-
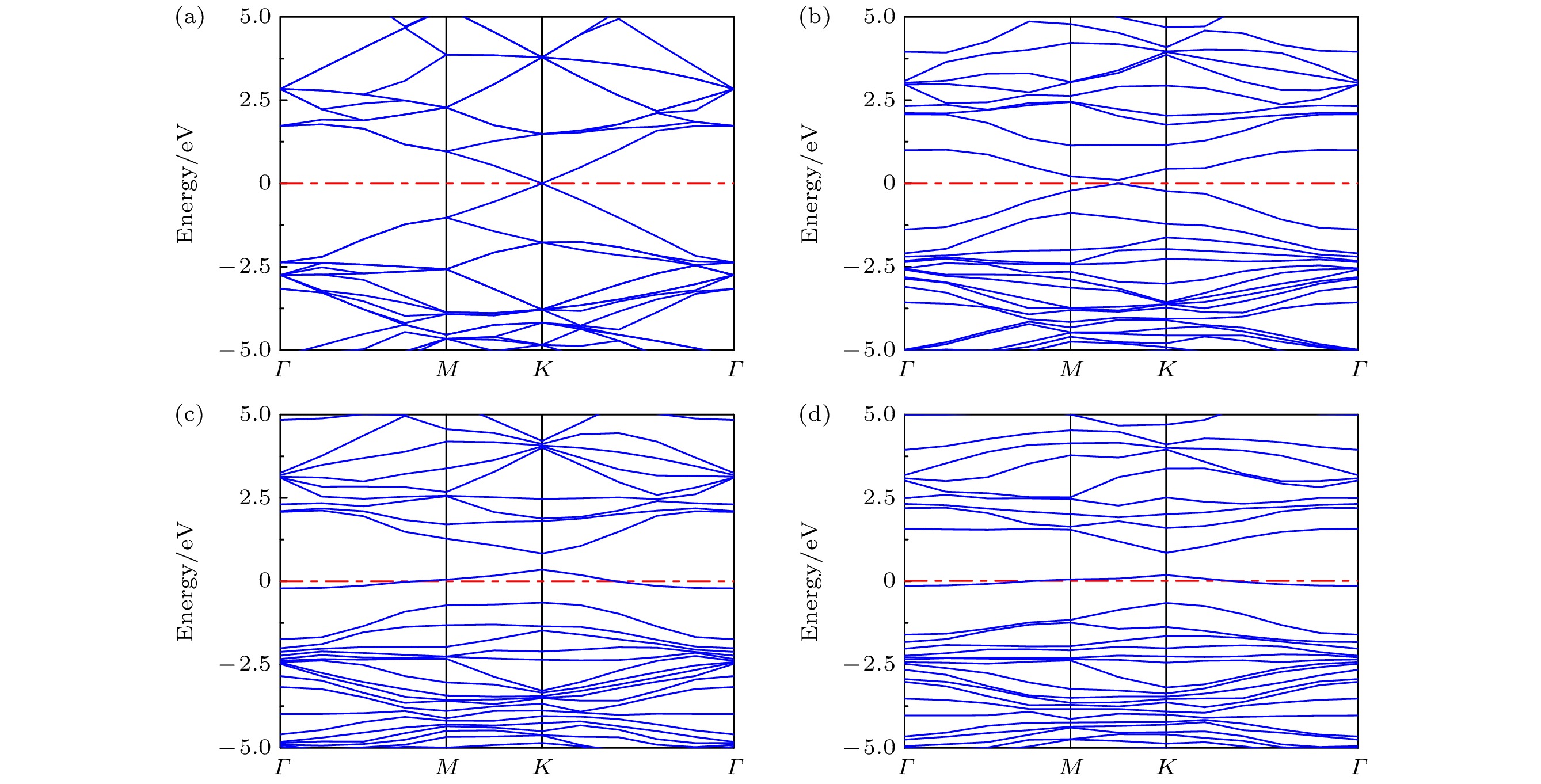
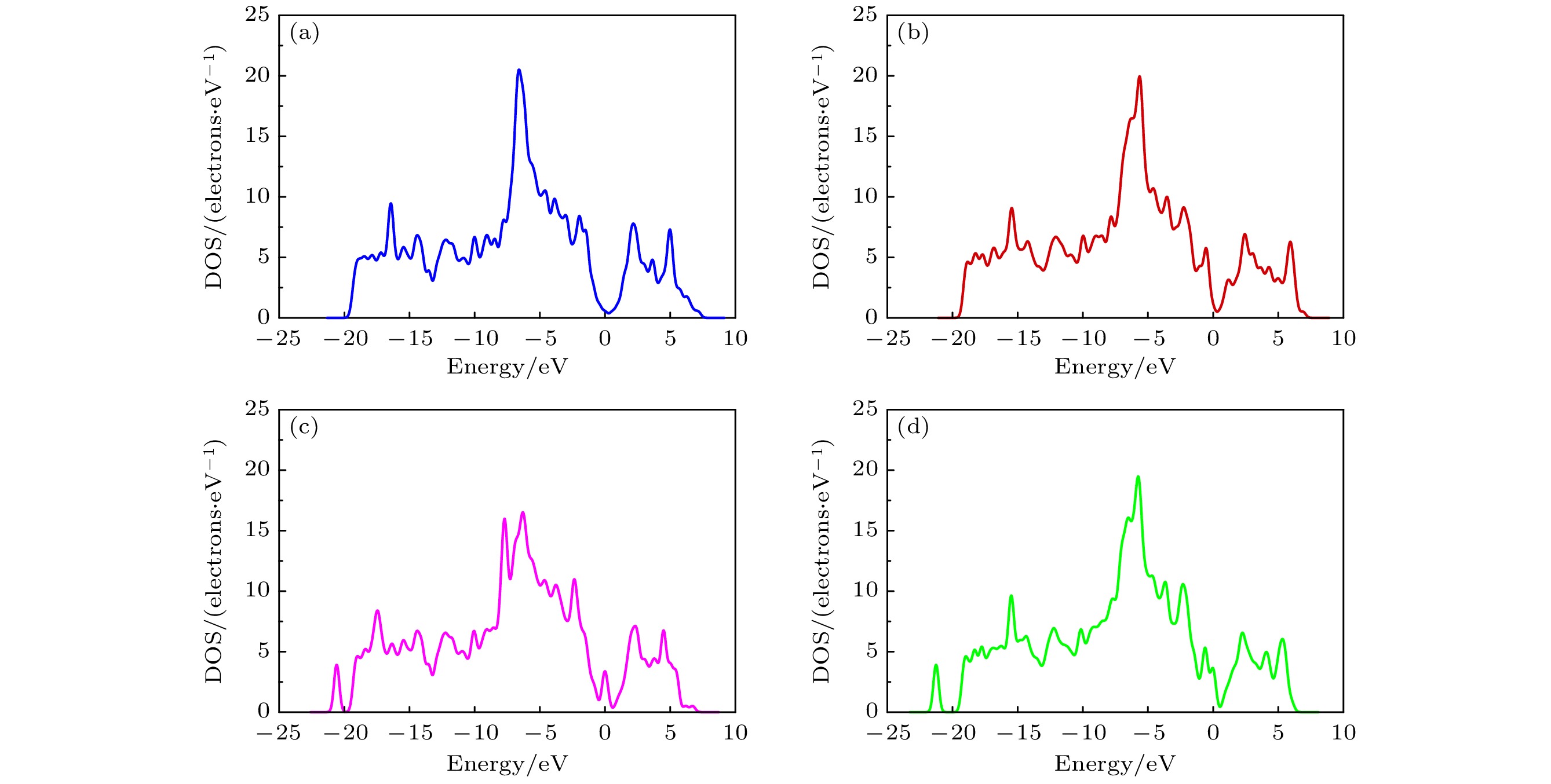
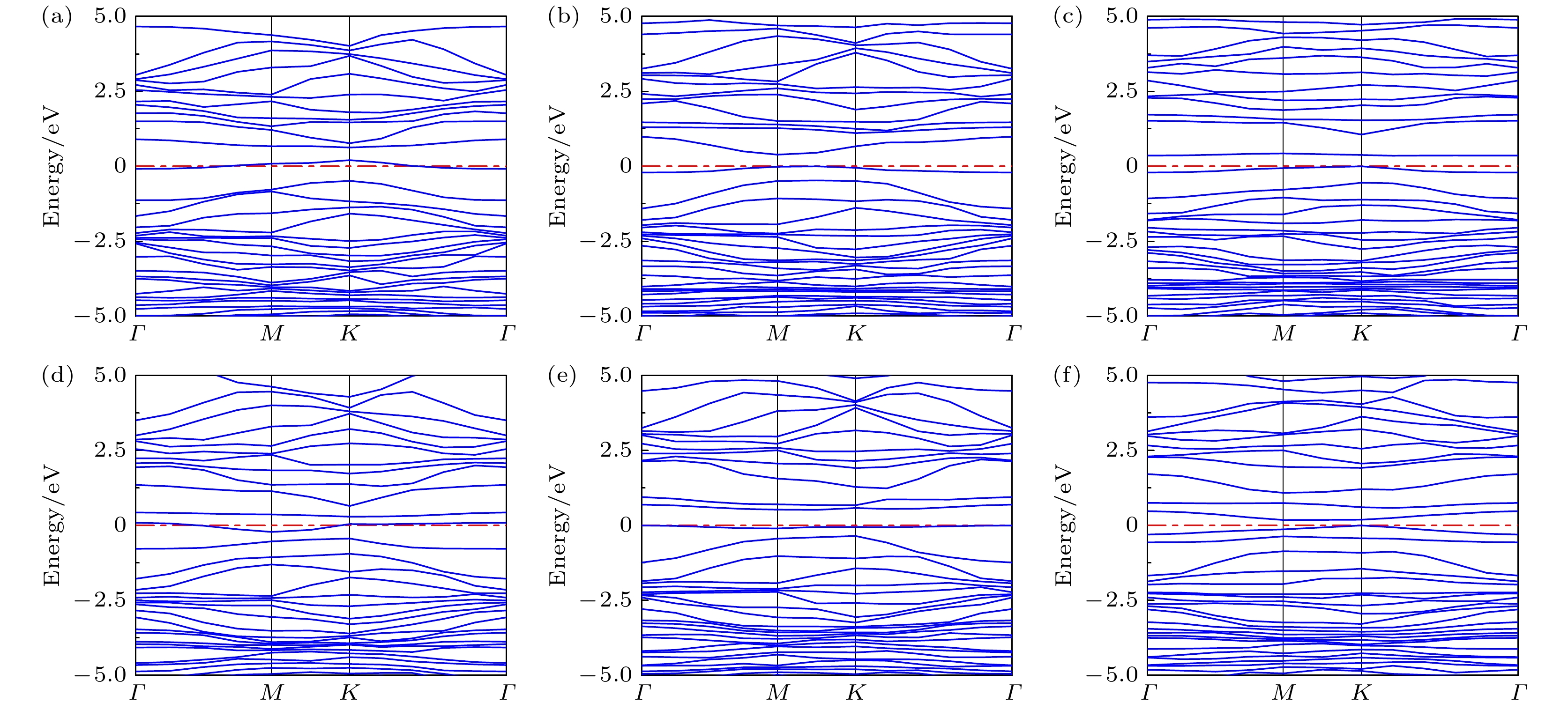
Graphene has attracted great attention due to its large specific surface area, high charge carrier mobility, and excellent electrical conductivity. However, the inherent structural integrity and zero bandgap characteristics of graphene limit its gas sensing properties. Consequently, researchers have embarked on exploring avenues such as doping graphene or using graphene oxide as a gas-sensitive material to design gas sensors that respond optimally to ammonia. This work, based on first-principle density functional theory, focuses on the field of ammonia gas sensors, investigating in detail the adsorption characteristics of ammonia molecules on graphene oxide (GO) and graphene oxide doped with Ag and Cu (AgGO, CuGO). By calculating parameters including charge distribution, density of states, band structures, and adsorption energy, this work delves into the influences of diverse oxygen-containing groups and metal doping on the gas sensing properties of graphene oxide. The research results show that there is a substantial charge density overlap between the density of states of hydroxyl groups in graphene oxide and NH3 molecules, indicating a clear tendency towards chemical adsorption. It is particularly noteworthy that after NH3 adsorption, the graphene oxide containing hydroxyl shows the highest charge transfer (0.078e) and adsorption energy (0.60 eV), which indicates that the adsorption efficacy of NH3 is higher, followed by carboxyl groups and epoxy groups, which mainly participate in physical adsorption. Furthermore, this work delves into the influence of metal doping on graphene oxide, demonstrating that the adsorption capability of doped graphene oxide hinges upon the synergistic influence of oxygen-containing groups and metal atoms, with Ag-doped graphene oxide showing a several-fold increase in adsorption energy. Through the analysis of density of states, it is found that Ag atoms resonate with s, p, and d orbitals of the N atom in NH3, proving the formation of a chemical bond between Ag atom and N atom. Moreover, a comparative analysis shows that Cu-doped graphene oxide (CuGO) has an increased charge transfer of about 0.020e and slightly higher adsorption energy than Ag-doped graphene oxide (AgGO) when adsorbing NH3. Intriguingly, under the same doping concentration, CuGO exhibits superior adsorption performance to NH3. It is worth noting that in graphene oxide doped with Ag or Cu, the adsorption mechanism of carboxyl and epoxy groups transforms from physical adsorption into chemical adsorption, while the hydroxyl groups maintain consistent chemical adsorption properties before and after doping. This indicates that doping with Ag or Cu atoms can significantly enhance the adsorption capability of graphene oxide to NH3.
-
Keywords:
- doping /
- graphene oxide /
- first principle /
- adsorption
[1] Yu Z, Wang B, Li Y, et al. 2017 RSC Adv. 7 22599
 Google Scholar
Google Scholar
[2] Hibbard T, Killard A J 2011 Crit. Rev. Anal. Chem. 41 21
 Google Scholar
Google Scholar
[3] Risby T H, Solga S F 2006 Appl. Phys. B 85 421
 Google Scholar
Google Scholar
[4] Ishpal I, Kaur A 2013 J. Appl. Phys. 113 938
 Google Scholar
Google Scholar
[5] Wang J, Yang P, Wei X 2015 ACS Appl. Mater. Interfaces 7 3816
 Google Scholar
Google Scholar
[6] Li Y, Li H, Zhao F L 2024 Phys. Status Solidi RRL 18 2400015
 Google Scholar
Google Scholar
[7] Mirzaei M, Roohollahi H, Bagheri H 2024 Progresses in Ammonia: Science, Technology and Membranes (1st Ed.) (Amsterdam: Elsevier) pp69–94
[8] Kwak D, Lei Y, Maric R 2019 Talanta 204 713
 Google Scholar
Google Scholar
[9] Zhu Y, Murali S, Cai W, Li X, Suk J W, Potts J R 2010 Adv. Mater. 22 3906
 Google Scholar
Google Scholar
[10] Wu J, Lin H, Moss D J 2023 Nat. Rev. Chem. 7 162
 Google Scholar
Google Scholar
[11] Bi J, Du Z, Sun J 2023 Adv. Mater. 35 2210734
 Google Scholar
Google Scholar
[12] Schedin F, Geim A K, Morozov S V 2007 Nat. Mater. 6 652
 Google Scholar
Google Scholar
[13] Peng Y, Li J 2013 Front. Environ. Sci. Eng. 7 403
 Google Scholar
Google Scholar
[14] Luo H, Zhang L, Xu S 2021 Appl. Surf. Sci. 537 147542
 Google Scholar
Google Scholar
[15] Park M S, Kim K H, Kim M J 2016 Colloid Surface A 490 104
 Google Scholar
Google Scholar
[16] Raza W, Krupanidhi S B 2018 ACS Appl. Mater. Interfaces 10 25285
 Google Scholar
Google Scholar
[17] Tran Q T, Hoa H T M, Yoo D H 2014 Sens. Actuators, B 194 45
 Google Scholar
Google Scholar
[18] Karaduman I, Er E, Çelikkan H 2017 J. Alloys Compd. 722 569
 Google Scholar
Google Scholar
[19] Zhang L, Tan Q, Kou H 2019 Sci. Rep. 9 9942
 Google Scholar
Google Scholar
[20] Saleh A M, Albiss B A 2024 ChemistrySelect 9 e202401500
 Google Scholar
Google Scholar
[21] Li Q, Liu Y, Chen D 2021 Chemosensors 9 227
 Google Scholar
Google Scholar
[22] Rawat S, Bamola P, Negi S 2023 ACS Appl. Nano Mater. 7 746
 Google Scholar
Google Scholar
[23] Sinnott S B 2013 J. Vac. Sci. Technol. , A 31 050812
 Google Scholar
Google Scholar
[24] Delley B 1990 J. Chem. Phys. 92 508
 Google Scholar
Google Scholar
[25] Delley B 2000 J. Chem. Phys. 113 7756
 Google Scholar
Google Scholar
[26] Lerf A, He H, Forster M 1998 J. Phys. Chem. B 102 4477
 Google Scholar
Google Scholar
[27] Szabó T, Berkesi O, Forgó P 2006 Chem. Mater. 18 2740
 Google Scholar
Google Scholar
[28] Liu H, Liu Y, Zhu D 2011 J. Mater. Chem. 21 3335
 Google Scholar
Google Scholar
[29] Guo B, Fang L, Zhang B 2011 Insciences J. 1 80
 Google Scholar
Google Scholar
[30] Geim A K, Novoselov K S 2007 Nat. Mater. 6 183
 Google Scholar
Google Scholar
[31] Wei D, Zhao C, Khan A 2019 Chem. Eng. J. 375 121964
 Google Scholar
Google Scholar
[32] Yan J A, Chou M Y 2010 Phys. Rev. B 82 125403
 Google Scholar
Google Scholar
[33] 王晓, 黄生祥, 罗衡, 邓联文, 吴昊, 徐运超, 贺军, 贺龙辉 2019 68 187301
 Google Scholar
Google Scholar
Wang X, Huang S X, Luo H, Deng L W, Wu H, Xu Y C, He J, He L H 2019 Acta. Phys. Sin. 68 187301
 Google Scholar
Google Scholar
[34] Giovannetti G, Khomyakov P A, Brocks G 2008 Phys. Rev. Lett. 101 026803
 Google Scholar
Google Scholar
-
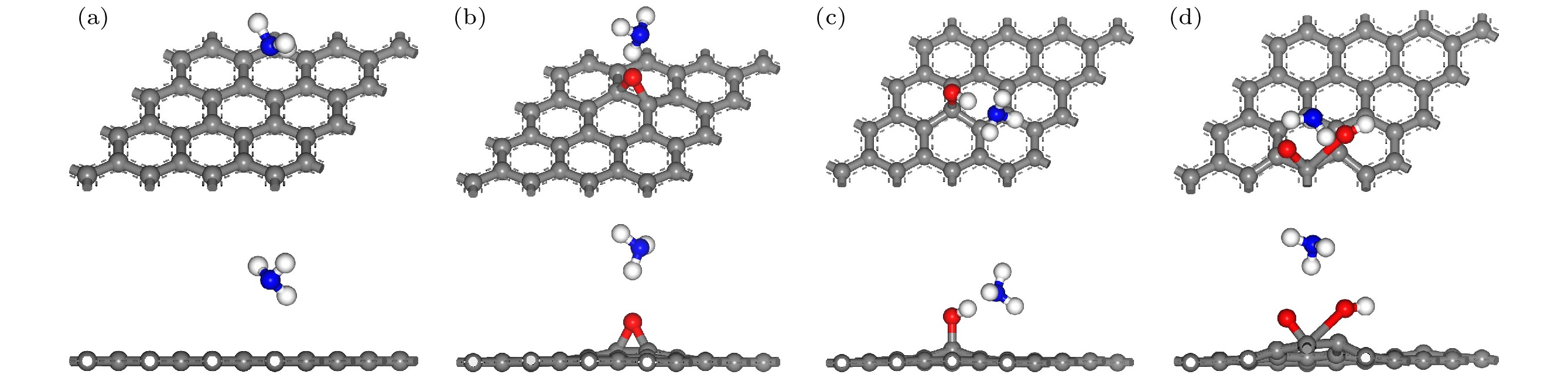
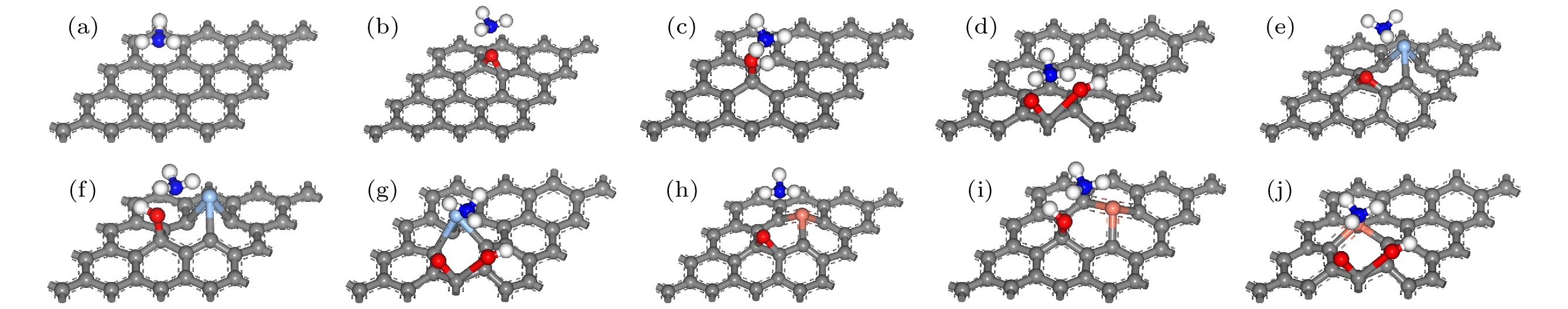
图 1 优化前相关建模图 (a) G-NH3; (b) GO-O-NH3; (c) GO-OH-NH3; (d) GO-COOH-NH3; (e) AgGO-O-NH3; (f) AgGO-OH-NH3; (g) AgGO-COOH-NH3; (h) CuGO-O-NH3; (i) CuGO-OH-NH3; (j) CuGO-COOH-NH3
Figure 1. Related model diagrams before optimization: (a) G-NH3; (b) GO-O-NH3; (c) GO-OH-NH3; (d) GO-COOH-NH3; (e) AgGO-O-NH3; (f) AgGO-OH-NH3; (g) AgGO-COOH-NH3; (h) CuGO-O-NH3; (i) CuGO-OH-NH3; (j) CuGO-COOH-NH3.
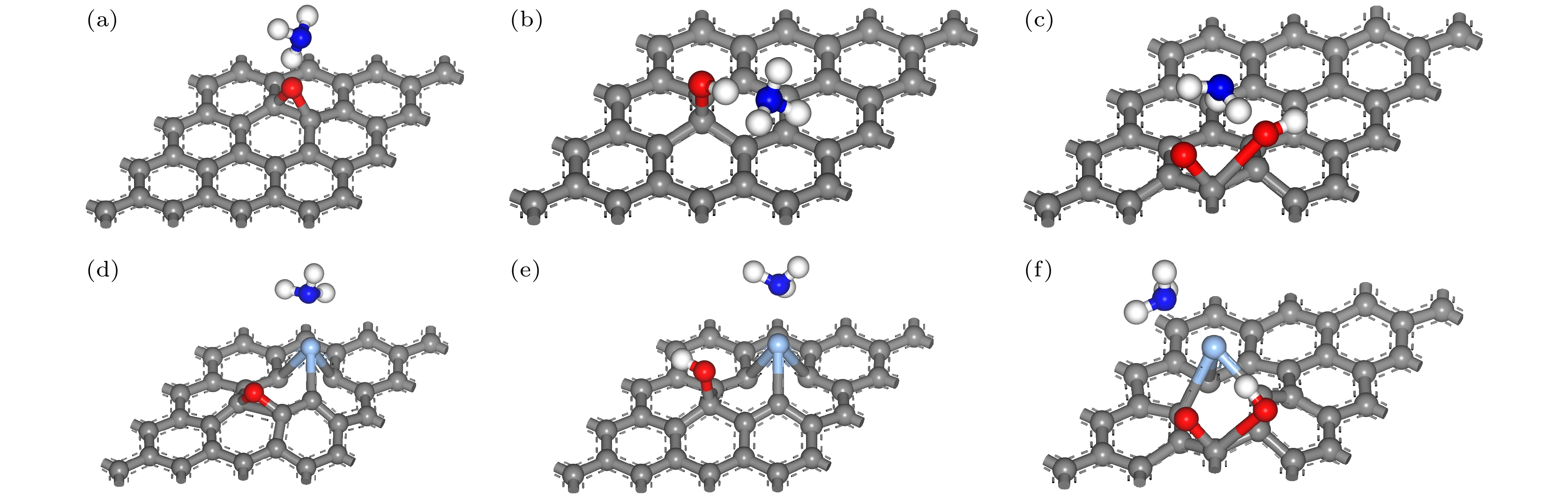
图 7 优化后含不同含氧基团的AgGO和GO吸附NH3的俯视图 (a) GO-O-NH3; (b) GO-OH-NH3; (c) GO-COOH-NH3; (d) AgGO-O-NH3; (e) AgGO-OH-NH3; (f) AgGO-COOH-NH3
Figure 7. Top views of optimized AgGO and GO with different oxygen-containing groups adsorbing NH3: (a) GO-O-NH3; (b) GO-OH-NH3; (c) GO-COOH-NH3; (d) AgGO-O-NH3; (e) AgGO-OH-NH3; (f) AgGO-COOH-NH3.
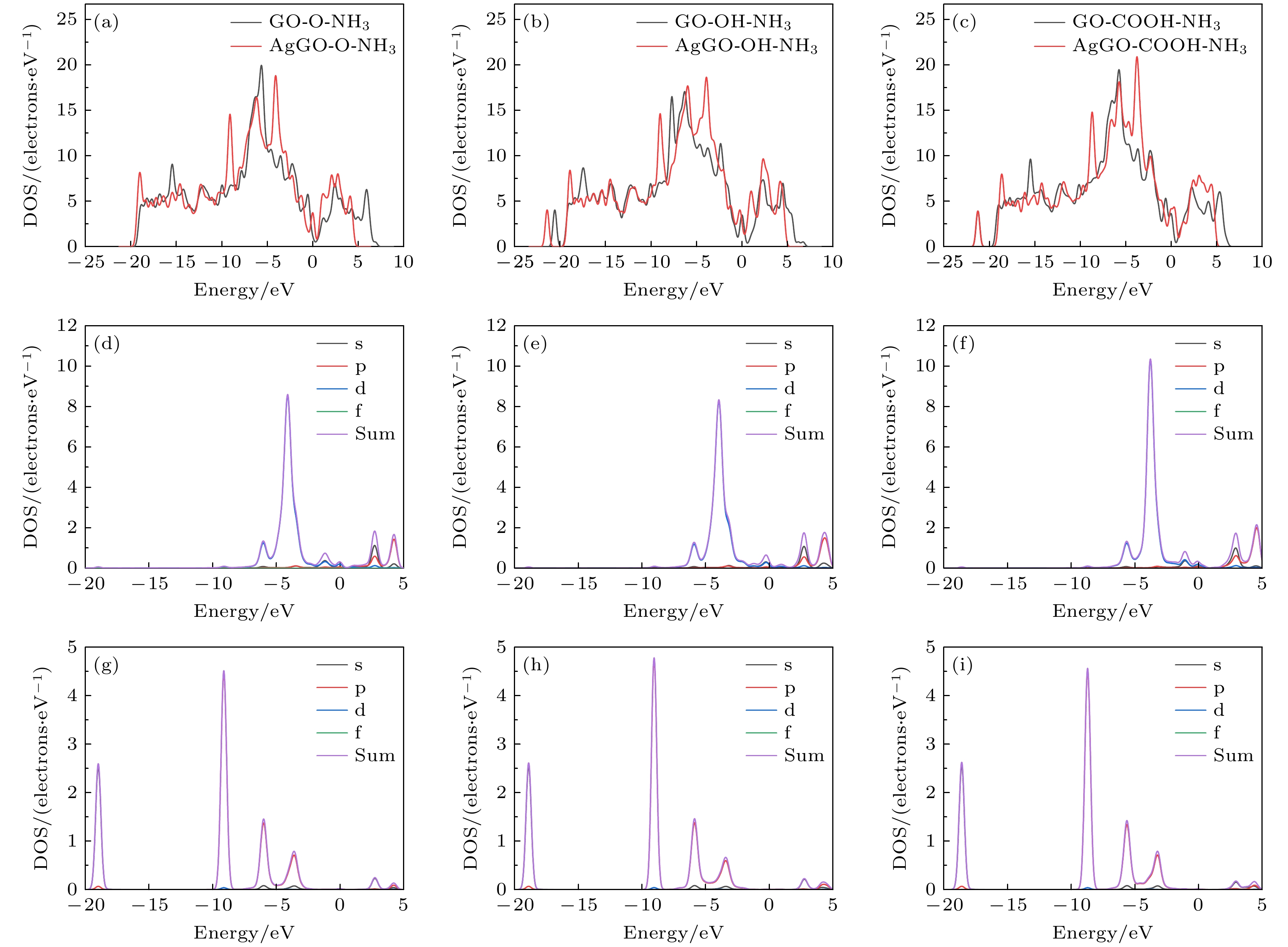
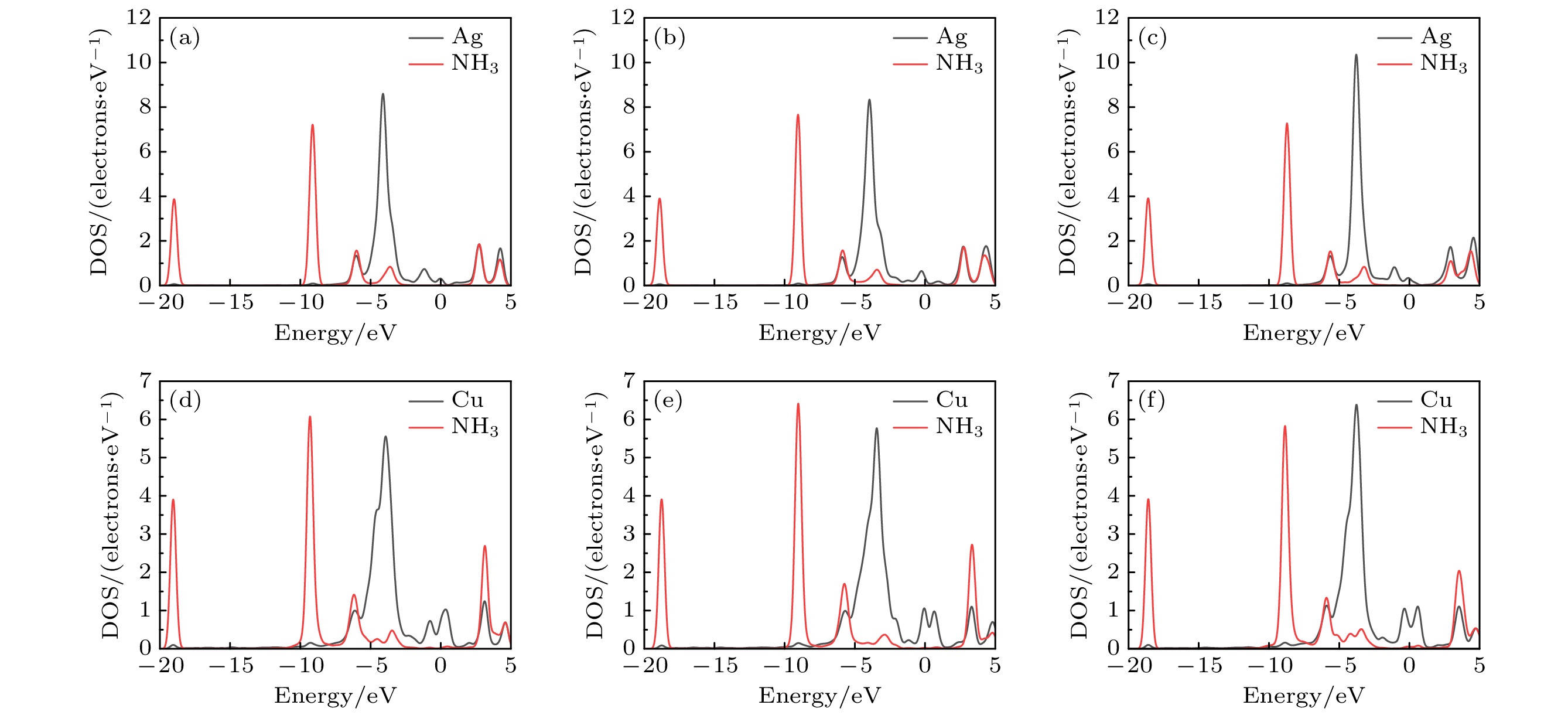
图 8 GO与AgGO的DOS和PDOS (a) GO-O-NH3和AgGO-O-NH3的DOS; (b) GO-OH-NH3和AgGO-OH-NH3的DOS; (c) GO-COOH-NH3和AgGO-COOH-NH3的DOS; (d) AgGO-O-NH3中Ag的DOS和PDOS; (e) AgGO-OH-NH3中Ag的DOS和PDOS; (f) AgGO-COOH-NH3中Ag的DOS和PDOS; (g) AgGO-O-NH3中N的DOS和PDOS; (h) AgGO-OH-NH3中N的DOS和PDOS; (i) AgGO-COOH-NH3中N的DOS和PDOS
Figure 8. DOS and PDOS of GO and AgGO: (a) DOS of GO-O-NH3 and AgGO-O-NH3; (b) DOS of GO-OH-NH3 and AgGO-OH-NH3; (c) DOS of GO-COOH-NH3 and AgGO-COOH-NH3; (d) DOS and PDOS of Ag in AgGO-O-NH3; (e) DOS and PDOS of Ag in AgGO-OH-NH3; (f) DOS and PDOS of Ag in AgGO-COOH-NH3; (g) DOS and PDOS of N in AgGO-O-NH3; (h) DOS and PDOS of N in AgGO-OH-NH3; (i) DOS and PDOS of N in AgGO-COOH-NH3.
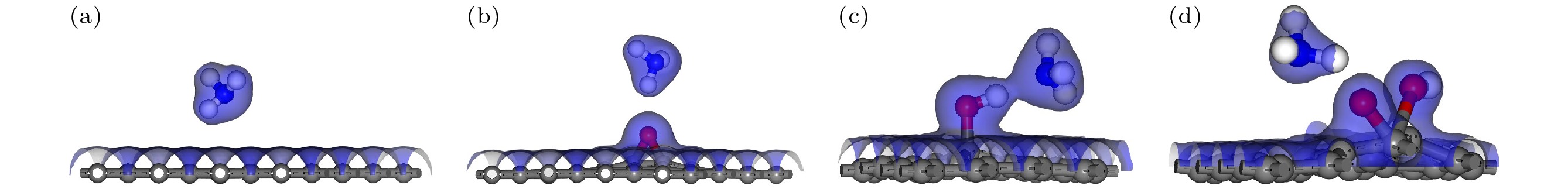
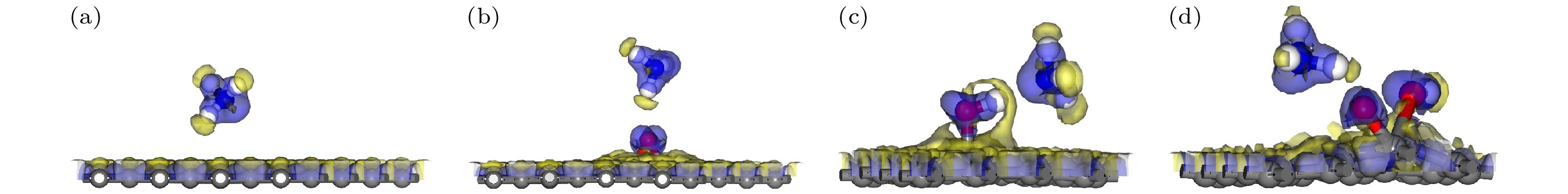
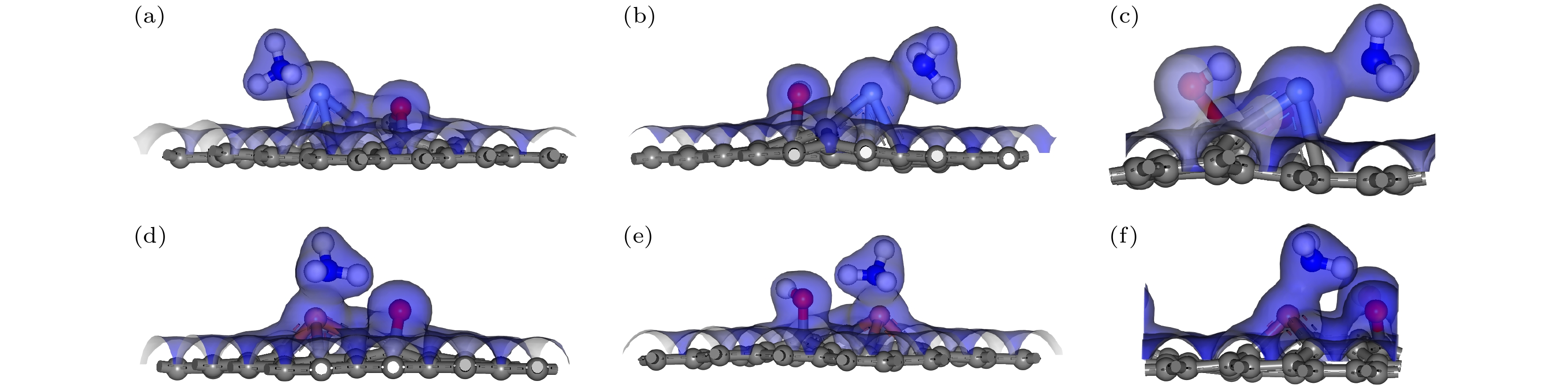
图 9 含有不同含氧基团的AgGO和CuGO吸附NH3的电荷总密度图(等值面为0.2) (a) AgGO-O-NH3; (b)AgGO-OH-NH3; (c) AgGO-COOH-NH3; (d) CuGO-O-NH3; (e) CuGO-OH-NH3; (f) CuGO-COOH-NH3.
Figure 9. Total charge density of AgGO and GO with different oxygen-containing groups adsorbing NH3 (The isosurface value is 0.2): (a) AgGO-O-NH3; (b) AgGO-OH-NH3; (c) AgGO-COOH-NH3; (d) CuGO-O-NH3; (e) CuGO-OH-NH3; (f) CuGO-COOH-NH3.
图 11 AgGO与CuGO的DOS (a) AgGO-O-NH3中Ag和NH3的DOS; (b) AgGO-OH-NH3中Ag和NH3的DOS; (c) AgGO-COOH-NH3中Ag和NH3的DOS; (d) CuGO-O-NH3中Cu和NH3的DOS; (e) CuGO-OH-NH3中Cu和NH3的DOS; (f) CuGO-COOH-NH3中Cu和NH3的DOS
Figure 11. DOS of AgGO and CuGO: (a) DOS of Ag and NH3 in AgGO-O-NH3; (b) DOS of Ag and NH3 in AgGO-OH-NH3; (c) DOS of Ag and NH3 in AgGO-COOH-NH3; (d) DOS of Cu and NH3 in CuGO-O-NH3; (e) DOS of Cu and NH3 in CuGO-OH-NH3; (f) DOS of Cu and NH3 in CuGO-COOH-NH3.
表 1 G和含有不同含氧基团的GO吸附NH3的几何参数. C1, C2和C3为与含氧基团相连的最近的三个碳原子; D表示吸附距离
Table 1. Structural parameters of G and GO with different oxygen-containing groups adsorbing NH3: C1, C2, and C3 are the three nearest carbon atoms connected to the oxygen-containing group; D represents the adsorption distance.
Species Bond angles/(°) D/Å C1-C2 C2-C3 C3-C1 G-NH3 120.06 119.98 119.94 3.24 (N—C) GO-O-NH3 118.75 118.19 118.17 2.28 (H—O) GO-OH-NH3 112.95 112.72 112.77 1.80 (N—H) GO-COOH-NH3 119.57 — — 2.44 (H—O) 表 2 G和含有不同含氧基团的GO吸附NH3的吸附能和电荷布居
Table 2. Adsorption energy and Mulliken charge of G and GO with different oxygen-containing groups adsorbing NH3.
System Mulliken charge/e Eads/eV C1 C2 C3 NH3 G-NH3 0.013 0.004 –0.039 0.022 –0.15 GO-O-NH3 0.112 0.024 0.026 0.005 –0.11 GO-OH-NH3 0.004 –0.035 0.006 0.078 –0.60 GO-COOH-NH3 0.125 –0.016 — 0.036 –0.17 表 3 带有不同含氧基团的AgGO和GO吸附NH3的吸附距离D、电荷转移Q和吸附能Eads
Table 3. Adsorption distance D, charge transfer Q, and adsorption energy Eads of AgGO and GO with different oxygen-containing groups adsorbing NH3.
Species D/Å Q/e Eads/eV NH3 Ag GO-O-NH3 2.28 (H-O) 0.005 — –0.11 AgGO-O-NH3 2.29 (N-Ag) 0.171 –0.038 –1.25 GO-OH-NH3 1.80 (N-H) 0.078 — –0.60 AgGO-OH-NH3 2.29 (N-Ag) 0.160 –0.033 –1.26 GO-COOH-NH3 2.44 (H-O) 0.036 — –0.17 AgGO-COOH-NH3 2.29 (N-Ag) 0.170 –0.082 –1.39 表 4 带有不同含氧基团AgGO和CuGO吸附NH3的电荷转移Q和吸附能Eads
Table 4. Charge transfer Q and adsorption energy Eads of AgGO and GO with different oxygen-containing groups adsorbing NH3.
Species Q/e Eads/eV NH3 Ag/Cu AgGO-O-NH3 0.171 –0.038 –1.25 CuGO-O-NH3 0.192 0.020 –1.39 AgGO-OH-NH3 0.160 –0.033 –1.26 CuGO-OH-NH3 0.180 –0.001 –1.43 AgGO-COOH-NH3 0.170 –0.082 –1.39 CuGO-COOH-NH3 0.192 0.034 –1.34 -
[1] Yu Z, Wang B, Li Y, et al. 2017 RSC Adv. 7 22599
 Google Scholar
Google Scholar
[2] Hibbard T, Killard A J 2011 Crit. Rev. Anal. Chem. 41 21
 Google Scholar
Google Scholar
[3] Risby T H, Solga S F 2006 Appl. Phys. B 85 421
 Google Scholar
Google Scholar
[4] Ishpal I, Kaur A 2013 J. Appl. Phys. 113 938
 Google Scholar
Google Scholar
[5] Wang J, Yang P, Wei X 2015 ACS Appl. Mater. Interfaces 7 3816
 Google Scholar
Google Scholar
[6] Li Y, Li H, Zhao F L 2024 Phys. Status Solidi RRL 18 2400015
 Google Scholar
Google Scholar
[7] Mirzaei M, Roohollahi H, Bagheri H 2024 Progresses in Ammonia: Science, Technology and Membranes (1st Ed.) (Amsterdam: Elsevier) pp69–94
[8] Kwak D, Lei Y, Maric R 2019 Talanta 204 713
 Google Scholar
Google Scholar
[9] Zhu Y, Murali S, Cai W, Li X, Suk J W, Potts J R 2010 Adv. Mater. 22 3906
 Google Scholar
Google Scholar
[10] Wu J, Lin H, Moss D J 2023 Nat. Rev. Chem. 7 162
 Google Scholar
Google Scholar
[11] Bi J, Du Z, Sun J 2023 Adv. Mater. 35 2210734
 Google Scholar
Google Scholar
[12] Schedin F, Geim A K, Morozov S V 2007 Nat. Mater. 6 652
 Google Scholar
Google Scholar
[13] Peng Y, Li J 2013 Front. Environ. Sci. Eng. 7 403
 Google Scholar
Google Scholar
[14] Luo H, Zhang L, Xu S 2021 Appl. Surf. Sci. 537 147542
 Google Scholar
Google Scholar
[15] Park M S, Kim K H, Kim M J 2016 Colloid Surface A 490 104
 Google Scholar
Google Scholar
[16] Raza W, Krupanidhi S B 2018 ACS Appl. Mater. Interfaces 10 25285
 Google Scholar
Google Scholar
[17] Tran Q T, Hoa H T M, Yoo D H 2014 Sens. Actuators, B 194 45
 Google Scholar
Google Scholar
[18] Karaduman I, Er E, Çelikkan H 2017 J. Alloys Compd. 722 569
 Google Scholar
Google Scholar
[19] Zhang L, Tan Q, Kou H 2019 Sci. Rep. 9 9942
 Google Scholar
Google Scholar
[20] Saleh A M, Albiss B A 2024 ChemistrySelect 9 e202401500
 Google Scholar
Google Scholar
[21] Li Q, Liu Y, Chen D 2021 Chemosensors 9 227
 Google Scholar
Google Scholar
[22] Rawat S, Bamola P, Negi S 2023 ACS Appl. Nano Mater. 7 746
 Google Scholar
Google Scholar
[23] Sinnott S B 2013 J. Vac. Sci. Technol. , A 31 050812
 Google Scholar
Google Scholar
[24] Delley B 1990 J. Chem. Phys. 92 508
 Google Scholar
Google Scholar
[25] Delley B 2000 J. Chem. Phys. 113 7756
 Google Scholar
Google Scholar
[26] Lerf A, He H, Forster M 1998 J. Phys. Chem. B 102 4477
 Google Scholar
Google Scholar
[27] Szabó T, Berkesi O, Forgó P 2006 Chem. Mater. 18 2740
 Google Scholar
Google Scholar
[28] Liu H, Liu Y, Zhu D 2011 J. Mater. Chem. 21 3335
 Google Scholar
Google Scholar
[29] Guo B, Fang L, Zhang B 2011 Insciences J. 1 80
 Google Scholar
Google Scholar
[30] Geim A K, Novoselov K S 2007 Nat. Mater. 6 183
 Google Scholar
Google Scholar
[31] Wei D, Zhao C, Khan A 2019 Chem. Eng. J. 375 121964
 Google Scholar
Google Scholar
[32] Yan J A, Chou M Y 2010 Phys. Rev. B 82 125403
 Google Scholar
Google Scholar
[33] 王晓, 黄生祥, 罗衡, 邓联文, 吴昊, 徐运超, 贺军, 贺龙辉 2019 68 187301
 Google Scholar
Google Scholar
Wang X, Huang S X, Luo H, Deng L W, Wu H, Xu Y C, He J, He L H 2019 Acta. Phys. Sin. 68 187301
 Google Scholar
Google Scholar
[34] Giovannetti G, Khomyakov P A, Brocks G 2008 Phys. Rev. Lett. 101 026803
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 2830
- PDF Downloads: 76
- Cited By: 0














 DownLoad:
DownLoad: