-
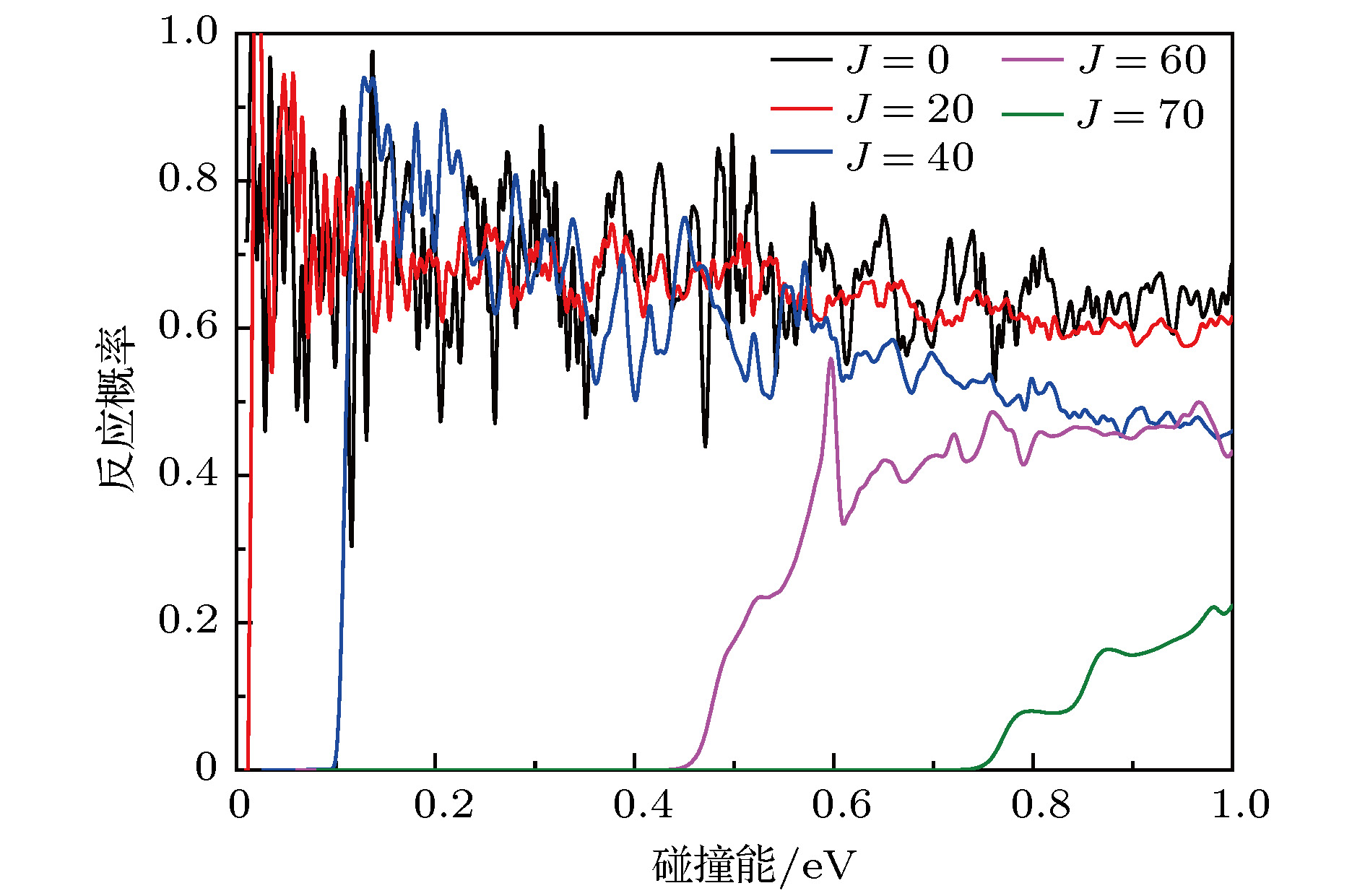
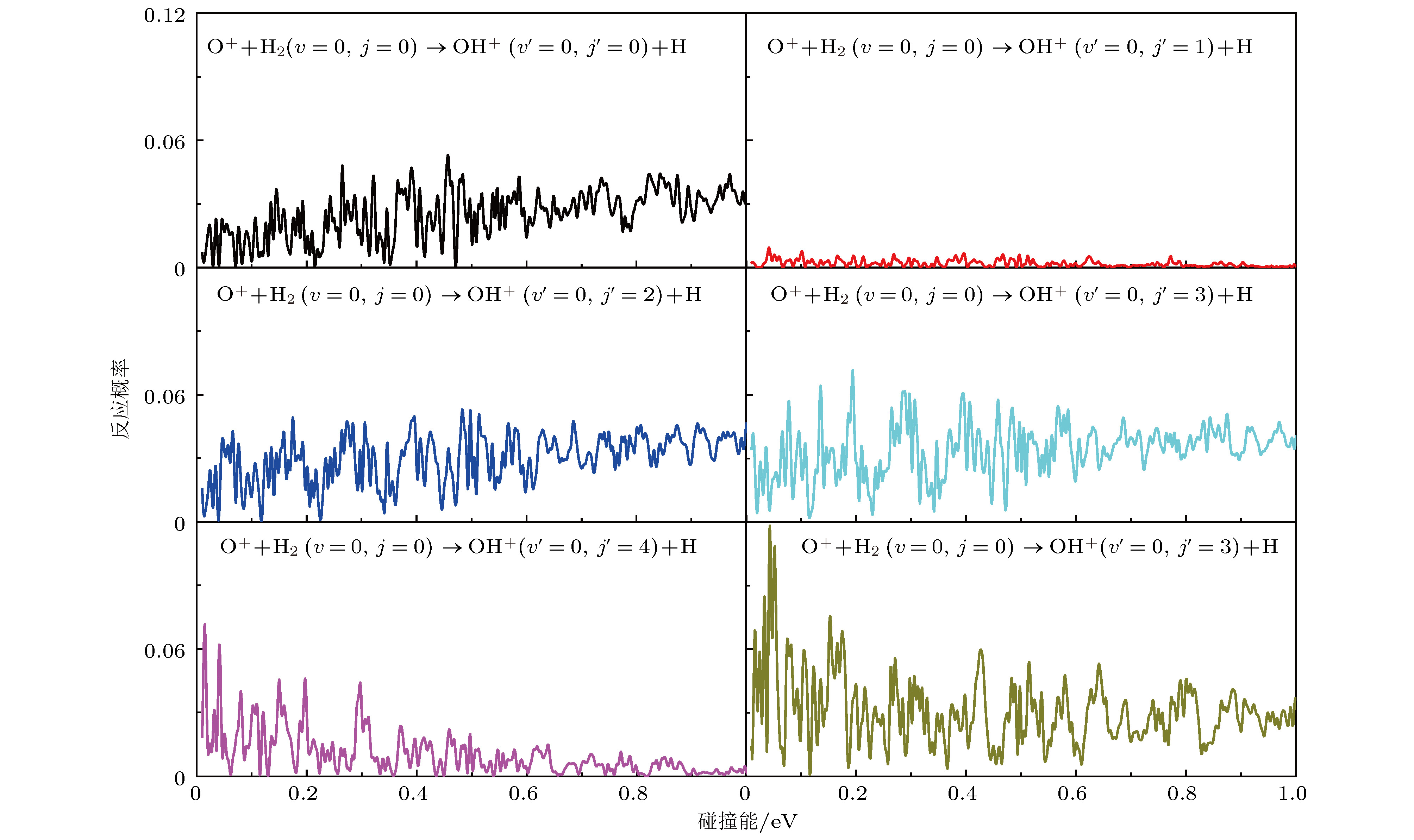
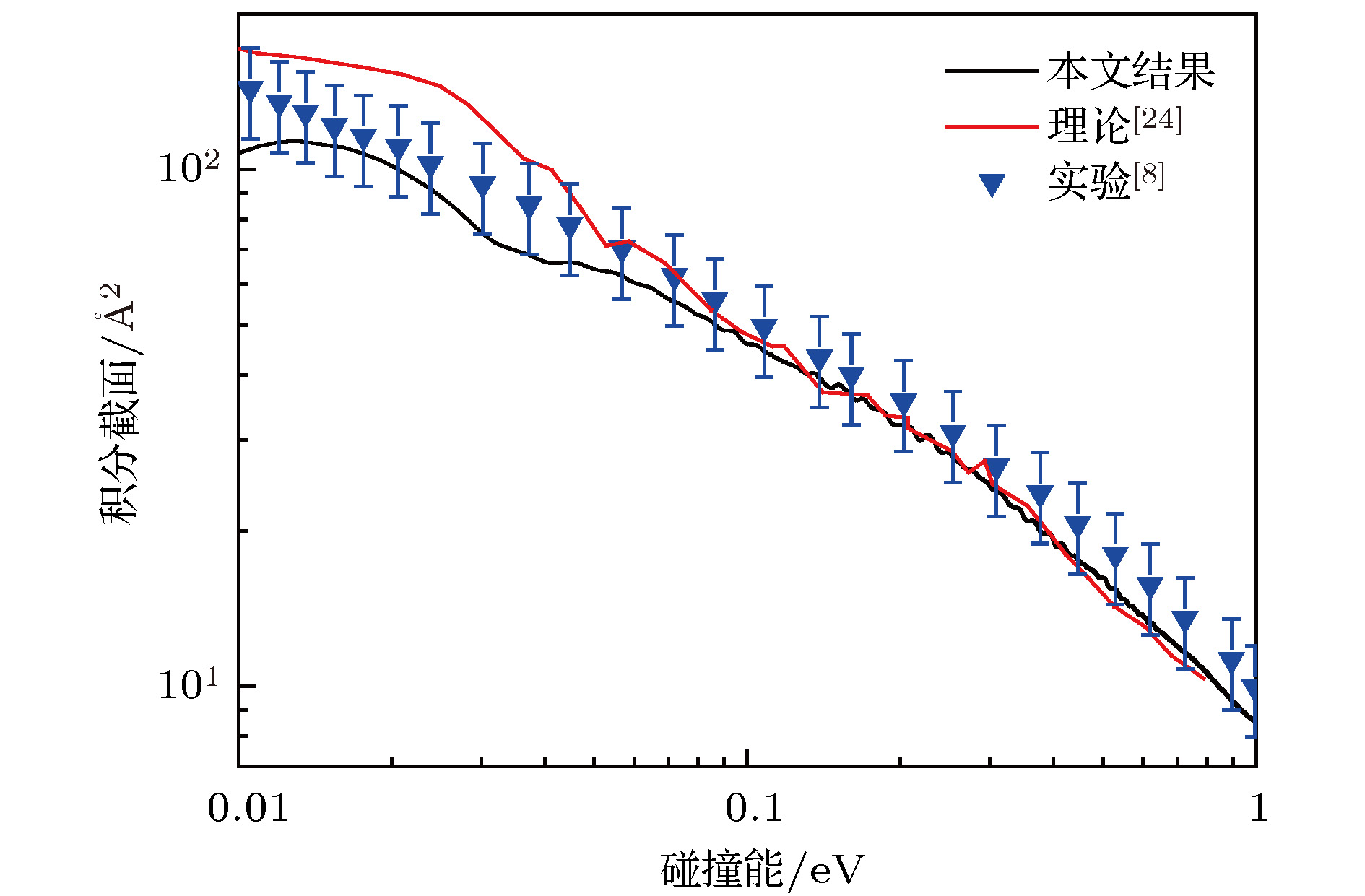
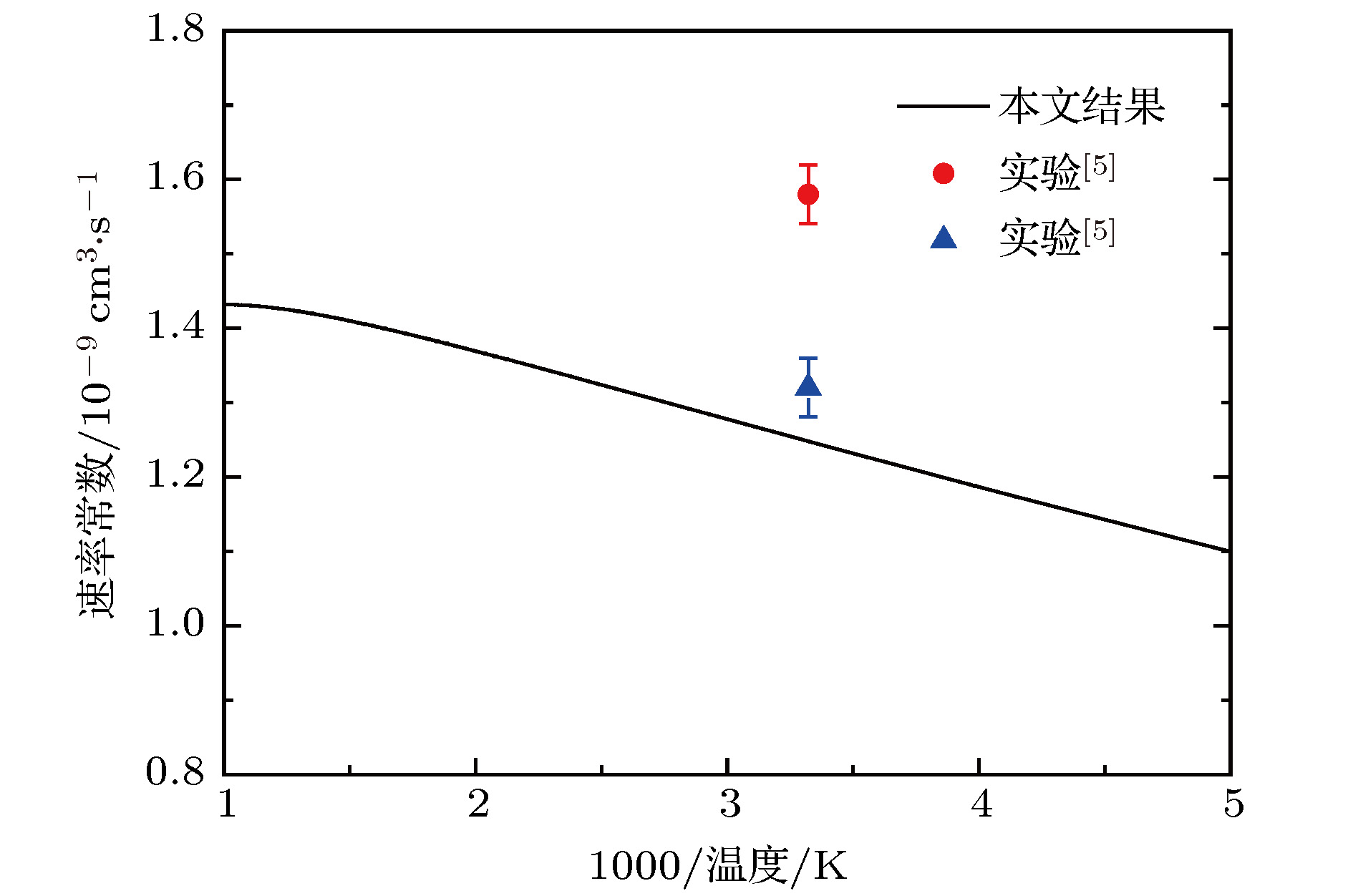
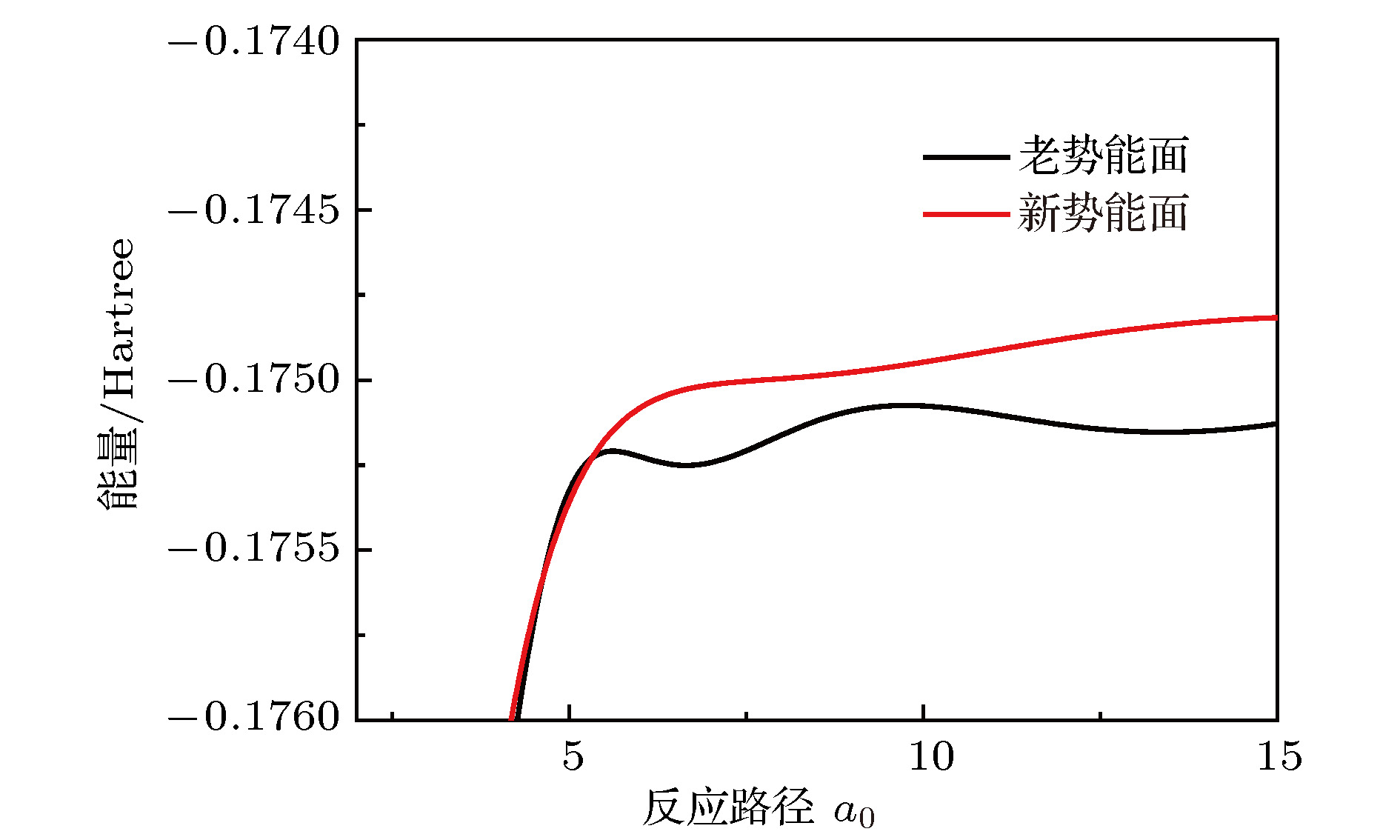
In the present work, the long-range interaction potential part of potential energy surface (PES) of OH2+ system is revised and the new resulting PES apparently is more reasonable than the old one in the long-range part. Based on the new PES, the dynamics calculations of O+ +H2→ OH+ + H reaction are carried out at a state-to-state level of theory by using time-dependent quantum wave packet method with second order split operator in a collision energy range from 0.01 to 1.0 eV. The dynamic properties such as reaction probability, ro-vibrational resolved statereaction probability, integral cross section, differential cross section, and state specific rate constant are calculated and compared with available theoretical and experimental results. The results of ro-vibrational resolved state reaction probability reflect some dynamic properties such as resonances which is attributed to the deep well located on the reaction path. The vibrational resolved state reaction probability indicates that the excitation efficiency of the OH+ product is relatively low. The results of integral cross sections indicate that the present results are in better agreement with the experimental values than with previous theoretical calculations, especially in the low collision energy region. However, the state specific rate constant results underestimate the experimental values. The comparison betweenour calculations and the experimental results indicates that the contribution of the rotational excitation of H2 molecule should be included in the calculations. However, only the initial state v = 0, j = 0 is calculated in the present work. We suppose that the deviation of the present results from the experimental data is due to the fact that the rotational excitation of reactant isnot included in the present calculation. The differential cross section signals indicate that the complex-forming reaction mechanism isdominated in the case of low collision energy, but it transforms into abstract reaction mechanism as the collision energy further increases.
[1] Duley W W, Williams D A 1984 Interstellar Chemistry (New York: Academic Press) p251
[2] Armentrout P 2000 Int. J. Mass Spectrom. Ion Process. 200 21933
[3] Jambrina P G, Alvarino J M, Gerlich D, Hankel M, Herrero V J, Saez-Rabanos V, Aoiz F J 2012 Phys. Chem. Chem. Phys. 14 3346
 Google Scholar
Google Scholar
[4] Wang B B, Han Y C, Gao W, Cong S L 2017 Phys. Chem. Chem. Phys. 19 22926
 Google Scholar
Google Scholar
[5] Fehsenfeld F C, Schmeltekopf A L, Ferguson E E 1967 J. Chem. Phys. 46 2802
 Google Scholar
Google Scholar
[6] Kim J K, Theard L P, Huntress W T 1975 J. Chem. Phys. 62 45
 Google Scholar
Google Scholar
[7] Federer W, Villinger H, Howorka F, Lindinger W, Tosi P, Bassi D, Ferguson E 1984 Phys. Rev. Lett. 52 2084
 Google Scholar
Google Scholar
[8] Smith D, Adams N G, Miller T M 1978 J. Chem. Phys. 69 308
 Google Scholar
Google Scholar
[9] Burley J, Ervin K M, Armentrout P 1987 Int. J. Mass Spectrom. Ion Process 80 153
 Google Scholar
Google Scholar
[10] Sunderlin L, Armentrout P 1990 Chem. Phys. Lett. 167 188
 Google Scholar
Google Scholar
[11] Flesch G D, Ng C Y 1991 J. Chem. Phys. 94 2372
 Google Scholar
Google Scholar
[12] Li X, Huang Y L, Flesch G D, Ng C Y 1997 J. Chem. Phys. 106 564
 Google Scholar
Google Scholar
[13] Gillen K T, Mahan B H, Winn J S 1973 J. Chem. Phys. 58 5373
 Google Scholar
Google Scholar
[14] Gillen K T, Mahan B H, Winn J S 1973 J. Chem. Phys. 59 6380
 Google Scholar
Google Scholar
[15] Ng C Y 2002 J. Phys. Chem. A 106 5953
 Google Scholar
Google Scholar
[16] Martínez R, Millán J, González M 2004 J. Chem. Phys. 120 4705
 Google Scholar
Google Scholar
[17] Martínez R, Sierra J D, González M 2005 J. Chem. Phys. 123 174312
 Google Scholar
Google Scholar
[18] Martínez R, Lucas J M, Giménez X, Aguilar A, González M 2006 J. Chem. Phys. 124 144301
 Google Scholar
Google Scholar
[19] Martínez R, Sierra J D, Gray S K, González M 2006 J. Chem. Phys. 125 164305
 Google Scholar
Google Scholar
[20] Kłos J, Bulut N, Akpinar S 2012 Chem. Phys. Lett. 532 22
 Google Scholar
Google Scholar
[21] Xu W, Li W, Lv S, Zhai H, Duan Z, Zhang P 2012 J. Phys. Chem. A 116 10882
 Google Scholar
Google Scholar
[22] Gómez-Carrasco S, Godard B, Lique F, Bulut N, Kłos J, Roncero O, Aguado A, Aoiz F J, Castillo J F, Goicoechea J R 2014 Astrophys. J. 794 33
 Google Scholar
Google Scholar
[23] Bulut N, Castillo J F, Jambrina P G, Kłos J, Roncero O, Aoiz F J, Bañares L 2015 J. Phys. Chem. A 119 11951
 Google Scholar
Google Scholar
[24] Li W T, Yuan J C, Yuan M L, Zhang Y, Yao M H, Sun Z G 2018 Phys. Chem. Chem. Phys. 20 1039
 Google Scholar
Google Scholar
[25] 段志欣, 邱明辉, 姚翠霞 2014 63 063402
 Google Scholar
Google Scholar
Duan Z X, Qiu M H, Yao C X 2014 Acta Phys. Sin. 63 063402
 Google Scholar
Google Scholar
[26] 李文涛, 于文涛, 姚明海 2018 67 103401
 Google Scholar
Google Scholar
Li W T, Yu W T, Yao M H 2018 Acta Phys. Sin. 67 103401
 Google Scholar
Google Scholar
[27] 张静, 魏巍, 高守宝, 孟庆田 2015 64 063101
 Google Scholar
Google Scholar
Zhang J, Wei W, Gao S B, Meng Q T 2015 Acta Phys. Sin. 64 063101
 Google Scholar
Google Scholar
[28] Zhao B, Sun Z G, Guo H 2016 J. Chem. Phys. 144 214303
 Google Scholar
Google Scholar
[29] Zhao B, Sun Z G, Guo H 2016 J. Chem. Phys. 144 064104
 Google Scholar
Google Scholar
[30] Li W T, Chen M D, Sun Z G 2015 Chin. J. Chem. Phys. 28 415
 Google Scholar
Google Scholar
[31] Fleck J A, Morris J R, Feit M D 1976 Appl. Phys. 10 129
-
表 1 计算中使用的参数(除了特殊声明, 均采用原子单位a.u.)
Table 1. Parameters used in the calculation (The atomic unit is used in the calculation unless otherwise stated)
格点范围和大小 $R \in \left[ {0.1,16} \right],{N_R} = 279,N_R^{{\rm{int}}} = 159$ $r \in \left[ {0.1,15} \right],{N_r} = 279,N_r^{{\rm{asy}}} = 159$ 初始波包 Rc = 11.0, ${k_0} = \sqrt {2{E_0}{\mu_R}} $ ${\varDelta _R} = 0.2 {\rm{exp}}\left[ { - \dfrac{{{{\left( {R - {R_{\rm{c}}}} \right)}^2}}}{{2\varDelta _R^2}}} \right]{\rm{cos}}\left( {{k_0}R} \right)$;
其中E0 = 0.5 eV总传播时间 30000 最大总角
动量J70 -
[1] Duley W W, Williams D A 1984 Interstellar Chemistry (New York: Academic Press) p251
[2] Armentrout P 2000 Int. J. Mass Spectrom. Ion Process. 200 21933
[3] Jambrina P G, Alvarino J M, Gerlich D, Hankel M, Herrero V J, Saez-Rabanos V, Aoiz F J 2012 Phys. Chem. Chem. Phys. 14 3346
 Google Scholar
Google Scholar
[4] Wang B B, Han Y C, Gao W, Cong S L 2017 Phys. Chem. Chem. Phys. 19 22926
 Google Scholar
Google Scholar
[5] Fehsenfeld F C, Schmeltekopf A L, Ferguson E E 1967 J. Chem. Phys. 46 2802
 Google Scholar
Google Scholar
[6] Kim J K, Theard L P, Huntress W T 1975 J. Chem. Phys. 62 45
 Google Scholar
Google Scholar
[7] Federer W, Villinger H, Howorka F, Lindinger W, Tosi P, Bassi D, Ferguson E 1984 Phys. Rev. Lett. 52 2084
 Google Scholar
Google Scholar
[8] Smith D, Adams N G, Miller T M 1978 J. Chem. Phys. 69 308
 Google Scholar
Google Scholar
[9] Burley J, Ervin K M, Armentrout P 1987 Int. J. Mass Spectrom. Ion Process 80 153
 Google Scholar
Google Scholar
[10] Sunderlin L, Armentrout P 1990 Chem. Phys. Lett. 167 188
 Google Scholar
Google Scholar
[11] Flesch G D, Ng C Y 1991 J. Chem. Phys. 94 2372
 Google Scholar
Google Scholar
[12] Li X, Huang Y L, Flesch G D, Ng C Y 1997 J. Chem. Phys. 106 564
 Google Scholar
Google Scholar
[13] Gillen K T, Mahan B H, Winn J S 1973 J. Chem. Phys. 58 5373
 Google Scholar
Google Scholar
[14] Gillen K T, Mahan B H, Winn J S 1973 J. Chem. Phys. 59 6380
 Google Scholar
Google Scholar
[15] Ng C Y 2002 J. Phys. Chem. A 106 5953
 Google Scholar
Google Scholar
[16] Martínez R, Millán J, González M 2004 J. Chem. Phys. 120 4705
 Google Scholar
Google Scholar
[17] Martínez R, Sierra J D, González M 2005 J. Chem. Phys. 123 174312
 Google Scholar
Google Scholar
[18] Martínez R, Lucas J M, Giménez X, Aguilar A, González M 2006 J. Chem. Phys. 124 144301
 Google Scholar
Google Scholar
[19] Martínez R, Sierra J D, Gray S K, González M 2006 J. Chem. Phys. 125 164305
 Google Scholar
Google Scholar
[20] Kłos J, Bulut N, Akpinar S 2012 Chem. Phys. Lett. 532 22
 Google Scholar
Google Scholar
[21] Xu W, Li W, Lv S, Zhai H, Duan Z, Zhang P 2012 J. Phys. Chem. A 116 10882
 Google Scholar
Google Scholar
[22] Gómez-Carrasco S, Godard B, Lique F, Bulut N, Kłos J, Roncero O, Aguado A, Aoiz F J, Castillo J F, Goicoechea J R 2014 Astrophys. J. 794 33
 Google Scholar
Google Scholar
[23] Bulut N, Castillo J F, Jambrina P G, Kłos J, Roncero O, Aoiz F J, Bañares L 2015 J. Phys. Chem. A 119 11951
 Google Scholar
Google Scholar
[24] Li W T, Yuan J C, Yuan M L, Zhang Y, Yao M H, Sun Z G 2018 Phys. Chem. Chem. Phys. 20 1039
 Google Scholar
Google Scholar
[25] 段志欣, 邱明辉, 姚翠霞 2014 63 063402
 Google Scholar
Google Scholar
Duan Z X, Qiu M H, Yao C X 2014 Acta Phys. Sin. 63 063402
 Google Scholar
Google Scholar
[26] 李文涛, 于文涛, 姚明海 2018 67 103401
 Google Scholar
Google Scholar
Li W T, Yu W T, Yao M H 2018 Acta Phys. Sin. 67 103401
 Google Scholar
Google Scholar
[27] 张静, 魏巍, 高守宝, 孟庆田 2015 64 063101
 Google Scholar
Google Scholar
Zhang J, Wei W, Gao S B, Meng Q T 2015 Acta Phys. Sin. 64 063101
 Google Scholar
Google Scholar
[28] Zhao B, Sun Z G, Guo H 2016 J. Chem. Phys. 144 214303
 Google Scholar
Google Scholar
[29] Zhao B, Sun Z G, Guo H 2016 J. Chem. Phys. 144 064104
 Google Scholar
Google Scholar
[30] Li W T, Chen M D, Sun Z G 2015 Chin. J. Chem. Phys. 28 415
 Google Scholar
Google Scholar
[31] Fleck J A, Morris J R, Feit M D 1976 Appl. Phys. 10 129
计量
- 文章访问数: 10699
- PDF下载量: 62
- 被引次数: 0













 下载:
下载: