] Sharafi A, Meyer H M, Nanda J, Wolfenstine J, Sakamoto J 2016 J. Power Sources 302 135
 Google Scholar
Google Scholar
[37] Yu S, Siegel D J 2017 Chem. Mater. 29 9639
 Google Scholar
Google Scholar
[38] Pesci F M, Brugge R H, Hekselman A K O, Cavallaro A, Chater R J, Aguadero A 2018 J. Mater. Chem. A 6 19817
 Google Scholar
Google Scholar
[39] Han F, Westover A S, Yue J, Fan X, Wang F, Chi M, Leonard D N, Dudney N J, Wang H, Wang C 2019 Nat. Energy 4 187
 Google Scholar
Google Scholar
[40] Haruyama J, Sodeyama K, Tateyama Y 2017 ACS Appl. Mater. Interfaces 9 286
 Google Scholar
Google Scholar
[41] Zarabian M, Bartolini M, Pereira-Almao P, Thangadurai V 2017 J. Electrochem. Soc. 164 A1133
 Google Scholar
Google Scholar
[42] Liu Y, Sun Q, Liu J, Norouzi Banis M, Zhao Y, Wang B, Adair K, Hu Y, Xiao Q, Zhang C, Zhang L, Lu S, Huang H, Song X, Sun X 2020 ACS Appl. Mater. Interfaces 12 2293
 Google Scholar
Google Scholar
[43] Trevey J E, Jung Y S, Lee S H 2011 Electrochim. Acta 56 4243
 Google Scholar
Google Scholar
[44] Mizuno F, Hayashi A, Tadanaga K, Tatsumisago M 2005 Adv. Mater. 17 918
 Google Scholar
Google Scholar
[45] Kanno R, Murayama M 2001 J. Electrochem. Soc. 148 A742
 Google Scholar
Google Scholar
[46] Kanno R, Hata T, Kawamoto Y, Irie M 2000 Solid State Ionics 130 97
 Google Scholar
Google Scholar
[47] Kamaya N, Homma K, Yamakawa Y, Hirayama M, Kanno R, Yonemura M, Kamiyama T, Kato Y, Hama S, Kawamoto K, Mitsui A 2011 Nat. Mater. 10 682
 Google Scholar
Google Scholar
[48] Kudu Ö U, Famprikis T, Fleutot B, Braida M-D, Le Mercier T, Islam M S, Masquelier C 2018 J. Power Sources 407 31
 Google Scholar
Google Scholar
[49] Mizuno F, Hayashi A, Tadanaga K, Tatsumisago M 2006 Solid State Ionics 177 2721
 Google Scholar
Google Scholar
[50] Liu Z, Fu W, Payzant E A, Yu X, Wu Z, Dudney N J, Kiggans J, Hong K, Rondinone A J, Liang C 2013 J. Am. Chem. Soc. 135 975
 Google Scholar
Google Scholar
[51] Dietrich C, Weber D A, Culver S, Senyshyn A, Sedlmaier S J, Indris S, Janek J, Zeier W G 2017 Inorg. Chem. 56 6681
 Google Scholar
Google Scholar
[52] Seino Y, Ota T, Takada K, Hayashi A, Tatsumisago M 2014 Energy Environ. Sci. 7 627
 Google Scholar
Google Scholar
[53] Wenzel S, Randau S, Leichtweiß T, Weber D A, Sann J, Zeier W G, Janek J 2016 Chem. Mater. 28 2400
 Google Scholar
Google Scholar
[54] Zhou P, Wang J, Cheng F, Li F, Chen J 2016 Chem. Commun. 52 6091
 Google Scholar
Google Scholar
[55] Boulineau S, Courty M, Tarascon J M, Viallet V 2012 Solid State Ionics 221 1
 Google Scholar
Google Scholar
[56] Zhu Y, He X, Mo Y 2015 ACS Appl. Mater. Interfaces 7 23685
 Google Scholar
Google Scholar
[57] Auvergniot J, Cassel A, Foix D, Viallet V, Seznec V, Dedryvère R 2017 Solid State Ionics 300 78
 Google Scholar
Google Scholar
[58] Gao Y, Wang D, Li Y C, Yu Z, Mallouk T E, Wang D 2018 Angew. Chem. Int. Ed. 57 13608
 Google Scholar
Google Scholar
[59] Koerver R, Walther F, Aygün I, Sann J, Dietrich C, Zeier W G, Janek J 2017 J. Mater. Chem. A 5 22750
 Google Scholar
Google Scholar
[60] Du M, Liao K, Lu Q, Shao Z 2019 Energy Environ. Sci. 12 1780
 Google Scholar
Google Scholar
[61] Ni X, Qian T, Liu X, Xu N, Liu J, Yan C 2018 Adv. Funct. Mater. 28 1706513
 Google Scholar
Google Scholar
[62] Zhang L, Zhang K, Shi Z, Zhang S 2017 Langmuir 33 11164
 Google Scholar
Google Scholar
[63] Shi X, Pang Y, Wang B, Sun H, Wang X, Li Y, Yang J, Li H W, Zheng S 2020 Mater. Today Nano 10 100079
 Google Scholar
Google Scholar
[64] Fan X, Ji X, Han F, Yue J, Chen J, Chen L, Deng T, Jiang J, Wang C 2018 Sci. Adv. 4 eaau9245
 Google Scholar
Google Scholar
[65] Chang X, Liu H, Yang H, Di J, Tang W, Fu H, Li M, Liu R 2020 J. Materiomics 6 54
 Google Scholar
Google Scholar
[66] Nagao K, Suyama M, Kato A, Hotehama C, Deguchi M, Sakuda A, Hayashi A, Tatsumisago M 2019 ACS Appl. Energy Mater. 2 3042
 Google Scholar
Google Scholar
[67] Liang J Y, Zhang X D, Zeng X X, Yan M, Yin Y X, Xin S, Wang W P, Wu X W, Shi J L, Wan L J, Guo Y G 2020 Angew. Chem. Int. Ed. 59 6585
 Google Scholar
Google Scholar
[68] Tripathi A M, Su W N, Hwang B J 2018 Chem. Soc. Rev. 47 736
 Google Scholar
Google Scholar
[69] Sicolo S, Fingerle M, Hausbrand R, Albe K 2017 J. Power Sources 354 124
 Google Scholar
Google Scholar
[70] Wenzel S, Leichtweiss T, Krüger D, Sann J, Janek J 2015 Solid State Ionics. 278 98
 Google Scholar
Google Scholar
[71] Liu Z, Li G, A. Borodin, Liu X, Li Y, Endres F 2019 J. Solid State Electrochem. 23 2107
 Google Scholar
Google Scholar
[72] Jones S D, Akridge J R, Shokoohi F K 1994 Solid State Ionics 69 357
 Google Scholar
Google Scholar
[73] Lacivita V, Artrith N, Ceder G 2018 Chem. Mater. 30 7077
 Google Scholar
Google Scholar
[74] Lacivita V, Westover A S, Kercher A, Phillip N D, Yang G, Veith G, Ceder G, Dudney N J 2018 J. Am. Chem. Soc. 140 11029
 Google Scholar
Google Scholar
[75] Yu X, Bates J B, Jellison G E, Hart F X 1997 J. Electrochem. Soc. 144 524
 Google Scholar
Google Scholar
[76] Schwöbel A, Hausbrand R, Jaegermann W 2015 Solid State Ionics 273 51
 Google Scholar
Google Scholar
[77] Nazri G 1988 MRS Proc. 135 117
[78] Nazri G 1989 Solid State Ionics 34 97
 Google Scholar
Google Scholar
[79] Maier J, Bunsenges B 1985 Phys. Chem. 89 355
[80] Guo X, Chen C 2016 Acta. Chim. Slov. 63 3
[81] Bañares M A 2005 Catal. Today 100 71
 Google Scholar
Google Scholar
[82] Palomares V, Sharma N 2019 Front. Energy Res. 7 10
 Google Scholar
Google Scholar
[83] Wu X, Villevieille C, Novák P, El Kazzi M 2018 Phys. Chem. Chem. Phys. 20 11123
 Google Scholar
Google Scholar
[84] Nelson Weker J, Toney M F 2015 Adv. Funct. Mater. 25 1622
 Google Scholar
Google Scholar
[85] Wood K N, Steirer K X, Hafner S E, et al. 2018 Nat. Commun. 9 2490
 Google Scholar
Google Scholar
-
图 4 (a) 原始和放电后的Li7La3Zr2O12(LLZO)表面的Zr 3d分峰拟合结果[31]; (b)原始Li1.3Al0.3Ti1.7(PO4)3(LATP)、化学失效LATP、电化学失效LATP的Ti 2p谱图[35]; LiCoO2衬底上LLZO薄膜不同刻蚀深度的(c)Co 2p峰以及(d)Li 1s和Co 3p峰的XPS深度剖析谱图[41]
Figure 4. (a) Peak deconvolution of Zr 3d spectra of fresh and discharged Li7La3Zr2O12 (LLZO)[31]; (b) Ti 2p XPS spectra of the Li1.3Al0.3Ti1.7(PO4)3 (LATP)-pristine, chem-LATP(chemical failure), and EC-LATP(electrochemical failure) surface[35]; XPS depth profiles of (c) Co 2p spectra, (d) Li 1s and Co 3p spectra of LLZO thin film on LiCoO2 at different depth[41].
图 6 (a) 循环后的Li/Li10GeP2S12界面的XPS深度剖析谱图, S 2p, Ge 3d, P 2p, Li 1s[58]; (b) 在不同截止电压(4.0—5.0 V)循环25次后的集流体/正极界面随时间变化的S 2p深度剖析谱图[59]
Figure 6. (a) XPS depth profiles of cycled Li/Li10GeP2S12 interface: XPS spectra of S 2p, Ge 3d, P 2p, Li 1s[58]; (b) S 2p XPS depth profiles after 25 cycles for different upper cut-off voltage (4.0–5.0 V) as a function of different etching time[59].
图 7 (a) 循环后LiFSI修饰的Li3PS4的F 1s和P 2p(右)谱图, 循环后未处理的Li3PS4的P 2p(左)谱图[64]; (b) Li/Li2.9B0.9S0.1O3.1对称电池恒流充放电循环前后的阻抗图[66]; (c)刻蚀得到的Li/Li2.9B0.9S0.1O3.1区域表面/界面/体相的B 1s, S 2p, O 1s, Li 1s谱图[66]
Figure 7. (a) F 1s and P 2p (right) spectra of LiFSI-treated Li3PS4 from cycled cell, P 2p (left) spectra of untreated Li3PS4 from cycled cell[64]; (b) impedance plots of the Li/ Li2.9B0.9S0.1O3.1 symmetric cell before and after the galvanostatic test for cycles[66]; (c) XPS spectra of B 1s, S 2p, O 1s, Li 1s for the outermost surface, interface, and electrolyte bulk regions uncovered by etching of Li/Li2.9B0.9S0.1O3.1 interface area[66].
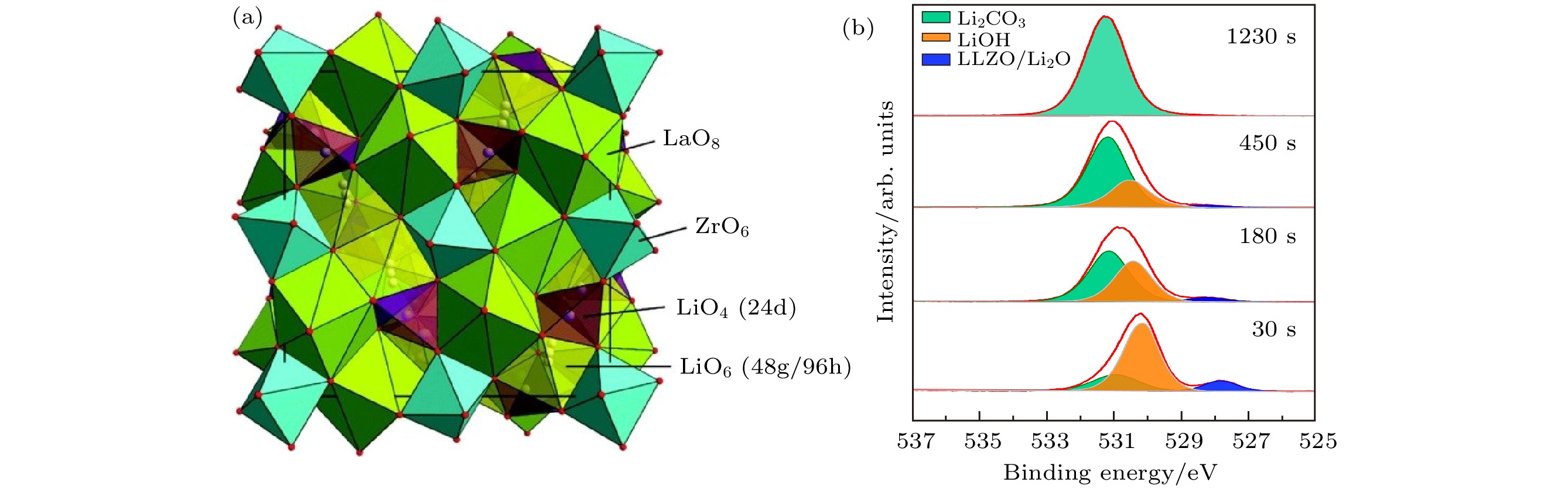
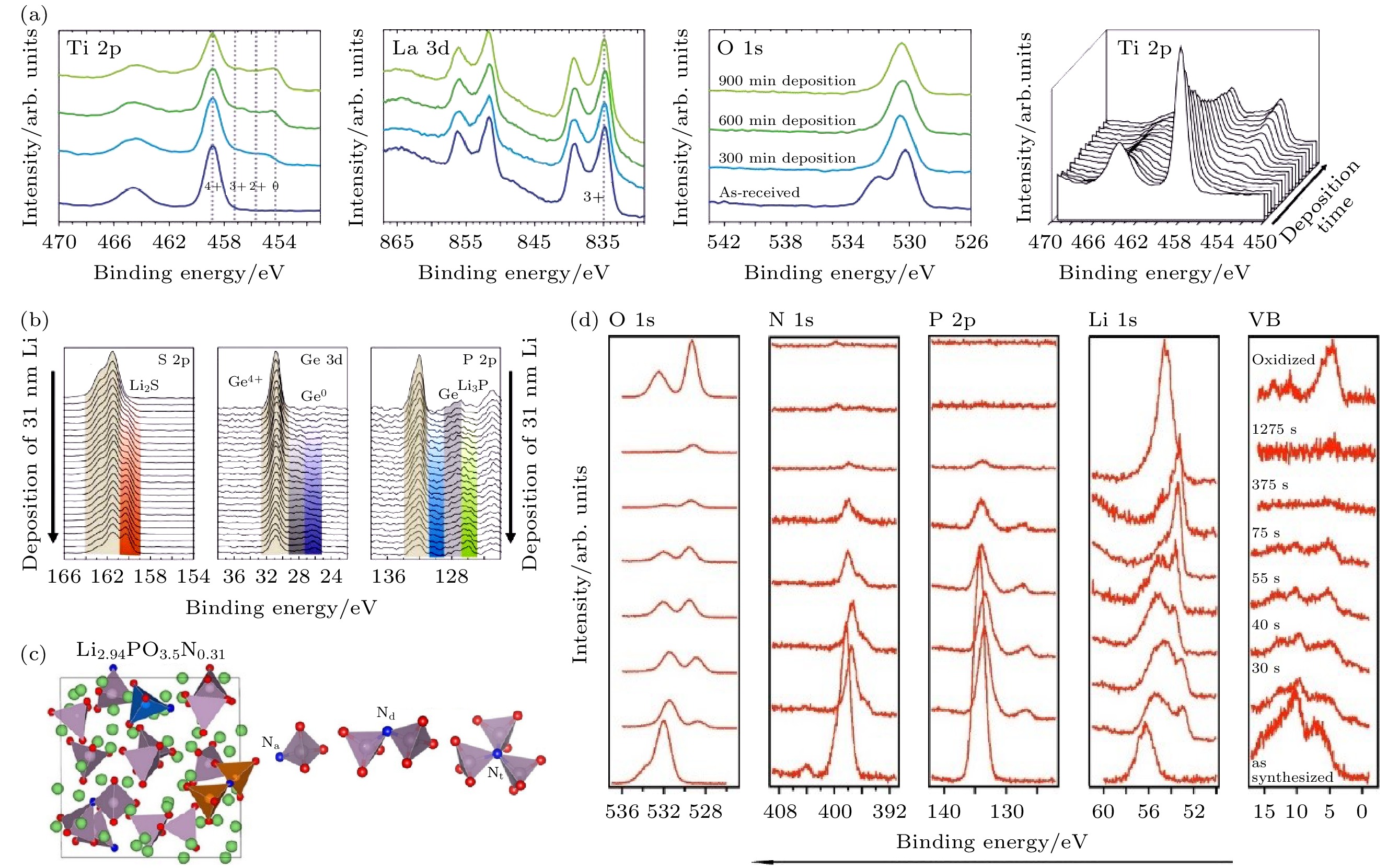
图 9 (a) LLTO表面不同锂沉积时间的Ti 2p, La 3d, O 1s谱以及Ti 2p瀑布图[70]; (b) 31 nm金属锂沉积过程中Li10GeP2S12表面的S 2p, Ge 3d和P 2p-Ge 3p谱图[53]; (c) Li2.94PO3.5N0.31的结构和磷酸盐结构中N的结合情况示意图[73,74]; (d)金属锂沉积过程中LiPON表面的O 1s, N 1s, P 2p, Li 1s和价带图[76]
Figure 9. (a) Ti 2p, La 3d, O 1s detail spectra and Ti 2p waterfall plot for different Li metal deposition times on LLTO surface[70]; (b) S 2p, Ge 3d, and P 2p-Ge 3p detail spectra during 31 nm Li metal deposition on Li10GeP2S12 surface[53]; (c) scheme of Li2.94PO3.5N0.31 structure and possible N configurations in phosphate structures[73,74]; (d) O 1s, N 1s, P 2p, Li 1s, and valence band spectra of LiPON surface during Li metal deposition[76].
图 10 (a)电化学电池的负极界面原位XPS测试示意图; (b)不同电势下Li/Ga-LLZO界面的O 1s, Li 1s, La 4d, Zr 3d谱图[71]; (c)原位XPS装置示意图; (d)电化学极化中混合正极的S 2p谱图[59]
Figure 10. (a) Scheme of the configuration of the anode surface of electrochemical cell for in situ XPS; (b) in situ XPS spectra of O 1s, Li 1s, La 4d, and Zr 3d at the Li/Ga-doped LLZO interphase as a function of potential[71]; (c) scheme of the measurement setup for in situ XPS; (d) S 2p spectra of composite cathode during electrochemical polarization[59].
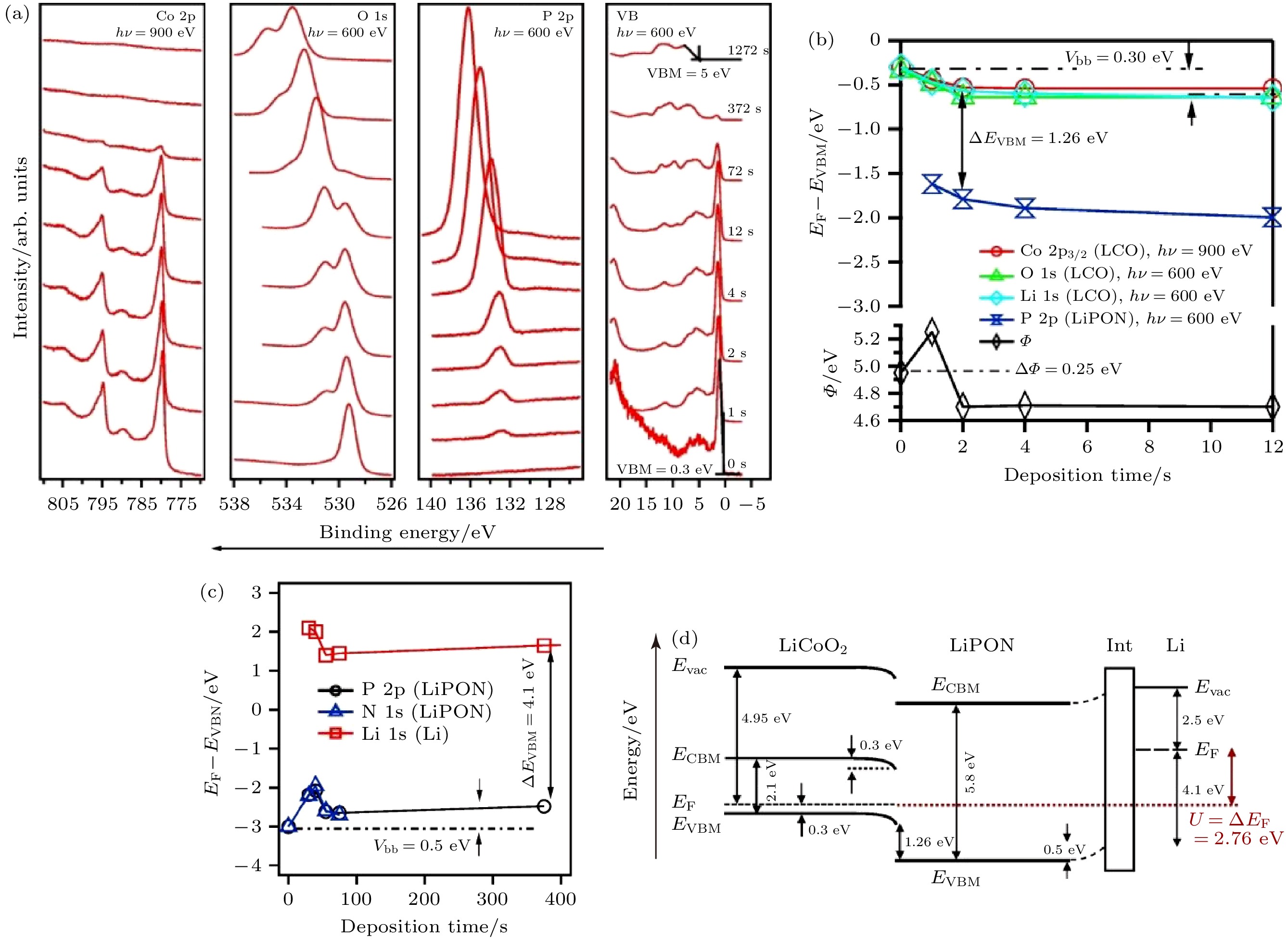
图 11 (a) LiPON沉积过程中LiCoO2表面的Co 2p, O 1s, P 2p和价带谱图; (b) LiCoO2内层电子结合能, LiPON P 2p电子结合能和表面功函数在沉积过程中的变化; (c)金属锂沉积过程中LiPON内层电子结合能的变化; (d)Li/LiPON/LiCoO2 SSLIB的能带图[12]
Figure 11. (a) Co 2p, O 1s, P 2p and valence band spectra of LiCoO2 surface during the deposition of LiPON; (b) evolution of the core level binding energies of the LiCoO2 substrate and the P 2p binding energy of the covering LiPON layer as a function of deposition time; (c) evolution of the binding energies during the stepwise evaporation of lithium on top of LiPON; (d) energy band diagram of Li/LiPON/LiCoO2 SSLIB[12].
图 12 (a)全固态operando XPS电池的结构示意图[83]; (b) Operando XPS电池和标准电池工作电极的循环伏安测试结果[83]; (c) S 2p, P 2p, Co 3p-Li 1s-Fe 3p谱图在operando XPS测试过程中的变化[83]
Figure 12. (a) An operando XPS cell design for all-solid-state batteries[83]; (b) cyclic voltammetry measurements of the work electrodes of the operando XPS cell and a standard test cell[83]; (c) evolution of S 2p, P 2p, and Co 3p-Li 1s-Fe 3p core level spectra recorded during the operando XPS measurements[83].
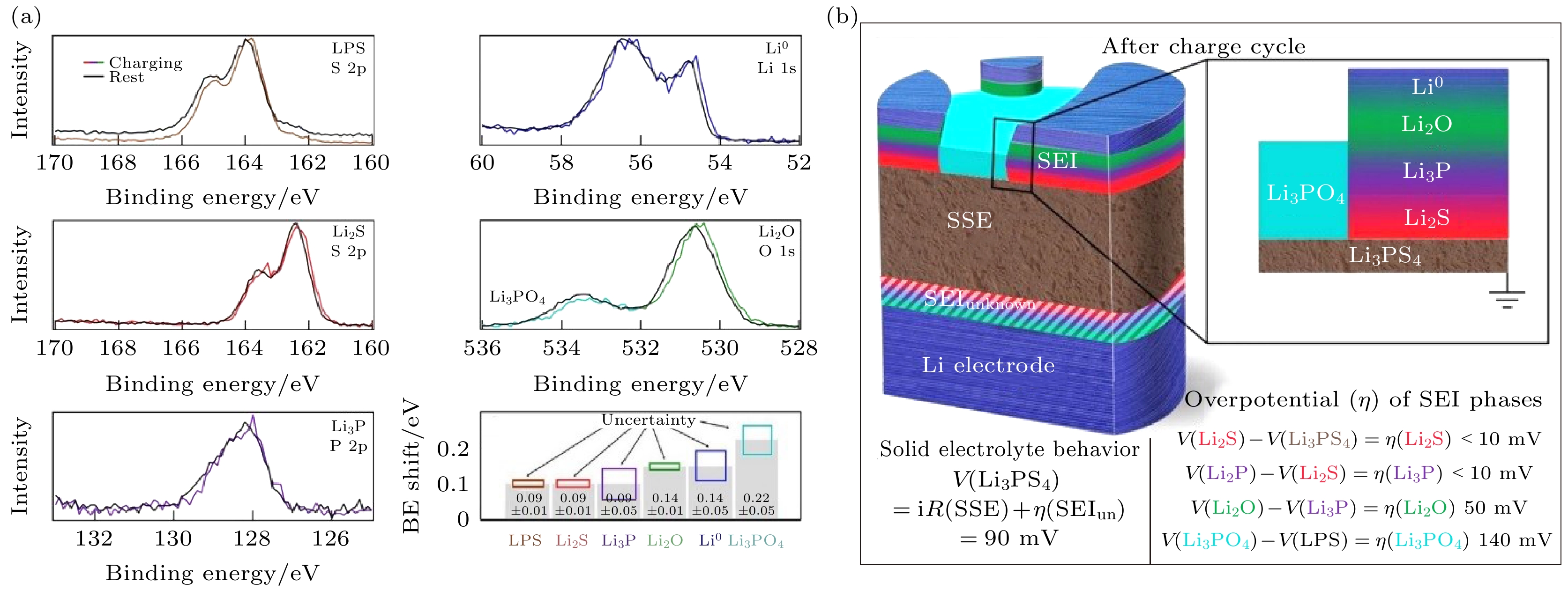
图 13 (a)虚拟电极法实现operando XPS测试的原理图和原始、充电、放电状态下Li2S-P2S5表面的Li 1s谱图变化[85]; (b)各XPS谱图及拟合结果在operando XPS过程中的变化[85]
Figure 13. (a) Schematic of operando XPS measurements via virtual electrode cycling and the evolution of Li 1s spectra on Li2S-P2S5 surface during the cycling process[85]; (b) evolution of XPS spectra showing peak deconvolution during operando XPS measurements[85].
图 14 (a) SEI各组分充电过程中结合能的位移[85]; (b) SEI各组分的过电位及得到的SEI结构示意图[85]
Figure 14. (a) Binding energy shifts of each SEI phase composition during charging process[85]; (b) overpotential of each SEI phase composition during charging process and scheme of SEI structure after charging based on the overpotential[85].
-
[1] Scrosati B, Garche J 2010 J. Power Sources 195 2419
 Google Scholar
Google Scholar
[2] Wen J, Yu Y, Chen C 2012 Mater. Express 2 197
 Google Scholar
Google Scholar
[3] Xiang Y, Li X, Cheng Y, Sun X, Yang Y 2020 Mater. Today 36 139
 Google Scholar
Google Scholar
[4] Wang H, Liu Y, Li Y, Cui Y 2019 Electrochem. Energy Rev. 2 509
 Google Scholar
Google Scholar
[5] Kim J G, Son B, Mukherjee S, Schuppert N, Bates A, Kwon O, Choi M J, Chung H Y, Park S 2015 J. Power Sources 282 299
 Google Scholar
Google Scholar
[6] Wang P, Qu W, Song W L, Chen H, Chen R, Fang D 2019 Adv. Funct. Mater. 29 1900950
[7] Shen Z, Zhang W, Zhu G, Huang Y, Feng Q, Lu Y 2020 Small Methods 4 1900592
 Google Scholar
Google Scholar
[8] Xu L, Tang S, Cheng Y, Wang K, Liang J, Liu C, Cao Y, Wei F, Mai L 2018 Joule 2 1991
 Google Scholar
Google Scholar
[9] Colclasure A M, Smith K A, Kee R J 2011 Electrochim. Acta 58 33
 Google Scholar
Google Scholar
[10] Chen W, Ou Z, Tang H, Wang H, Yang Y 2008 Electrochim. Acta 53 4414
 Google Scholar
Google Scholar
[11] Wang A, Kadam S, Li H, Shi S, Qi Y 2018 NPJ Comput. Mater. 4 15
 Google Scholar
Google Scholar
[12] Schwöbel A, Jaegermann W, Hausbrand R 2016 Solid State Ionics 288 224
 Google Scholar
Google Scholar
[13] Brundle C R 1974 J. Vac. Sci. Technol. 11 212
 Google Scholar
Google Scholar
[14] Watts J F, Wolstenholme J 2003 An Introduction to Surface Analysis by XPS and AES (Chichester: John Wiley & Sons) pp1–14
[15] Watts J F, Wolstenholme J 2003 An Introduction to Surface Analysis by XPS and AES (Chichester: John Wiley & Sons) pp59–76
[16] Meunier G, Dormoy R, Levasseur A 1989 Mater. Sci. Eng., B 3 19
 Google Scholar
Google Scholar
[17] Ueda S 2013 J. Electron Spectrosc. Relat. Phenom. 190 235
 Google Scholar
Google Scholar
[18] Zhao W, Yi J, He P, Zhou H 2019 Electrochem. Energy Rev. 2 574
 Google Scholar
Google Scholar
[19] Reddy M V, Julien C M, Mauger A, Zaghib K 2020 Nanomaterials 10 1606
 Google Scholar
Google Scholar
[20] Manthiram A, Yu X, Wang S 2017 Nat. Rev. Mater. 2 16103
 Google Scholar
Google Scholar
[21] Murugan R, Thangadurai V, Weppner W 2007 Angew. Chem. Int. Ed. 46 7778
 Google Scholar
Google Scholar
[22] Sudo R, Nakata Y, Ishiguro K, Matsui M, Hirano A, Takeda Y, Yamamoto O, Imanishi N 2014 Solid State Ionics 262 151
 Google Scholar
Google Scholar
[23] Luo W, Gong Y, Zhu Y, Fu K K, Dai J, Lacey S D, Wang C, Liu B, Han X, Mo Y, Wachsman E D, Hu L 2016 J. Am. Chem. Soc. 138 12258
 Google Scholar
Google Scholar
[24] Meng J, Zhang Y, Zhou X, Lei M, Li C 2020 Nat. Commun. 11 3716
 Google Scholar
Google Scholar
[25] Sharafi A, Yu S, Naguib M, Lee M, Ma C, Meyer H M, Nanda J, Chi M, Siegel D J, Sakamoto J 2017 J. Mater. Chem. A 5 13475
 Google Scholar
Google Scholar
[26] Wu J F, Pu B W, Wang D, Shi S Q, Zhao N, Guo X, Guo X 2019 ACS Appl. Mater. Interfaces 11 898
 Google Scholar
Google Scholar
[27] Samson A J, Hofstetter K, Bag S, Thangadurai V 2019 Energy Environ. Sci. 12 2957
 Google Scholar
Google Scholar
[28] Li Y, Wang Z, Cao Y, Du F, Chen C, Cui Z, Guo X 2015 Electrochim. Acta 180 37
 Google Scholar
Google Scholar
[29] Ohta S, Kobayashi T, Asaoka T 2011 J. Power Sources 196 3342
 Google Scholar
Google Scholar
[30] Zhu Y, He X, Mo Y 2016 J. Mater. Chem. A 4 3253
 Google Scholar
Google Scholar
[31] Han F, Zhu Y, He X, Mo Y, Wang C 2016 Adv. Energy Mater. 6 1501590
 Google Scholar
Google Scholar
[32] Wolfenstine J, Allen J L, Read J, Sakamoto J 2013 J. Mater. Sci. 48 5846
 Google Scholar
Google Scholar
[33] Tan D H S, Banerjee A, Chen Z, Meng Y S 2020 Nat. Nanotechnol. 15 170
 Google Scholar
Google Scholar
[34] Connell J G, Zhu Y, Zapol P, Tepavcevic S, Sharafi A, Sakamoto J, Curtiss L A, Fong D D, Freeland J W, Markovic N M 2018 ACS Appl. Mater. Interfaces 10 17471
 Google Scholar
Google Scholar
[35] Zhu J, Zhao J, Xiang Y, Lin M, Wang H, Zheng B, He H, Wu Q, Huang J Y, Yang Y 2020 Chem. Mater. 32 4998
 Google Scholar
Google Scholar
[36] Sharafi A, Meyer H M, Nanda J, Wolfenstine J, Sakamoto J 2016 J. Power Sources 302 135
 Google Scholar
Google Scholar
[37] Yu S, Siegel D J 2017 Chem. Mater. 29 9639
 Google Scholar
Google Scholar
[38] Pesci F M, Brugge R H, Hekselman A K O, Cavallaro A, Chater R J, Aguadero A 2018 J. Mater. Chem. A 6 19817
 Google Scholar
Google Scholar
[39] Han F, Westover A S, Yue J, Fan X, Wang F, Chi M, Leonard D N, Dudney N J, Wang H, Wang C 2019 Nat. Energy 4 187
 Google Scholar
Google Scholar
[40] Haruyama J, Sodeyama K, Tateyama Y 2017 ACS Appl. Mater. Interfaces 9 286
 Google Scholar
Google Scholar
[41] Zarabian M, Bartolini M, Pereira-Almao P, Thangadurai V 2017 J. Electrochem. Soc. 164 A1133
 Google Scholar
Google Scholar
[42] Liu Y, Sun Q, Liu J, Norouzi Banis M, Zhao Y, Wang B, Adair K, Hu Y, Xiao Q, Zhang C, Zhang L, Lu S, Huang H, Song X, Sun X 2020 ACS Appl. Mater. Interfaces 12 2293
 Google Scholar
Google Scholar
[43] Trevey J E, Jung Y S, Lee S H 2011 Electrochim. Acta 56 4243
 Google Scholar
Google Scholar
[44] Mizuno F, Hayashi A, Tadanaga K, Tatsumisago M 2005 Adv. Mater. 17 918
 Google Scholar
Google Scholar
[45] Kanno R, Murayama M 2001 J. Electrochem. Soc. 148 A742
 Google Scholar
Google Scholar
[46] Kanno R, Hata T, Kawamoto Y, Irie M 2000 Solid State Ionics 130 97
 Google Scholar
Google Scholar
[47] Kamaya N, Homma K, Yamakawa Y, Hirayama M, Kanno R, Yonemura M, Kamiyama T, Kato Y, Hama S, Kawamoto K, Mitsui A 2011 Nat. Mater. 10 682
 Google Scholar
Google Scholar
[48] Kudu Ö U, Famprikis T, Fleutot B, Braida M-D, Le Mercier T, Islam M S, Masquelier C 2018 J. Power Sources 407 31
 Google Scholar
Google Scholar
[49] Mizuno F, Hayashi A, Tadanaga K, Tatsumisago M 2006 Solid State Ionics 177 2721
 Google Scholar
Google Scholar
[50] Liu Z, Fu W, Payzant E A, Yu X, Wu Z, Dudney N J, Kiggans J, Hong K, Rondinone A J, Liang C 2013 J. Am. Chem. Soc. 135 975
 Google Scholar
Google Scholar
[51] Dietrich C, Weber D A, Culver S, Senyshyn A, Sedlmaier S J, Indris S, Janek J, Zeier W G 2017 Inorg. Chem. 56 6681
 Google Scholar
Google Scholar
[52] Seino Y, Ota T, Takada K, Hayashi A, Tatsumisago M 2014 Energy Environ. Sci. 7 627
 Google Scholar
Google Scholar
[53] Wenzel S, Randau S, Leichtweiß T, Weber D A, Sann J, Zeier W G, Janek J 2016 Chem. Mater. 28 2400
 Google Scholar
Google Scholar
[54] Zhou P, Wang J, Cheng F, Li F, Chen J 2016 Chem. Commun. 52 6091
 Google Scholar
Google Scholar
[55] Boulineau S, Courty M, Tarascon J M, Viallet V 2012 Solid State Ionics 221 1
 Google Scholar
Google Scholar
[56] Zhu Y, He X, Mo Y 2015 ACS Appl. Mater. Interfaces 7 23685
 Google Scholar
Google Scholar
[57] Auvergniot J, Cassel A, Foix D, Viallet V, Seznec V, Dedryvère R 2017 Solid State Ionics 300 78
 Google Scholar
Google Scholar
[58] Gao Y, Wang D, Li Y C, Yu Z, Mallouk T E, Wang D 2018 Angew. Chem. Int. Ed. 57 13608
 Google Scholar
Google Scholar
[59] Koerver R, Walther F, Aygün I, Sann J, Dietrich C, Zeier W G, Janek J 2017 J. Mater. Chem. A 5 22750
 Google Scholar
Google Scholar
[60] Du M, Liao K, Lu Q, Shao Z 2019 Energy Environ. Sci. 12 1780
 Google Scholar
Google Scholar
[61] Ni X, Qian T, Liu X, Xu N, Liu J, Yan C 2018 Adv. Funct. Mater. 28 1706513
 Google Scholar
Google Scholar
[62] Zhang L, Zhang K, Shi Z, Zhang S 2017 Langmuir 33 11164
 Google Scholar
Google Scholar
[63] Shi X, Pang Y, Wang B, Sun H, Wang X, Li Y, Yang J, Li H W, Zheng S 2020 Mater. Today Nano 10 100079
 Google Scholar
Google Scholar
[64] Fan X, Ji X, Han F, Yue J, Chen J, Chen L, Deng T, Jiang J, Wang C 2018 Sci. Adv. 4 eaau9245
 Google Scholar
Google Scholar
[65] Chang X, Liu H, Yang H, Di J, Tang W, Fu H, Li M, Liu R 2020 J. Materiomics 6 54
 Google Scholar
Google Scholar
[66] Nagao K, Suyama M, Kato A, Hotehama C, Deguchi M, Sakuda A, Hayashi A, Tatsumisago M 2019 ACS Appl. Energy Mater. 2 3042
 Google Scholar
Google Scholar
[67] Liang J Y, Zhang X D, Zeng X X, Yan M, Yin Y X, Xin S, Wang W P, Wu X W, Shi J L, Wan L J, Guo Y G 2020 Angew. Chem. Int. Ed. 59 6585
 Google Scholar
Google Scholar
[68] Tripathi A M, Su W N, Hwang B J 2018 Chem. Soc. Rev. 47 736
 Google Scholar
Google Scholar
[69] Sicolo S, Fingerle M, Hausbrand R, Albe K 2017 J. Power Sources 354 124
 Google Scholar
Google Scholar
[70] Wenzel S, Leichtweiss T, Krüger D, Sann J, Janek J 2015 Solid State Ionics. 278 98
 Google Scholar
Google Scholar
[71] Liu Z, Li G, A. Borodin, Liu X, Li Y, Endres F 2019 J. Solid State Electrochem. 23 2107
 Google Scholar
Google Scholar
[72] Jones S D, Akridge J R, Shokoohi F K 1994 Solid State Ionics 69 357
 Google Scholar
Google Scholar
[73] Lacivita V, Artrith N, Ceder G 2018 Chem. Mater. 30 7077
 Google Scholar
Google Scholar
[74] Lacivita V, Westover A S, Kercher A, Phillip N D, Yang G, Veith G, Ceder G, Dudney N J 2018 J. Am. Chem. Soc. 140 11029
 Google Scholar
Google Scholar
[75] Yu X, Bates J B, Jellison G E, Hart F X 1997 J. Electrochem. Soc. 144 524
 Google Scholar
Google Scholar
[76] Schwöbel A, Hausbrand R, Jaegermann W 2015 Solid State Ionics 273 51
 Google Scholar
Google Scholar
[77] Nazri G 1988 MRS Proc. 135 117
[78] Nazri G 1989 Solid State Ionics 34 97
 Google Scholar
Google Scholar
[79] Maier J, Bunsenges B 1985 Phys. Chem. 89 355
[80] Guo X, Chen C 2016 Acta. Chim. Slov. 63 3
[81] Bañares M A 2005 Catal. Today 100 71
 Google Scholar
Google Scholar
[82] Palomares V, Sharma N 2019 Front. Energy Res. 7 10
 Google Scholar
Google Scholar
[83] Wu X, Villevieille C, Novák P, El Kazzi M 2018 Phys. Chem. Chem. Phys. 20 11123
 Google Scholar
Google Scholar
[84] Nelson Weker J, Toney M F 2015 Adv. Funct. Mater. 25 1622
 Google Scholar
Google Scholar
[85] Wood K N, Steirer K X, Hafner S E, et al. 2018 Nat. Commun. 9 2490
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 17630
- PDF Downloads: 637
- Cited By: 0














 DownLoad:
DownLoad: