-
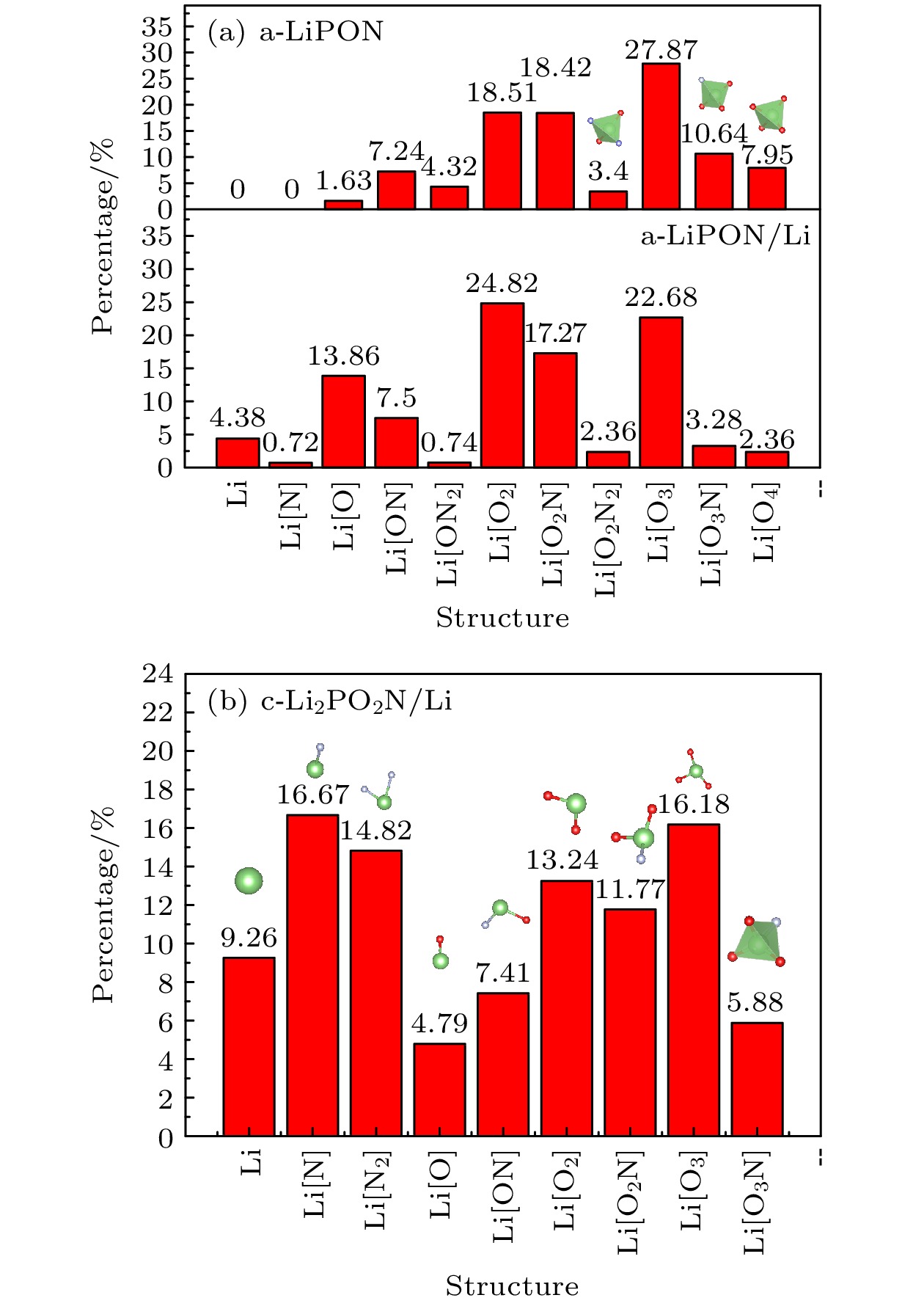
近年来, 全固态锂离子电池因其高安全性、高能量密度、简单的电池结构等优势成为研究热点. 然而电极与电解质的固固界面问题严重影响电池性能的进一步提升, 从而受到了广泛关注. 本文采用从头算分子动力学对LiPON/ Li界面进行了模拟. 研究发现, 界面处原子互扩散现象明显, 并形成薄界面层. 相比LiPON体相结构, 界面层以Li为中心原子的Li[O2N2], Li[O3N], Li[O4]四面体局域结构占比明显减少, 并且界面层Li-O, Li-N, P-O和P-N的平均配位数均有所减小. 由于界面层结构和配位数的变化使得Li受到O, N的离子键作用更弱, Li离子扩散过程中受到的阻碍更小. 这一点对于LiPON电解质在实际电池应用中的性能起到了积极促进作用.In recent years, all-solid-state thin-film batteries have been used to power low-energy devices such as microchips, smart cards, microelectromechanical systems, wireless sensors, and implantable medical devices. All-solid-state thin-film batteries have become an important research direction of rechargeable solid-state batteries (SSBs). However, the solid-solid interface between electrodes and electrolytes seriously affects the further improvement of battery performance, which has attracted extensive attention. Lithium phosphorus oxynitride (LiPON) was found to be a useful inorganic electrolyte in lithium batteries because of its favorable electrochemical properties. For instance, LiPON has good electrical and chemical stability, negligible electronic conductivity and excellent cyclability as well as ease of integration with thin film battery with an electrochemical stability window. The LiPON can present two states, i.e. amorphous state and crystalline state. Here, we adopt ab initio molecular dynamics to study amorphous-LiPON/Li(100) interface and crystalline-Li2PO2N(100)/Li(100) interface. Our results demonstrate that the atomic inter-diffusion occurs in the interfacial region, forming a thin interfacial layer, and the ionic conductivity is increased after the interface layer has formed. Meanwhile, comparing with the Lipon bulk phase structure, the proportion of Li[O2N2], Li[O3N], and Li[O4] tetrahedral local structure in the interface layer with Li atom as the center decrease obviously, and the average coordination number of Li-O, Li-N, P-O, and P-N in the interfacial layers also decrease in the LiPON/Li interface. Due to the change of structure and coordination number at the interface, the ionic bonds between Li and O, N are weaker, which explains the increase of ionic conductivity at the LiPON/Li interface. Previous experiments showed that element interdiffusion occurs at the LiPON/Li interface and the interface layer is formed, and found that the decrease in impedance of the interface layer can confirm that the ionic conductivity of the interface layer indeed increases. In addition, the tetrahedral structure of the interface layer will be decomposed into other smaller structures. Our computational results are consistent with the previous experimental results, which indicates the rationality and reliability of our conclusion. This feature plays a positive role in promoting the performance of LiPON electrolytes in practical battery applications.
-
Keywords:
- solid electrolyte /
- lithium phosphorus oxynitride /
- interface /
- structural evolution /
- Li ion diffusion
[1] Bates J B, Dudney N J, Gruzalski G R, Zuhr R A, Choudhury A, Luck C F 1992 Solid State Ionics 53 647
[2] Yu X, Bates J B, Jellison G E, Hart F X 1997 J. Electrochem. Soc. 144 524
 Google Scholar
Google Scholar
[3] Le Van-Jodin L, Ducroquet F, Sabary F, Chevalier I 2013 Solid State Ionics 253 151
 Google Scholar
Google Scholar
[4] Zhao S l, Wen J b, Zhu Y m, Qin Q 2008 J. Funct. Mater. 39 91
[5] Nowak S, Berkemeier F, Schmitz G 2015 J. Power Sources 275 144
 Google Scholar
Google Scholar
[6] Kim H T, Mun T, Park C, Jin S W, Park H Y 2013 J. Power Sources 244 641
 Google Scholar
Google Scholar
[7] Nisula M, Shindo Y, Koga H, Karppinen M 2015 Chem. Mater. 27 6987
 Google Scholar
Google Scholar
[8] Du Y A, Holzwarth N A W 2010 Phys. Rev. B 81
[9] Senevirathne K, Day C S, Gross M D, Lachgar A, Holzwarth N A W 2013 Solid State Ionics 233 95
 Google Scholar
Google Scholar
[10] Sagane F, Ikeda K I, Okita K, Sano H, Sakaebe H, Iriyama Y 2013 J. Power Sources 233 34
 Google Scholar
Google Scholar
[11] Schwöbel A, Hausbrand R, Jaegermann W 2015 Solid State Ionics 273 51
 Google Scholar
Google Scholar
[12] Sicolo S, Fingerle M, Hausbrand R, Albe K 2017 J. Power Sources 354 124
 Google Scholar
Google Scholar
[13] Albertus P, Babinec S, Litzelman S, Newman A 2017 Nat. Energy 3 16
[14] Bates J B, Dudney N J, Gruzalski G R, Zuhr R A, Choudhury A, Luck C F 1993 J. Power Sources 43 103
 Google Scholar
Google Scholar
[15] Suzuki N, Inaba T, Shiga T 2012 Thin Solid Films 520 1821
 Google Scholar
Google Scholar
[16] Han F, Westover A S, Yue J, Fan X, Wang F, Chi M, Leonard D N, Dudney N J, Wang H, Wang C 2019 Nat. Energy 4 187
 Google Scholar
Google Scholar
[17] Su Y, Falgenhauer J, Polity A, Leichtweiß T, Kronenberger A, Obel J, Zhou S, Schlettwein D, Janek J, Meyer B K 2015 Solid State Ionics 282 63
 Google Scholar
Google Scholar
[18] Li G, Li M, Dong L, Li X, Li D 2014 Int. J. Hydrogen Energy 39 17466
 Google Scholar
Google Scholar
[19] Le Van-Jodin L, Claudel A, Secouard C, Sabary F, Barnes J P, Martin S 2018 Electrochim. Acta 259 742
 Google Scholar
Google Scholar
[20] Hamon Y, Douard A, Sabary F, Marcel C, Vinatier P, Pecquenard B, Levasseur A 2006 Solid State Ionics 177 257
 Google Scholar
Google Scholar
[21] Tian H K, Xu B, Qi Y 2018 J. Power Sources 392 79
 Google Scholar
Google Scholar
[22] Kresse G, Furthmiiller J 1998 Phys. Rev. B 59 1758
[23] Kresse G, Furthmuller 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[24] Kresse G, Furthmuller 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[25] Blochl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[26] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[27] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[28] Martyna G J, Klein M L, Tuckerman M 1992 J. Chem. Phys. 97 2635
 Google Scholar
Google Scholar
[29] Cheng D, Wynn T A, Wang X, Wang S, Zhang M, Shimizu R, Bai S, Nguyen H, Fang C, Kim M C, Li W, Lu B, Kim S J, Meng Y S 2020 Joule 4 2484
 Google Scholar
Google Scholar
[30] Mani P D, Saraf S, Singh V, Real-Robert M, Vijayakumar A, Duranceau S J, Seal S, Coffey K R 2016 Solid State Ionics 287 48
 Google Scholar
Google Scholar
[31] Larfaillou S, Guy-Bouyssou D, le Cras F, Franger S 2016 J. Power Sources 319 139
 Google Scholar
Google Scholar
-
图 1 (a)−(c) 三种a-LiPON/Li(100)界面沿Z方向的Li原子互扩散图; (d), (e) 两种Li2PO2N(100)/Li(100)界面沿Z方向的Li原子互扩散图. 灰色虚线表示原始的界面位置. 结构图中的红色虚线框标注的是固定的末端原子, 蓝色虚线框标注的是形成的界面区域
Fig. 1. (a)−(c) Inter-diffusion of Li atoms along the Z direction of three a-LiPON/Li(100) interfaces; (d), (e) inter-diffusion of Li atoms along the Z direction of two Li2PO2N(100)/Li(100) interfaces. The gray dotted line indicates the original interface location. The red dotted frames in the structure diagram mark fixed terminal atoms, and the blue dotted frames mark the formed interface areas.
图 2 (a)−(c)三种a-LiPON/Li(100)界面和(d) a-LiPON在不同高温下Li+的均方位移(MSD); (e) a-LiPON和三种a-LiPON/Li(100)界面体系中温度与Li+扩散系数(DLi)的关系. 图中灰色虚线为300 K对应的位置
Fig. 2. (a)−(c) the three a-LiPON /Li(100) and (d) a-LiPON MSD of Li+ at different temperatures, (e) Arrhenius plot of Li diffusivity (DLi) as a function of temperature in a-LiPON and three kinds of a-LiPON/Li (100). The corresponding positions of 300 K are presented by dotted lines.
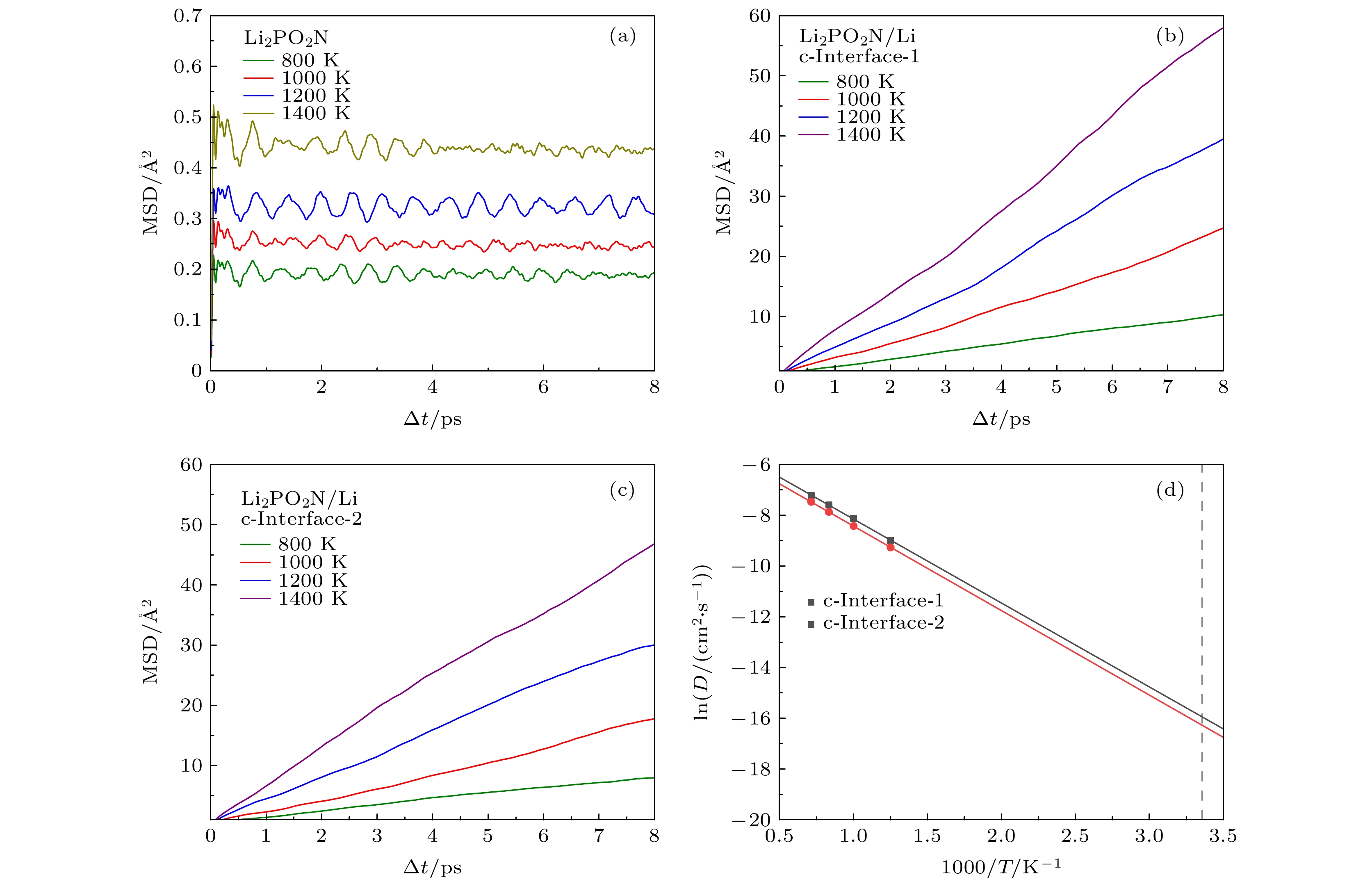
图 3 (a) Li2PO2N、(b), (c) Li2PO2N(100)/Li(100)界面在不同高温下的MSD; (d)两种Li2PO2N(100)/Li(100)界面体系中温度与Li+扩散系数(DLi)的关系. 图中灰色虚线为300 K对应的位置
Fig. 3. (a) Li2PO2N and (b), (c) Li2PO2N(100)/Li(100) MSD of Li+ at different temperatures. Arrhenius plot of Li diffusivity (DLi) as a function of temperature in two kinds of Li2PO2N(100)/Li(100). The corresponding positions of 300 K are presented by dotted lines.
表 1 a-LiPON、三种a-LiPON/Li(100)界面和两种Li2PO2N(100)/Li(100)界面的室温Li+扩散系数(DLi)与电导率(σLi)
Table 1. Li+ diffusion coefficient (DLi) and electrical conductivity (σLi) of a-LiPON, three a-LiPON/Li(100) interfaces and two Li2PO2N(100)/Li(100) interfaces at room temperature.
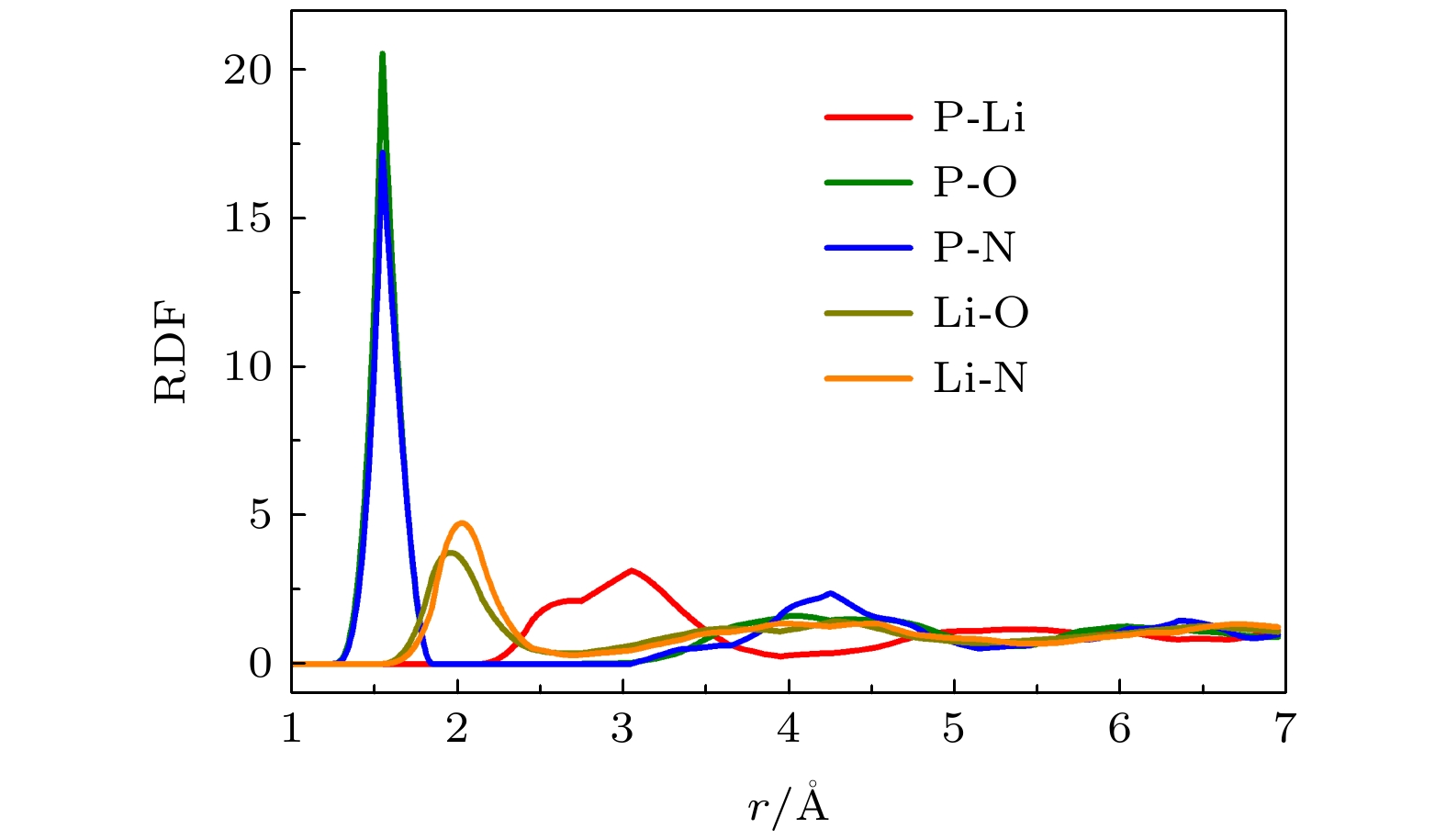
表 2 LiPON体相与LiPON/Li界面中原子间的平均配位数
Table 2. The average coordination number between atoms in LiPON bulk and LiPON/Li interface
Structure Li-O Li-N P-O P-N a-LiPON 2.49 0.50 2.43 0.51 a-LiPON/Li (100) 1.75 0.34 2.07 0.42 Li2PO2N 3 1 2 2 Li2PO2N (100)/Li (100) 1.63 0.71 1.04 1.72 -
[1] Bates J B, Dudney N J, Gruzalski G R, Zuhr R A, Choudhury A, Luck C F 1992 Solid State Ionics 53 647
[2] Yu X, Bates J B, Jellison G E, Hart F X 1997 J. Electrochem. Soc. 144 524
 Google Scholar
Google Scholar
[3] Le Van-Jodin L, Ducroquet F, Sabary F, Chevalier I 2013 Solid State Ionics 253 151
 Google Scholar
Google Scholar
[4] Zhao S l, Wen J b, Zhu Y m, Qin Q 2008 J. Funct. Mater. 39 91
[5] Nowak S, Berkemeier F, Schmitz G 2015 J. Power Sources 275 144
 Google Scholar
Google Scholar
[6] Kim H T, Mun T, Park C, Jin S W, Park H Y 2013 J. Power Sources 244 641
 Google Scholar
Google Scholar
[7] Nisula M, Shindo Y, Koga H, Karppinen M 2015 Chem. Mater. 27 6987
 Google Scholar
Google Scholar
[8] Du Y A, Holzwarth N A W 2010 Phys. Rev. B 81
[9] Senevirathne K, Day C S, Gross M D, Lachgar A, Holzwarth N A W 2013 Solid State Ionics 233 95
 Google Scholar
Google Scholar
[10] Sagane F, Ikeda K I, Okita K, Sano H, Sakaebe H, Iriyama Y 2013 J. Power Sources 233 34
 Google Scholar
Google Scholar
[11] Schwöbel A, Hausbrand R, Jaegermann W 2015 Solid State Ionics 273 51
 Google Scholar
Google Scholar
[12] Sicolo S, Fingerle M, Hausbrand R, Albe K 2017 J. Power Sources 354 124
 Google Scholar
Google Scholar
[13] Albertus P, Babinec S, Litzelman S, Newman A 2017 Nat. Energy 3 16
[14] Bates J B, Dudney N J, Gruzalski G R, Zuhr R A, Choudhury A, Luck C F 1993 J. Power Sources 43 103
 Google Scholar
Google Scholar
[15] Suzuki N, Inaba T, Shiga T 2012 Thin Solid Films 520 1821
 Google Scholar
Google Scholar
[16] Han F, Westover A S, Yue J, Fan X, Wang F, Chi M, Leonard D N, Dudney N J, Wang H, Wang C 2019 Nat. Energy 4 187
 Google Scholar
Google Scholar
[17] Su Y, Falgenhauer J, Polity A, Leichtweiß T, Kronenberger A, Obel J, Zhou S, Schlettwein D, Janek J, Meyer B K 2015 Solid State Ionics 282 63
 Google Scholar
Google Scholar
[18] Li G, Li M, Dong L, Li X, Li D 2014 Int. J. Hydrogen Energy 39 17466
 Google Scholar
Google Scholar
[19] Le Van-Jodin L, Claudel A, Secouard C, Sabary F, Barnes J P, Martin S 2018 Electrochim. Acta 259 742
 Google Scholar
Google Scholar
[20] Hamon Y, Douard A, Sabary F, Marcel C, Vinatier P, Pecquenard B, Levasseur A 2006 Solid State Ionics 177 257
 Google Scholar
Google Scholar
[21] Tian H K, Xu B, Qi Y 2018 J. Power Sources 392 79
 Google Scholar
Google Scholar
[22] Kresse G, Furthmiiller J 1998 Phys. Rev. B 59 1758
[23] Kresse G, Furthmuller 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[24] Kresse G, Furthmuller 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[25] Blochl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[26] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[27] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[28] Martyna G J, Klein M L, Tuckerman M 1992 J. Chem. Phys. 97 2635
 Google Scholar
Google Scholar
[29] Cheng D, Wynn T A, Wang X, Wang S, Zhang M, Shimizu R, Bai S, Nguyen H, Fang C, Kim M C, Li W, Lu B, Kim S J, Meng Y S 2020 Joule 4 2484
 Google Scholar
Google Scholar
[30] Mani P D, Saraf S, Singh V, Real-Robert M, Vijayakumar A, Duranceau S J, Seal S, Coffey K R 2016 Solid State Ionics 287 48
 Google Scholar
Google Scholar
[31] Larfaillou S, Guy-Bouyssou D, le Cras F, Franger S 2016 J. Power Sources 319 139
 Google Scholar
Google Scholar
计量
- 文章访问数: 16335
- PDF下载量: 380
- 被引次数: 0














 下载:
下载: