-
In recent decades, significant progress has been made in the precise theoretical investigation of gas-phase chemical reactions. Presently, a major challenge in the field of quantum dynamics is to develop the precise methodologies for studying chemical reactions involving more than four atoms. As a typical multi-atomic reaction system, the F+CH4 reaction and its isotopic substitution reactions have attracted widespread attention from both experimental and theoretical perspectives in recent years. Experimental studies on the reaction of F+CHD3 have revealed that the stretching vibration excitation of the C—H bond inhibits the bond dissociation, favoring the formation of DF+CHD2 product channels. In this study, we use a seven-dimensional quantum time-dependent wave packet method to investigate the dynamics of the F+CHD3 reaction in both the reactant vibrational ground state and the first stretching excited state of the C—H bond. In this work, the reaction probabilities under different vibrational conditions are analyzed, showing that when the collision energy is below 0.06 eV, the reaction probability curves exhibit numerous fast-oscillating peaks, supporting the experimentally suggested phenomena of dynamic resonance. In a collision energy range from 0.06 eV to 0.3 eV, the reaction probability for the HF product channel in the vibrational excited state is lower than that in the ground state, which is consistent with experimental observation. Through the analysis of the time-independent wave functions of product channels under low-energy collision conditions, it is found that for reactions involving vibrational ground states, the HF products in the product asymptotic region and the reaction transition state region are in the v' = 2 excited state and v' = 3 excited state of stretching vibration, respectively, which are consistent with previous experimental observations and six-dimensional quantum wave packet simulations. For reactions involving the first excited state of C—H stretching vibration, the HF products in the product asymptotic region and the reaction transition state region are both in the v' = 3 excited state of stretching vibration, which are consistent with the results obtained based on energy analysis. Simulation results indicate that in the case of low-energy collisions, the time-independent wave function for the C—H stretching vibrational excited state tends to be closer to the D atom side in the transition state region. This phenomenon is attributed to the more significant energy advantage of the vibrational excited state potential energy surface in the large collision angle region, explaining the inhibitory effect of stretching vibration excitation on the HF product channel. This study offers important theoretical support for explaining experimental results and contributes to a more in-depth understanding of the influence of vibrational mode excitations on the dynamical processes in poly-atomic reactions.
[1] Zhang D H, Collins M A, Lee S Y 2000 Science 290 961
 Google Scholar
Google Scholar
[2] Yang M, Zhang D H, Collins M A, Lee S Y 2001 J. Chem. Phys. 114 4759
 Google Scholar
Google Scholar
[3] Zhang D H 2006 J. Chem. Phys. 125 133102
 Google Scholar
Google Scholar
[4] Xiao C, Xu X, Liu S, Wang T, Dong W, Yang T, Sun Z, Dai D X, Xu X, Zhang D H, Yang X M 2011 Science 333 440
 Google Scholar
Google Scholar
[5] Roberto-Neto O, Machado F B C, Ornellas F R 2005 Chem. Phys. 315 27
 Google Scholar
Google Scholar
[6] Zhou J, Lin J J, Liu K P 2010 Mol. Phys. 108 957
 Google Scholar
Google Scholar
[7] Czakó G, Bowman J M 2011 Phys. Chem. Chem. Phys. 13 8306
 Google Scholar
Google Scholar
[8] Yang J Y, Zhang D, Chen Z, Blauert F, Jiang B, Dai D X, Wu G R, Zhang D H, Yang X M 2015 J. Chem. Phys. 143 044316
 Google Scholar
Google Scholar
[9] Palma J, Manthe U 2017 J. Chem. Phys. 146 214117
 Google Scholar
Google Scholar
[10] Ellerbrock R, Manthe U, Palma J 2019 J. Phys. Chem. A 123 7237
 Google Scholar
Google Scholar
[11] Marjollet A, Inhester L, Welsch R 2022 J. Chem. Phys. 156 044101
 Google Scholar
Google Scholar
[12] Shiu W C, Lin J J, Liu K P, Wu M, Parker D H 2003 J. Chem. Phys. 120 117
 Google Scholar
Google Scholar
[13] Shiu W C, Lin J J, Liu K P 2004 Phys. Rev. Lett. 92 103201
 Google Scholar
Google Scholar
[14] Zhou J G, Lin J J, Liu K P 2003 J. Chem. Phys. 119 8289
 Google Scholar
Google Scholar
[15] Zhou J G, Lin J J, Liu K P 2004 J. Chem. Phys. 121 813
 Google Scholar
Google Scholar
[16] Troya D, Millán J, Baños I, González M 2004 J. Chem. Phys. 120 5181
 Google Scholar
Google Scholar
[17] Espinosa-García J, Bravo J L, Rangel C 2007 J. Phys. Chem. A 111 2761
 Google Scholar
Google Scholar
[18] Czakó G, Shepler B C, Braams B J, Bowman J M 2009 J. Chem. Phys. 130 084301
 Google Scholar
Google Scholar
[19] Nyman G, Espinosa-Garcia J 2007 J. Phys. Chem. A 111 11943
 Google Scholar
Google Scholar
[20] Wang D, Czakó G 2013 J. Phys. Chem. A 117 7124
 Google Scholar
Google Scholar
[21] Westermann T, Eisfeld W, Manthe U 2013 J. Chem. Phys. 139 014309
 Google Scholar
Google Scholar
[22] von Horsten H F, Clary D C 2011 Phys. Chem. Chem. Phys. 13 4340
 Google Scholar
Google Scholar
[23] Qi J, Song H W, Yang M H, Palma J, Manthe U, Guo H 2016 J. Chem. Phys. 144 171101
 Google Scholar
Google Scholar
[24] Zhao B, Manthe U 2020 J. Chem. Phys. 152 231102
 Google Scholar
Google Scholar
[25] Chen J, Xu X, Liu S, Zhang D H 2018 Phys. Chem. Chem. Phys. 20 9090
 Google Scholar
Google Scholar
[26] Liu S, Chen J, Zhang X R, Zhang D H 2023 Chem. Sci. 14 7973
 Google Scholar
Google Scholar
[27] Zhang W, Kawamata H, Liu K 2009 Science 325 303
 Google Scholar
Google Scholar
[28] Czakó G, Shuai Q, Liu K P, Bowman J M 2010 J. Chem. Phys. 133 131101
 Google Scholar
Google Scholar
[29] Zhang W, Zhou Y, Wu G, Lu Y, Pan H, Fu B, Shuai Q, Liu L, Liu S, Zhang L, Jiang B, Dai D, Lee S Y, Xie Z, Braams B J, Bowman J M, Collins M A, Zhang D H, Yang X 2010 Proc. Natl. Acad. Sci. 107 12782
 Google Scholar
Google Scholar
[30] Zhou Y, Fu B N, Wang C R, Collins M A, Zhang D H 2011 J. Chem. Phys. 134 064323
 Google Scholar
Google Scholar
[31] Xu X, Chen J, Zhang D H 2014 Chin. J. Chem. Phys. 27 373
 Google Scholar
Google Scholar
[32] Zhou Y, Zhang D H 2014 J. Chem. Phys. 141 194307
 Google Scholar
Google Scholar
[33] Li J, Chen J, Zhao Z, Xie D, Zhang D H, Guo H 2015 J. Chem. Phys. 142 204302
 Google Scholar
Google Scholar
[34] Yang M, Lee S Y, Zhang D H 2007 J. Chem. Phys. 126 064303
 Google Scholar
Google Scholar
[35] Czakó G, Bowman J M 2009 J. Chem. Phys. 131 244302
 Google Scholar
Google Scholar
[36] Czakó G, Bowman J M 2009 J. Am. Chem. Soc. 131 17534
 Google Scholar
Google Scholar
[37] Zhang D H, Light J C 1996 J. Chem. Phys. 104 4544
 Google Scholar
Google Scholar
-
图 3 产物通道的非含时波函数 (a)振动基态, 碰撞能Ec = 0.018 eV; (b) C—H键伸缩振动第一激发态, 碰撞能Ec = 0.025 eV; 图中竖直方向和水平方向分别对应散射坐标和HF产物坐标
Figure 3. Time-independent wave function in product channel: (a) Ground vibrational state with collision energy Ec = 0.018 eV; (b) the first C—H stretching vibrational excited state with collision energy Ec = 0.025 eV. The vertical and horizontal directions correspond to the scattering coordinate and the HF product coordinate, respectively.
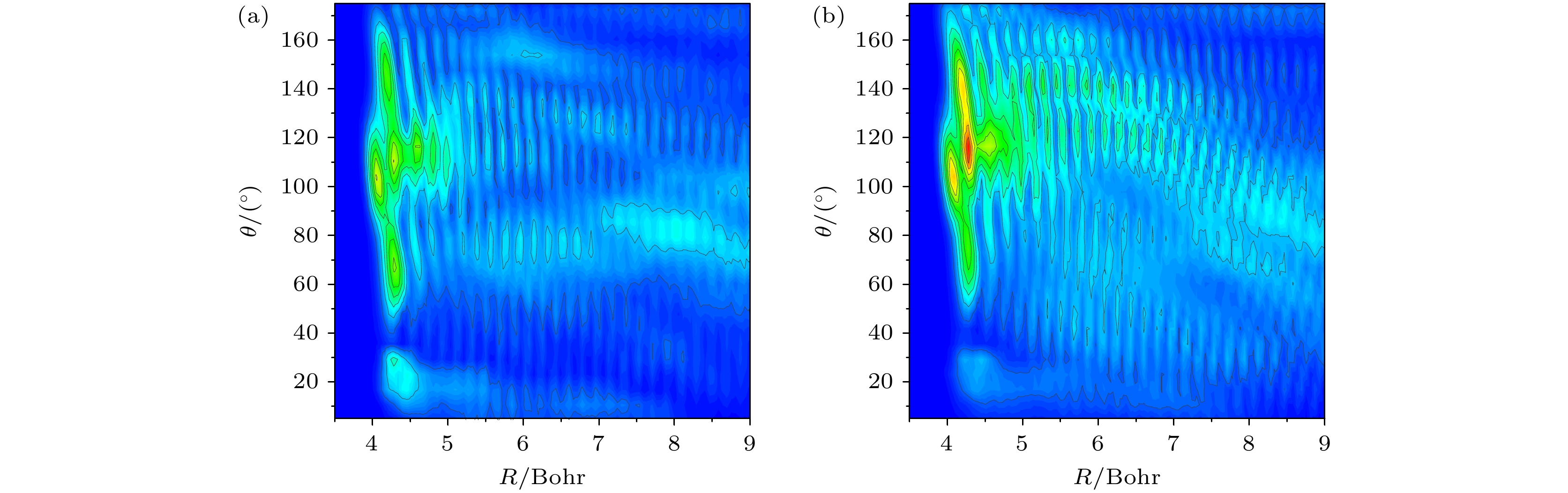
图 4 碰撞能Ec = 0.06 eV时, 反应物区的非含时波函数随反应坐标R和入射F原子倾斜角θ的变化 (a)振动基态; (b) C—H键伸缩振动第一激发态
Figure 4. Time-independent wave functions in the reactant region as a function of reaction coordinate R and the inclination angle θ of incoming F atom at a collision energy of Ec = 0.06 eV: (a) Ground vibrational state; (b) the first C—H stretching vibrational state.
图 5 碰撞能Ec = 0.2 eV时, 反应物区的非含时波函数随反应坐标R和入射F原子倾斜角θ的变化 (a)振动基态; (b) C—H键伸缩振动第一激发态
Figure 5. Time-independent wave functions in the reactant region as a function of reaction coordinate R and the inclination angle θ of incoming F atom at a collision energy of Ec = 0.2 eV: (a) Ground vibrational state; (b) the first C—H stretching vibrational state.
图 6 振动态平均的反应有效势能随反应坐标R和入射F原子倾斜角θ的变化 (a) 振动基态; (b) C—H键伸缩振动第一激发态
Figure 6. Averaged effective potential energy over vibrational states as a function of the reaction coordinate R and the inclination angle θ of incoming F atom: (a) Ground vibrational state; (b) the first C—H stretching vibrational excited state.
-
[1] Zhang D H, Collins M A, Lee S Y 2000 Science 290 961
 Google Scholar
Google Scholar
[2] Yang M, Zhang D H, Collins M A, Lee S Y 2001 J. Chem. Phys. 114 4759
 Google Scholar
Google Scholar
[3] Zhang D H 2006 J. Chem. Phys. 125 133102
 Google Scholar
Google Scholar
[4] Xiao C, Xu X, Liu S, Wang T, Dong W, Yang T, Sun Z, Dai D X, Xu X, Zhang D H, Yang X M 2011 Science 333 440
 Google Scholar
Google Scholar
[5] Roberto-Neto O, Machado F B C, Ornellas F R 2005 Chem. Phys. 315 27
 Google Scholar
Google Scholar
[6] Zhou J, Lin J J, Liu K P 2010 Mol. Phys. 108 957
 Google Scholar
Google Scholar
[7] Czakó G, Bowman J M 2011 Phys. Chem. Chem. Phys. 13 8306
 Google Scholar
Google Scholar
[8] Yang J Y, Zhang D, Chen Z, Blauert F, Jiang B, Dai D X, Wu G R, Zhang D H, Yang X M 2015 J. Chem. Phys. 143 044316
 Google Scholar
Google Scholar
[9] Palma J, Manthe U 2017 J. Chem. Phys. 146 214117
 Google Scholar
Google Scholar
[10] Ellerbrock R, Manthe U, Palma J 2019 J. Phys. Chem. A 123 7237
 Google Scholar
Google Scholar
[11] Marjollet A, Inhester L, Welsch R 2022 J. Chem. Phys. 156 044101
 Google Scholar
Google Scholar
[12] Shiu W C, Lin J J, Liu K P, Wu M, Parker D H 2003 J. Chem. Phys. 120 117
 Google Scholar
Google Scholar
[13] Shiu W C, Lin J J, Liu K P 2004 Phys. Rev. Lett. 92 103201
 Google Scholar
Google Scholar
[14] Zhou J G, Lin J J, Liu K P 2003 J. Chem. Phys. 119 8289
 Google Scholar
Google Scholar
[15] Zhou J G, Lin J J, Liu K P 2004 J. Chem. Phys. 121 813
 Google Scholar
Google Scholar
[16] Troya D, Millán J, Baños I, González M 2004 J. Chem. Phys. 120 5181
 Google Scholar
Google Scholar
[17] Espinosa-García J, Bravo J L, Rangel C 2007 J. Phys. Chem. A 111 2761
 Google Scholar
Google Scholar
[18] Czakó G, Shepler B C, Braams B J, Bowman J M 2009 J. Chem. Phys. 130 084301
 Google Scholar
Google Scholar
[19] Nyman G, Espinosa-Garcia J 2007 J. Phys. Chem. A 111 11943
 Google Scholar
Google Scholar
[20] Wang D, Czakó G 2013 J. Phys. Chem. A 117 7124
 Google Scholar
Google Scholar
[21] Westermann T, Eisfeld W, Manthe U 2013 J. Chem. Phys. 139 014309
 Google Scholar
Google Scholar
[22] von Horsten H F, Clary D C 2011 Phys. Chem. Chem. Phys. 13 4340
 Google Scholar
Google Scholar
[23] Qi J, Song H W, Yang M H, Palma J, Manthe U, Guo H 2016 J. Chem. Phys. 144 171101
 Google Scholar
Google Scholar
[24] Zhao B, Manthe U 2020 J. Chem. Phys. 152 231102
 Google Scholar
Google Scholar
[25] Chen J, Xu X, Liu S, Zhang D H 2018 Phys. Chem. Chem. Phys. 20 9090
 Google Scholar
Google Scholar
[26] Liu S, Chen J, Zhang X R, Zhang D H 2023 Chem. Sci. 14 7973
 Google Scholar
Google Scholar
[27] Zhang W, Kawamata H, Liu K 2009 Science 325 303
 Google Scholar
Google Scholar
[28] Czakó G, Shuai Q, Liu K P, Bowman J M 2010 J. Chem. Phys. 133 131101
 Google Scholar
Google Scholar
[29] Zhang W, Zhou Y, Wu G, Lu Y, Pan H, Fu B, Shuai Q, Liu L, Liu S, Zhang L, Jiang B, Dai D, Lee S Y, Xie Z, Braams B J, Bowman J M, Collins M A, Zhang D H, Yang X 2010 Proc. Natl. Acad. Sci. 107 12782
 Google Scholar
Google Scholar
[30] Zhou Y, Fu B N, Wang C R, Collins M A, Zhang D H 2011 J. Chem. Phys. 134 064323
 Google Scholar
Google Scholar
[31] Xu X, Chen J, Zhang D H 2014 Chin. J. Chem. Phys. 27 373
 Google Scholar
Google Scholar
[32] Zhou Y, Zhang D H 2014 J. Chem. Phys. 141 194307
 Google Scholar
Google Scholar
[33] Li J, Chen J, Zhao Z, Xie D, Zhang D H, Guo H 2015 J. Chem. Phys. 142 204302
 Google Scholar
Google Scholar
[34] Yang M, Lee S Y, Zhang D H 2007 J. Chem. Phys. 126 064303
 Google Scholar
Google Scholar
[35] Czakó G, Bowman J M 2009 J. Chem. Phys. 131 244302
 Google Scholar
Google Scholar
[36] Czakó G, Bowman J M 2009 J. Am. Chem. Soc. 131 17534
 Google Scholar
Google Scholar
[37] Zhang D H, Light J C 1996 J. Chem. Phys. 104 4544
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 4806
- PDF Downloads: 58
- Cited By: 0















 DownLoad:
DownLoad: