-
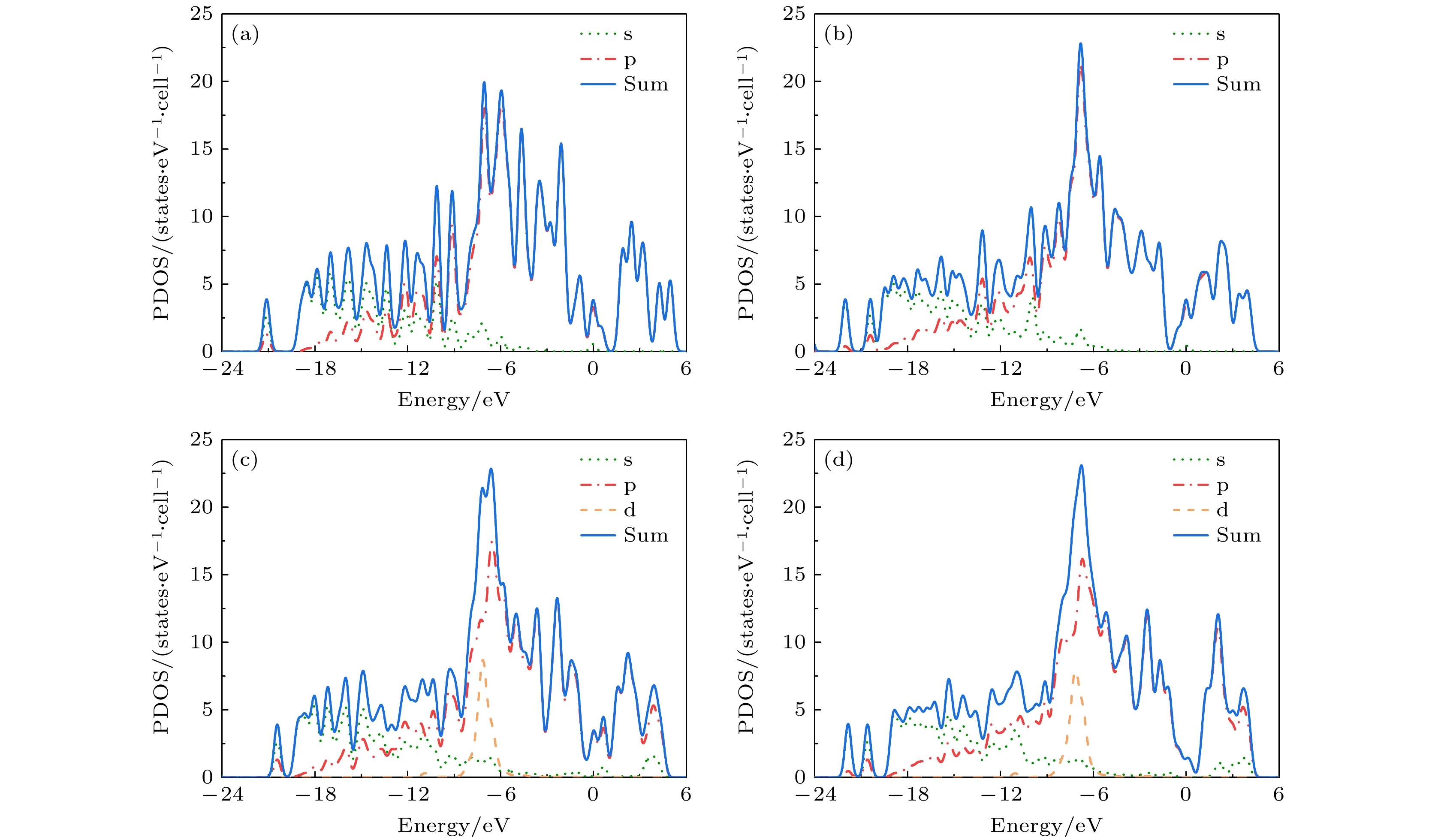
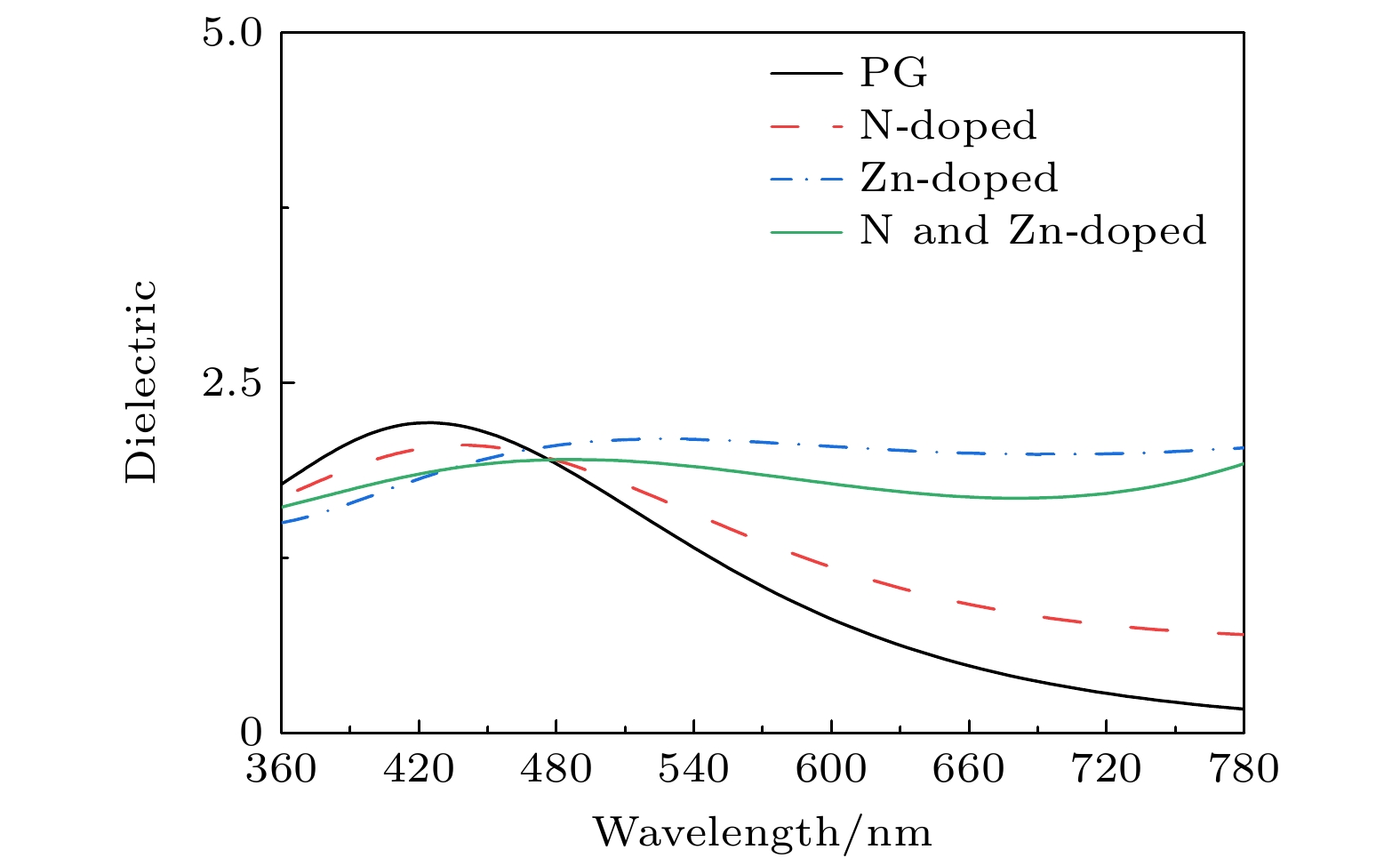
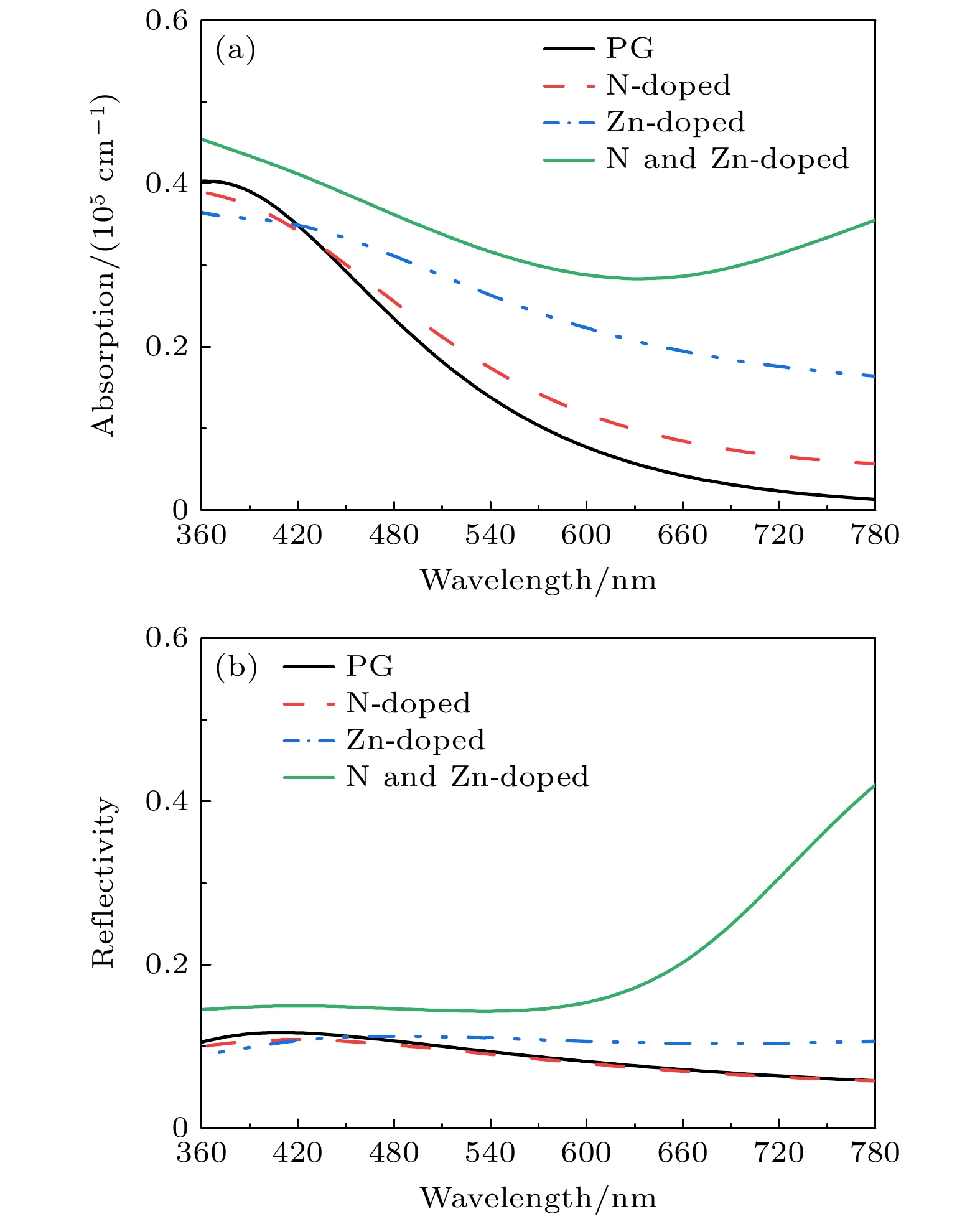
In order to study the adsorption of NO2 on pristine graphene and doped graphene (N-doped, Zn-doped, and N-Zn co-doped), we simulate the adsorption process by applying the first-principles plane-wave ultrasoft pseudopotentials of the density-functional theory in this work. The adsorption energy, Mulliken distribution, differential charge density, density of states, and optical properties of NO2 molecules adsorbed on the graphene surface are calculated. The results show that the doped graphene surface exhibits higher sensitivity to the adsorption of NO2 compared with the pristine graphene surface, and the order of adsorption energy is as follows: N-Zn co-doped surface > Zn-doped surface > N-doped surface > pristine surface. Pristine graphene surface and N-doped graphene surface have weak interactions with and physical adsorption of NO2. Zn-doped graphene surfac and N-Zn co-doped graphene surface form chemical bonds with NO2 and are chemisorbed. In the visible range, among the three doping modes, the N-Zn co-doped surface is the most effective for improving the optical properties of graphene, with the peak absorption and reflection coefficients improved by about 1.12 and 3.42 times, respectively, compared with pristine graphene. The N-Zn co-doped graphene not only enhances the interaction between the surface and NO2, but also improves the optical properties of the material, which provides theoretical support and experimental guidance for NO2 gas detection and sensing based on graphene substrate.
-
Keywords:
- NO2 /
- graphene /
- adsorption /
- first principles
[1] Cooper M J, Martin R V, Hammer M S, Levelt P F, Veefkind P, Lamsal L N, Krotkov N A, Brook J R, McLinden C A 2022 Nature 601 380
 Google Scholar
Google Scholar
[2] Gholami F, Tomas M, Gholami Z, Vakili M 2020 Sci. Total. Environ. 714 136712
 Google Scholar
Google Scholar
[3] Wang S, Liu J, Yi H, Tang X L, Yu Q J, Zhao S Z, Gao F Y, Zhou Y S, Zhong T T, Wang Y X 2022 Chemosphere 291 132917
 Google Scholar
Google Scholar
[4] Lim H, Kwon H, Kang H, Jang J E, Kwon H J 2023 Nat. Commun. 14 3114
 Google Scholar
Google Scholar
[5] Gao Z Y, Li L L, Huang H Y, Xu S P, Yan G, Zhao M L, Ding Z 2020 Appl. Surf. Sci. 527 146939
 Google Scholar
Google Scholar
[6] Geng X, Li S W, Mawella-Vithanage L, Ma T, Kilani M, Wang B W, Ma L, Hewa-Rahinduwage C C, Shafikova A, Nikolla E, Mao G Z, Brock S L, Zhang L, Luo L 2021 Nat. Commun. 12 4895
 Google Scholar
Google Scholar
[7] 熊枫, 彭志敏, 丁艳军, 杜艳君 2022 71 203302
 Google Scholar
Google Scholar
Xiong F, Peng Z M, Ding Y J, Du Y J 2022 Acta Phys. Sin. 71 203302
 Google Scholar
Google Scholar
[8] Zhao S K, Shen Y B, Zhou P F, Zhong X X, Han C, Zhao Q, Wei D Z 2019 Sens. Actuat. B-Chem. 282 917
 Google Scholar
Google Scholar
[9] Choi M S, Kim M Y, Mirzaei A, Kim S I, Baek S H, Chun D W, Jin C H, Lee K H 2021 Appl. Surf. Sci. 568 150910
 Google Scholar
Google Scholar
[10] Brophy R E, Junker B, Fakhri E A, Arnason H Ö, Svavarsson H G, Weimar U, Bârsan N, Manolescu A 2024 Sens. Actuat. B-Chem. 410 135648
 Google Scholar
Google Scholar
[11] Rani S, Kumar M, Garg P, Rani S, Kumar M, Garg P, Parmar R, Kumar A, Singh Y, Baloria V, Deshpande U, Singh V N 2022 ACS Appl. Mater. Interfaces 14 15381
 Google Scholar
Google Scholar
[12] Yu W, Sisi L, Haiyan Y, Luo J 2020 Rsc Adv. 10 15328
 Google Scholar
Google Scholar
[13] Dong Q C, Xiao M, Chu Z Y, Li G C, Zhang Y 2021 Sensors 21 3386
 Google Scholar
Google Scholar
[14] Gui Y G, Peng X, Liu K Ding Z Y 2020 Physicas E 119 113959
 Google Scholar
Google Scholar
[15] Zhu P C, Tang F, Wang S F, Cao W, Wang Q 2022 Mater. Today Commun. 33 104280
 Google Scholar
Google Scholar
[16] Li Q F, Chen W L, Liu W H, Sun M L, Xu M H, Peng H L, Wu H Y, Song S X, Li T H, Tang X H 2022 Appl. Surf. Sci. 586 152689
 Google Scholar
Google Scholar
[17] Hong H S, Ha N H, Thinh, D D, Nam N H, Huong N T, Hue N T, Hoang T V 2021 Nano Energy 87 106165
 Google Scholar
Google Scholar
[18] Zhang T, Sun H, Wang F D, Zhang W D, Tang S W, Ma J M, Gong H W, Zhang J P 2017 Appl. Surf. Sci. 425 340
 Google Scholar
Google Scholar
[19] Choudhuri I, Patra N, Mahata A, Ahuja R, Pathak B 2015 J. Phys. Chem. C 119 24827
 Google Scholar
Google Scholar
[20] Shukri M S M, Saimin M N S, Yaakob M K, Yahya M Z A, Taib M F M 2019 Appl. Surf. Sci. 494 817
 Google Scholar
Google Scholar
[21] Shamim S U D, Roy D, Alam S, Piya A A, Rahman M S, Hossain M K, Ahmed F 2022 Appl. Surf. Sci. 596 153603
 Google Scholar
Google Scholar
[22] Zhang X X, Yu L, Gui Y G, Hu W H 2016 Appl. Surf. Sci. 367 259
 Google Scholar
Google Scholar
[23] Jia X, An L 2019 Mod. Phys. Lett. B 33 1950044
 Google Scholar
Google Scholar
[24] Segall M D, Lindan P J D, Probert M J, Pickard C J, Hasnip P J, Clark S J, Payne M C 2002 J. Phys. Condens. Mat. 14 2717
 Google Scholar
Google Scholar
[25] Yang Z H, Wang Z Y, Su X P 2012 J. Cent. South Univ. 19 1796
 Google Scholar
Google Scholar
[26] Basiuk V A, Henao-Holguin L V 2014 J. Comput. Theor. Nanosci. 11 1609
 Google Scholar
Google Scholar
[27] 朱洪强, 冯庆 2014 63 133101
 Google Scholar
Google Scholar
Zhu H Q, Feng Q 2014 Acta Phys. Sin. 63 133101
 Google Scholar
Google Scholar
[28] Gao X, Zhou Q, Wang J X, Xu L N, Zeng W 2020 Appl. Surf. Sci. 517 146180
 Google Scholar
Google Scholar
[29] Zheng T, Traian D, Thomas F 2021 Phys. Chem. Chem. Phys. 23 19627
 Google Scholar
Google Scholar
[30] Ping L K, Mohamed M A, Mondal A K, Taib M F M, Samat M H, Berhanuddin D D, Menon P S, Bahru R 2021 Micromachines 12 348
 Google Scholar
Google Scholar
-
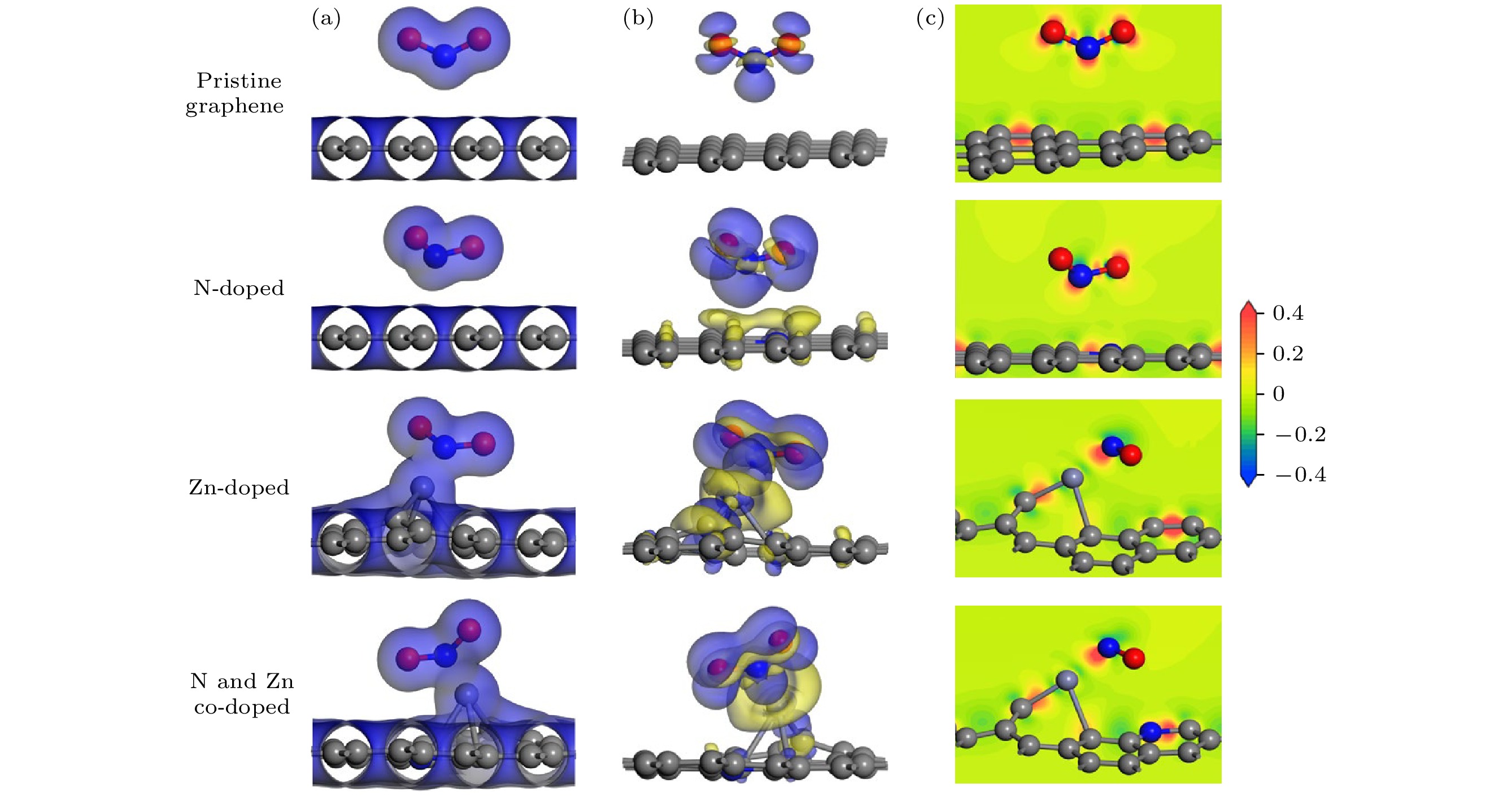
图 3 石墨烯表面吸附NO2分子 (a) 总电子密度等面图(TCD)、(b) 电荷密度差图(CDD)和(c)电子密度差图(ECD). TCD图等面设为0.02 e/Å3; CDD图等面设为0.01 e/Å3, 蓝色代表电子积累, 黄色代表电子耗尽; ECD图红色代表电荷聚集, 蓝色代表电荷耗尽
Figure 3. (a) Charge density difference (CDD), (b) total charge density (TCD), and (c) electron density difference (EDD) plots of NO2 molecules adsorbed on different graphene surfaces. The isosurfaces of TCD plots are set to 0.02 e/Å3; the isosurfaces of CDD plots are set to 0.01 e/Å3, blue represents electron accumulation and yellow represents electron depletion; in EDD plots, red represents charge accumulation and blue represents charge depletion.
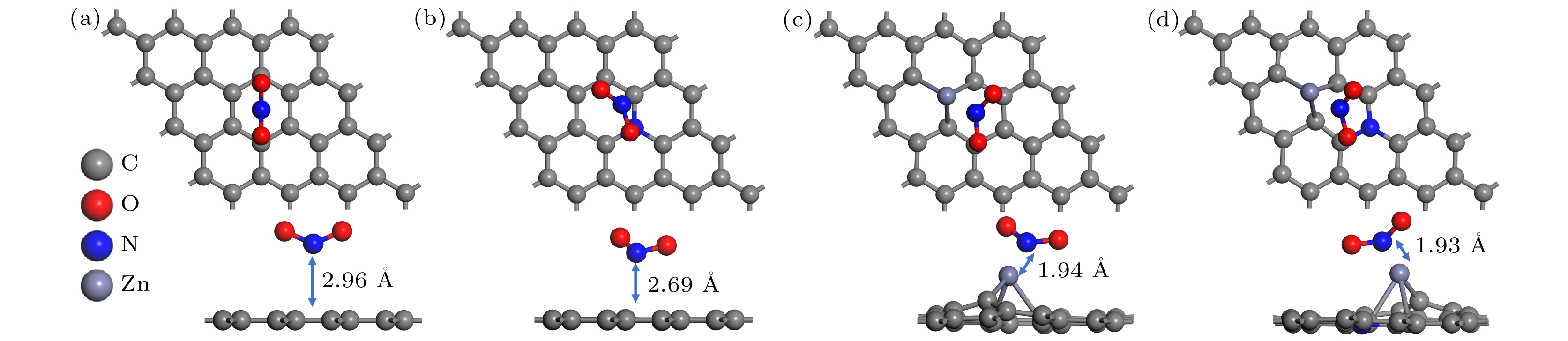
表 1 石墨烯表面吸附NO2的距离和吸附能
Table 1. The distance and adsorption energy of NO2 adsorption on graphene surface.
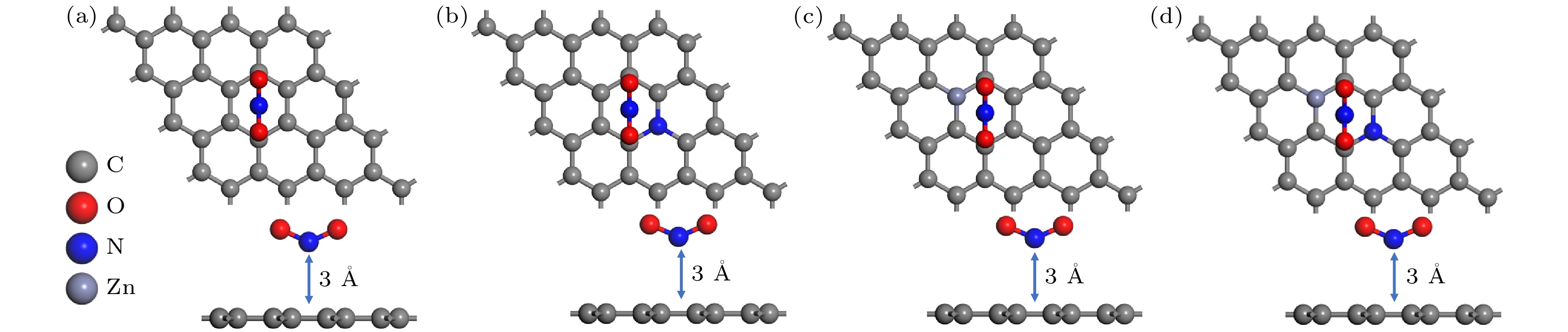
模型 初始距离/Å 优化后距离/Å 吸附能/eV 未掺杂石墨烯 3.00 2.96 –0.22 N掺杂 3.00 2.73 –0.69 Zn掺杂 3.00 1.94 –4.80 N-Zn双掺杂 3.00 1.93 –4.97 表 2 NO2分子的Mulliken的电荷分布
Table 2. Mulliken charge distribution of NO2.
模型 种类 s电子 p电子 总电子 电荷/e 分子带电荷/e 布居数 键长/Å NO2 N 1.39 3.18 4.57 0.44 0 0.68 1.23 O 1.85 4.36 6.22 –0.22 O 1.85 4.36 6.22 –0.22 未掺杂石墨烯 N 1.46 3.21 4.67 0.33 –0.25 0.63 1.24 O 1.86 4.44 6.29 –0.29 O 1.86 4.44 6.29 –0.29 N掺杂 N 1.51 3.23 4.74 0.26 –0.48 0.60 1.25 O 1.86 4.53 6.39 –0.38 O 1.86 4.51 6.36 –0.36 Zn掺杂 N 1.48 3.40 4.88 0.12 –0.59 0.67 1.26 O 1.86 4.50 6.36 –0.36 O 1.86 4.49 6.35 –0.35 N-Zn
双掺杂N 1.49 3.40 4.88 0.11 –0.60 0.67 1.27 O 1.86 4.51 6.37 –0.37 O 1.86 4.48 6.34 –0.34 -
[1] Cooper M J, Martin R V, Hammer M S, Levelt P F, Veefkind P, Lamsal L N, Krotkov N A, Brook J R, McLinden C A 2022 Nature 601 380
 Google Scholar
Google Scholar
[2] Gholami F, Tomas M, Gholami Z, Vakili M 2020 Sci. Total. Environ. 714 136712
 Google Scholar
Google Scholar
[3] Wang S, Liu J, Yi H, Tang X L, Yu Q J, Zhao S Z, Gao F Y, Zhou Y S, Zhong T T, Wang Y X 2022 Chemosphere 291 132917
 Google Scholar
Google Scholar
[4] Lim H, Kwon H, Kang H, Jang J E, Kwon H J 2023 Nat. Commun. 14 3114
 Google Scholar
Google Scholar
[5] Gao Z Y, Li L L, Huang H Y, Xu S P, Yan G, Zhao M L, Ding Z 2020 Appl. Surf. Sci. 527 146939
 Google Scholar
Google Scholar
[6] Geng X, Li S W, Mawella-Vithanage L, Ma T, Kilani M, Wang B W, Ma L, Hewa-Rahinduwage C C, Shafikova A, Nikolla E, Mao G Z, Brock S L, Zhang L, Luo L 2021 Nat. Commun. 12 4895
 Google Scholar
Google Scholar
[7] 熊枫, 彭志敏, 丁艳军, 杜艳君 2022 71 203302
 Google Scholar
Google Scholar
Xiong F, Peng Z M, Ding Y J, Du Y J 2022 Acta Phys. Sin. 71 203302
 Google Scholar
Google Scholar
[8] Zhao S K, Shen Y B, Zhou P F, Zhong X X, Han C, Zhao Q, Wei D Z 2019 Sens. Actuat. B-Chem. 282 917
 Google Scholar
Google Scholar
[9] Choi M S, Kim M Y, Mirzaei A, Kim S I, Baek S H, Chun D W, Jin C H, Lee K H 2021 Appl. Surf. Sci. 568 150910
 Google Scholar
Google Scholar
[10] Brophy R E, Junker B, Fakhri E A, Arnason H Ö, Svavarsson H G, Weimar U, Bârsan N, Manolescu A 2024 Sens. Actuat. B-Chem. 410 135648
 Google Scholar
Google Scholar
[11] Rani S, Kumar M, Garg P, Rani S, Kumar M, Garg P, Parmar R, Kumar A, Singh Y, Baloria V, Deshpande U, Singh V N 2022 ACS Appl. Mater. Interfaces 14 15381
 Google Scholar
Google Scholar
[12] Yu W, Sisi L, Haiyan Y, Luo J 2020 Rsc Adv. 10 15328
 Google Scholar
Google Scholar
[13] Dong Q C, Xiao M, Chu Z Y, Li G C, Zhang Y 2021 Sensors 21 3386
 Google Scholar
Google Scholar
[14] Gui Y G, Peng X, Liu K Ding Z Y 2020 Physicas E 119 113959
 Google Scholar
Google Scholar
[15] Zhu P C, Tang F, Wang S F, Cao W, Wang Q 2022 Mater. Today Commun. 33 104280
 Google Scholar
Google Scholar
[16] Li Q F, Chen W L, Liu W H, Sun M L, Xu M H, Peng H L, Wu H Y, Song S X, Li T H, Tang X H 2022 Appl. Surf. Sci. 586 152689
 Google Scholar
Google Scholar
[17] Hong H S, Ha N H, Thinh, D D, Nam N H, Huong N T, Hue N T, Hoang T V 2021 Nano Energy 87 106165
 Google Scholar
Google Scholar
[18] Zhang T, Sun H, Wang F D, Zhang W D, Tang S W, Ma J M, Gong H W, Zhang J P 2017 Appl. Surf. Sci. 425 340
 Google Scholar
Google Scholar
[19] Choudhuri I, Patra N, Mahata A, Ahuja R, Pathak B 2015 J. Phys. Chem. C 119 24827
 Google Scholar
Google Scholar
[20] Shukri M S M, Saimin M N S, Yaakob M K, Yahya M Z A, Taib M F M 2019 Appl. Surf. Sci. 494 817
 Google Scholar
Google Scholar
[21] Shamim S U D, Roy D, Alam S, Piya A A, Rahman M S, Hossain M K, Ahmed F 2022 Appl. Surf. Sci. 596 153603
 Google Scholar
Google Scholar
[22] Zhang X X, Yu L, Gui Y G, Hu W H 2016 Appl. Surf. Sci. 367 259
 Google Scholar
Google Scholar
[23] Jia X, An L 2019 Mod. Phys. Lett. B 33 1950044
 Google Scholar
Google Scholar
[24] Segall M D, Lindan P J D, Probert M J, Pickard C J, Hasnip P J, Clark S J, Payne M C 2002 J. Phys. Condens. Mat. 14 2717
 Google Scholar
Google Scholar
[25] Yang Z H, Wang Z Y, Su X P 2012 J. Cent. South Univ. 19 1796
 Google Scholar
Google Scholar
[26] Basiuk V A, Henao-Holguin L V 2014 J. Comput. Theor. Nanosci. 11 1609
 Google Scholar
Google Scholar
[27] 朱洪强, 冯庆 2014 63 133101
 Google Scholar
Google Scholar
Zhu H Q, Feng Q 2014 Acta Phys. Sin. 63 133101
 Google Scholar
Google Scholar
[28] Gao X, Zhou Q, Wang J X, Xu L N, Zeng W 2020 Appl. Surf. Sci. 517 146180
 Google Scholar
Google Scholar
[29] Zheng T, Traian D, Thomas F 2021 Phys. Chem. Chem. Phys. 23 19627
 Google Scholar
Google Scholar
[30] Ping L K, Mohamed M A, Mondal A K, Taib M F M, Samat M H, Berhanuddin D D, Menon P S, Bahru R 2021 Micromachines 12 348
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 3973
- PDF Downloads: 87
- Cited By: 0














 DownLoad:
DownLoad: