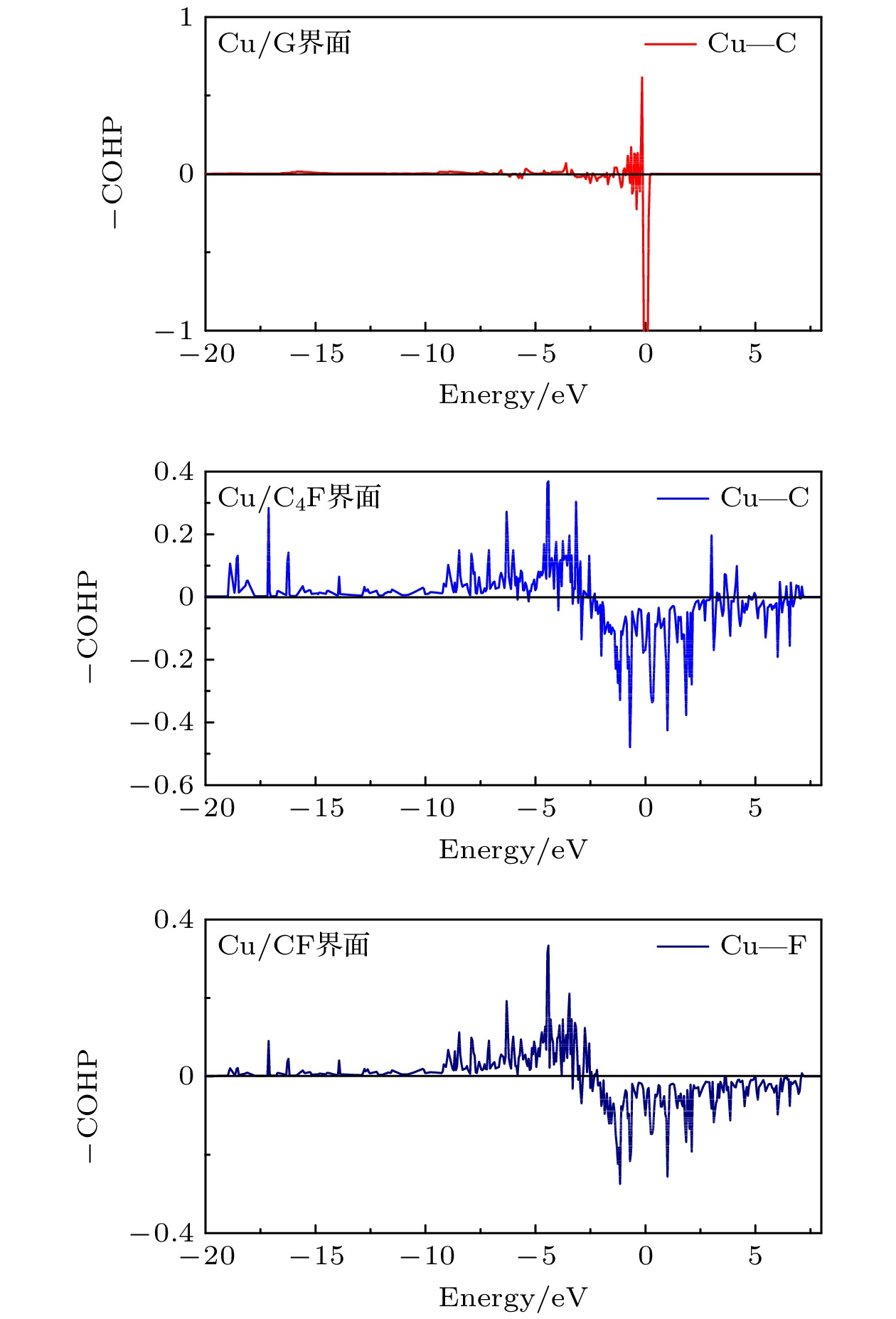
-
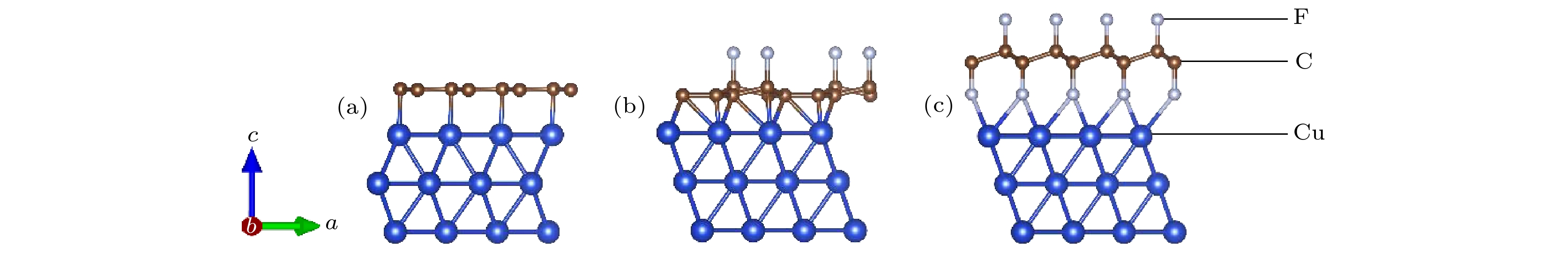
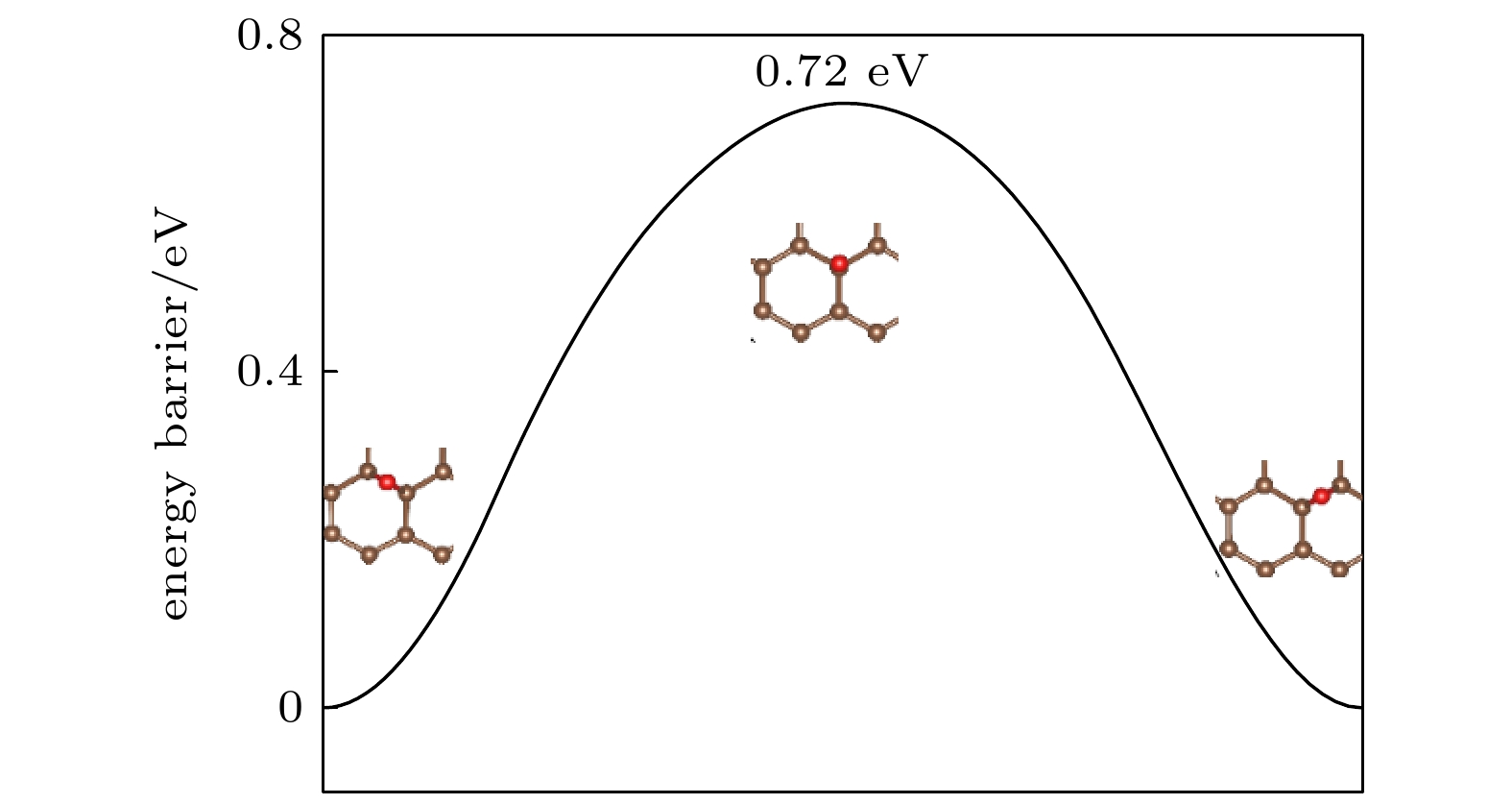
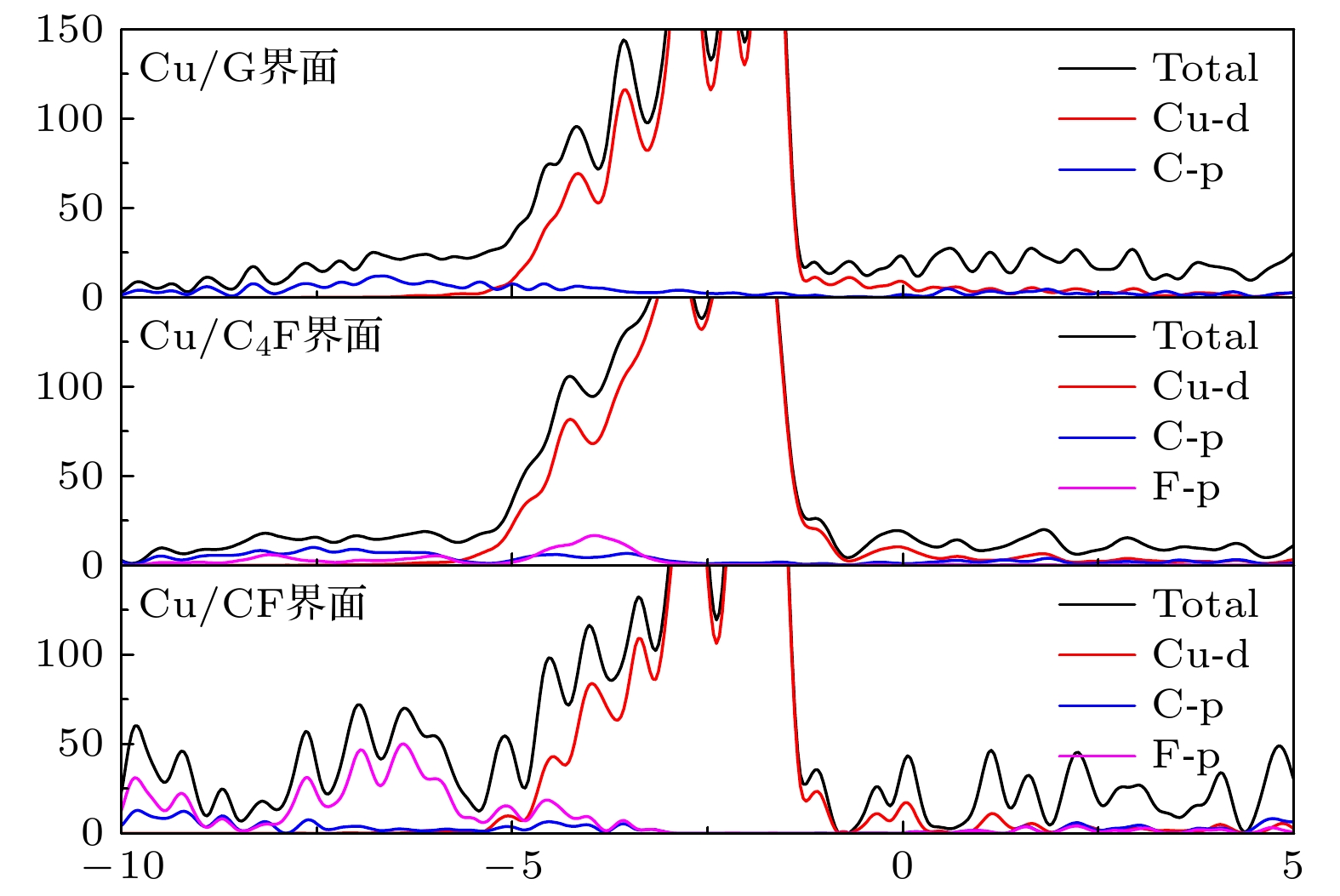
Fluorination of graphene is one of the most effective methods to improve the corrosion protection of graphene coatings. In this work, the diffusion and penetration behaviors of O atoms on fully fluorinated graphene (CF) and partially fluorinated graphene (C4F) are investigated by using the method of searching for NEB transition state . The effects of F atoms on the corrosion resistance of fluorinated graphene films are also analyzed r. The results show that the adsorption of F atoms can effectively inhibit the diffusion of O atoms on graphene. On C4F, the F atoms are distributed in a para-top position, which greatly increases the surface diffusion energy barrier of O atoms. Moreover, it is difficult for the adsorbed O atoms to diffuse to different sp2 C rings through the obstruction of F atoms. The energy barrier of the horizontal diffusion of O atoms even reaches 2.69 eV in CF. And with the increase of F atoms, the stable structure of graphene is gradually destroyed, the ability of C-atom layer to bar the penetration behaviors of O atoms decreases greatly. Furthermore, the interfacial adhesion work of pure graphene, CF and C4F films with Cu(111) surfaces are calculated, as well as the electronic structures of the composite interface are investigated by using first-principles calculations. The interfacial adhesion work of the Cu/G, Cu/C4F and Cu/CF interfaces are 2.626 J/m2, 3.529 J/m2 and 3.559 J/m2, respectively. The calculations show that the bonding of C4F and C4F with Cu substrate are stronger than pure graphene with Cu substrate, and the interfacial adhesion work increases with the augment of F atom adsorption concentration. The calculation of the density of states also conforms that the interaction between Cu and C atoms of the Cu/C4F interface is stronger than that at the Cu/CF interface. Bader charge analysis shows that the charge transfer at the Cu/C4F interface and the Cu/CF interface increase comparing with that at the Cu/G interface, and Cu/C4F interface has more charge transfer, in which Cu—C bonds are formed.
[1] Nine M J, Cole M A, Tran D N H, Losic D 2015 J. Mater. Chem. A Mater. 3 12580
 Google Scholar
Google Scholar
[2] Sun W, Yang Y J, Yang Z Q, Wang L D, Wang J, Xu D K, Liu G C 2021 J. Mater. Sci. Mater. Med. 91 278
[3] Bunch J S, Verbridge S S, Alden J S, van der Zande A M, Parpia J M, Craighead H G, McEuen P L 2008 Nano Lett. 8 2458
 Google Scholar
Google Scholar
[4] Tsetseris L, Pantelides S T 2014 Carbon 67 58
 Google Scholar
Google Scholar
[5] Miao M, Nardelli M B, Wang Q, Liu Y 2013 Phys. Chem. Chem. Phys. 15 16132
 Google Scholar
Google Scholar
[6] Zhang R Y, Yu X, Yang Q W, Cui G, Li Z L 2021 Constr. Build. Mater. 294 123613
 Google Scholar
Google Scholar
[7] Topsakal M, Sahin H, Ciraci S 2012 Phys. Rev. B 85 155445
 Google Scholar
Google Scholar
[8] 赵雯琪, 张岱, 崔明慧, 杜颖, 张树宇, 区琼荣 2021 70 095208
 Google Scholar
Google Scholar
Zhao W Q, Zhang D, Cui M H, Du Y, Zhang S Y, Qu Q R 2021 Acta Phys. Sin. 70 095208
 Google Scholar
Google Scholar
[9] Prasai D, Tuberquia J C, Harl R R, Jennings G K, Rogers B R, Bolotin K I 2012 ACS Nano 6 1102
 Google Scholar
Google Scholar
[10] 郭晓蒙, 青芳竹, 李雪松 2021 70 098102
 Google Scholar
Google Scholar
Guo X M, Qing F Z, Li X S 2021 Acta Phys. Sin. 70 098102
 Google Scholar
Google Scholar
[11] Cui C L, Lim A T O, Huang J X 2017 Nat. Nanotechnol. 12 834
 Google Scholar
Google Scholar
[12] Jihyung L, Diana B 2018 Carbon 126 225
 Google Scholar
Google Scholar
[13] Ding J H, Zhao H R, Zhao X P, Xu BY, Yu H B 2019 J. Mater. Chem. 7 13511
 Google Scholar
Google Scholar
[14] Boukhvalov D W, Kurmaev E Z, Urbańczyk E, Dercz G, Stolarczyk A, Simka W, Kukharenko A I, Zhidkov I S, Slesarev A I, Zatsepin A F, Cholakh S O 2018 Thin Solid Films 665 99
 Google Scholar
Google Scholar
[15] Ankit Y, Rajeev K, Pratap P U, Balaram S 2021 Carbon 173 350
 Google Scholar
Google Scholar
[16] Chauhan D S, Quraishi M A, Ansari K R, Saleh T A 2020 Prog. Org. Coat. 147 105741
 Google Scholar
Google Scholar
[17] 丁锐, 陈思, 吕静, 桂泰江, 王晓, 赵晓栋, 刘杰, 李秉钧, 宋立英, 李伟华 2019 化学学报 77 1140
Ding R, Chen S, Lv J, Gui T J, Wang X, Zhao X D, Liu J, Li B J, Song L Y, Li W H 2019 Acta Chem. Sin. 77 1140
[18] Karolina O, Marek L 2020 Coatings 10 883
 Google Scholar
Google Scholar
[19] Cui G, Bi Z X, Zhang R Y, Liu J G, Yu X, Li Z L 2019 Chem. Eng. J. 373 104
 Google Scholar
Google Scholar
[20] Li Q, Zheng S X, Pu J B, Sun J H, Huang L F, Wang L P, Xue Q J. 2019 Phys. Chem. Chem. Phys. 21 12121
 Google Scholar
Google Scholar
[21] Sadeghi A, Neek Amal M, Berdiyorov G R, Peeters F M 2015 Phys. Rev. B 91 014304
 Google Scholar
Google Scholar
[22] Shen L, Li Y, Zhao W J, Wang K, Ci X J, Wu Y M, Liu G, Liu C, Fang Z W 2020 J. Mater. Sci. Technol. 44 121
 Google Scholar
Google Scholar
[23] 窦宝捷, 付英奎, 高秀磊, 张颖君, 林修洲, 王兆华, 马兵, 方治文 2020 表面技术 49 241
Dou B J, Fu Y K, Gao X L, Zhang Y J, Lin X Z, Wang Z H, Ma B, Fang Z W 2020 Surf. Tech. 49 241
[24] Wu Y M, Jiang F W, Qiang Y J, Zhao W J 2021 Carbon 176 39
 Google Scholar
Google Scholar
[25] Yao W J, Zhou S G, Wang Z X, Lu Z B, Hou C J 2020 Appl. Surf. Sci. 499 143962
 Google Scholar
Google Scholar
[26] Widjaja H, Jiang Z T, Altarawneh M, Yin C Y, Goh B M, Mondinos N, Amri A, Dlugogorski B Z 2016 Appl. Surf. Sci. 373 65
 Google Scholar
Google Scholar
[27] Robinson J T, Burgess J S, Junkermeier C E, Badescu S C, Reinecke T L, Perkins F K, Zalalutdniov M K, Baldwin J W, Culbertson J C, Sheehan P E, Snow E S 2010 Nano Lett. 10 3001
 Google Scholar
Google Scholar
[28] Zbořil R, Karlický F, Bourlinos A B, Steriotis T A, Stubos A K, Georgakilas V, Šafářová K, Jančík D, Trapalis C, Otyepka M 2010 Small 6 2885
 Google Scholar
Google Scholar
[29] Samarakoon D K, Chen Z F, Nicolas C, Wang X Q 2011 Small 7 965
 Google Scholar
Google Scholar
[30] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[31] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[32] Perdew J P, Wang Y 1992 Phys. Rev. B 45 13244
 Google Scholar
Google Scholar
[33] Lee K, Murray É D, Kong L, Lundqvist B I, Langreth D C 2010 Phys. Rev. B 82 081101
 Google Scholar
Google Scholar
[34] Henkelman G, Jónsson H 2000 J. Chem. Phys. 113 9978
 Google Scholar
Google Scholar
[35] 吴迪, 杨永, 章小峰, 黄贞益, 王昭东 2022 71 086301
 Google Scholar
Google Scholar
Wu D, Yang Y, Zhang X F, Huang Z Y, Wang Z D 2022 Acta Phys. Sin. 71 086301
 Google Scholar
Google Scholar
[36] Chronopoulos D D, Bakandritsos A, Pykal M, Zbořil R, Otyepka M 2017 Appl. Mater. Today 9 60
[37] Li Y F, Pantoja B A, Chen Z F. 2014 J. Chem. Theory. Comput. 10 1265
 Google Scholar
Google Scholar
[38] 余金中, 王杏华 2001 物理 3 169
 Google Scholar
Google Scholar
Yu J Z, Wang X H 2001 Physics 3 169
 Google Scholar
Google Scholar
[39] Deringer V L, Tchougréeff A L, Dronskowski R 2011 J. Phys. Chem. A 115 5461
 Google Scholar
Google Scholar
-
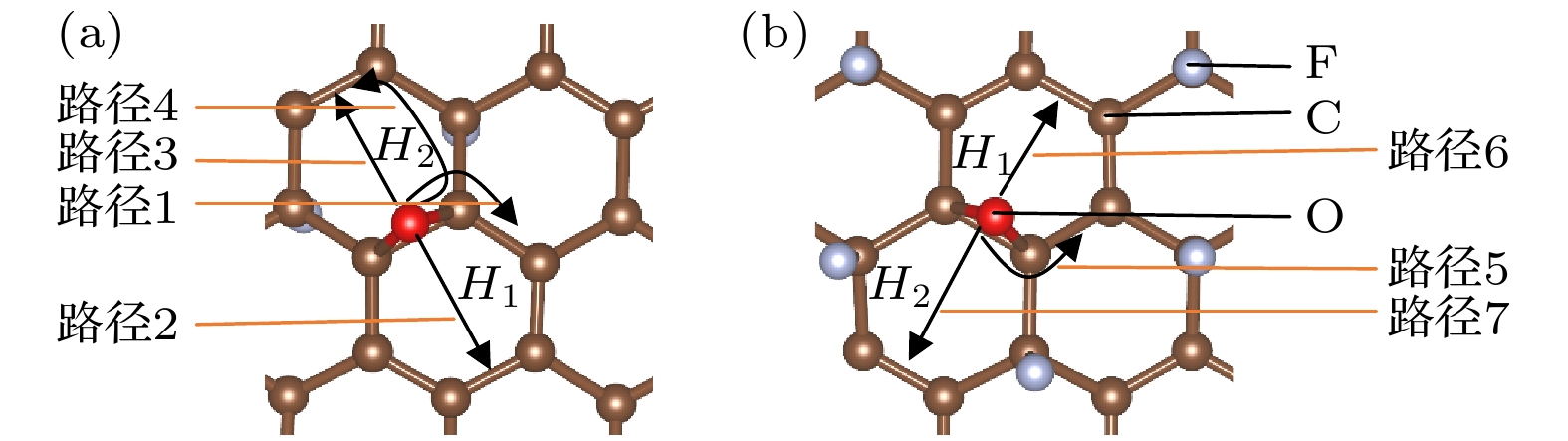
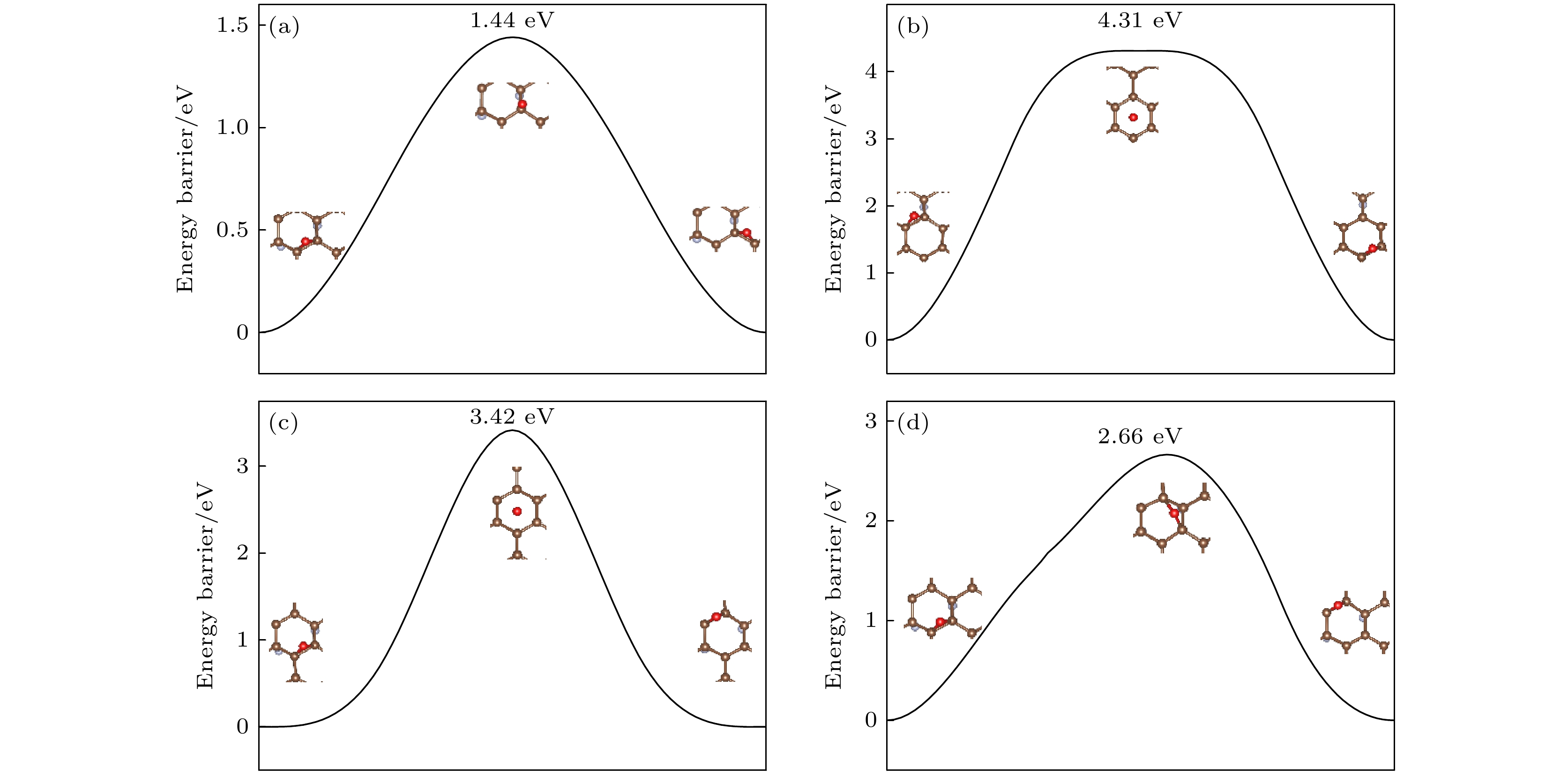
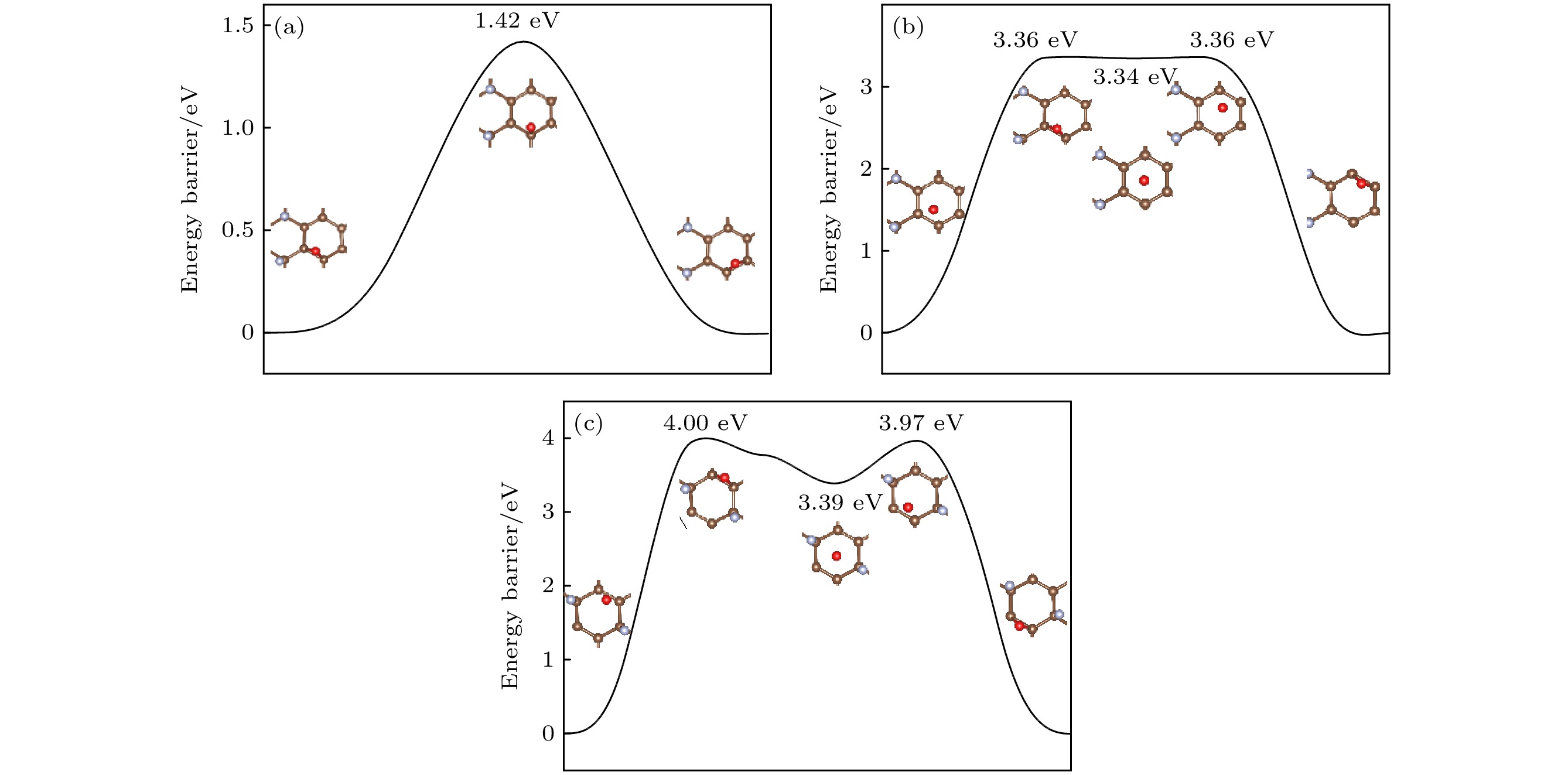
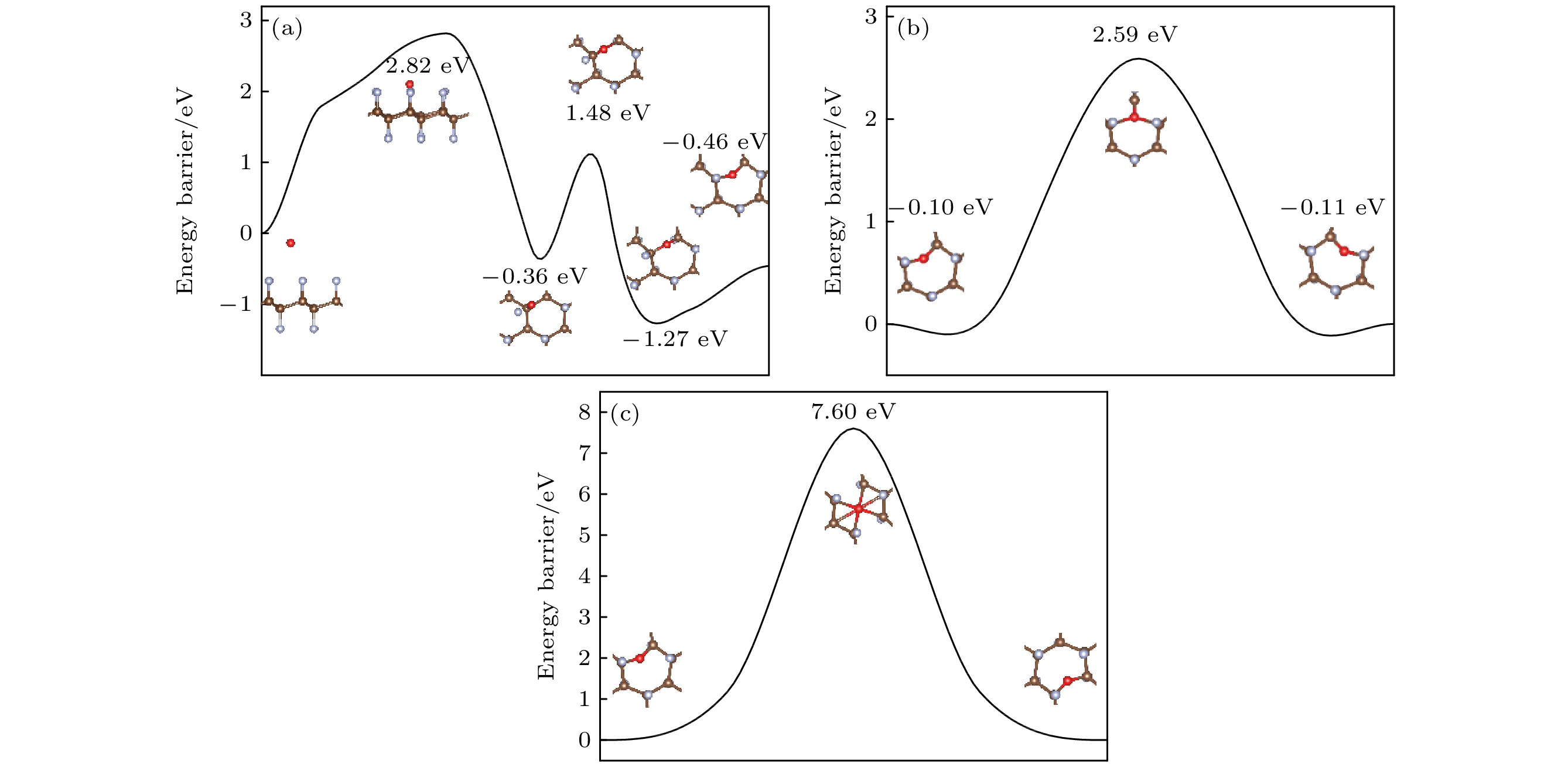
图 8 (a) O原子穿透CF的F原子层到达C原子层的能垒图; (b) O原子在CF内相邻桥位上的扩散的能垒图; (c) O原子在CF内沿空心位扩散的能垒图
Figure 8. (a) Diffusion barrier diagram of O atom penetrating the F atomic layer of CF to the C atomic layer; (b) diffusion barrier diagram of O atom diffusion on the adjacent bridge site in CF; (c) diffusion barrier diagram of O atom diffusion along the hollow site in CF.
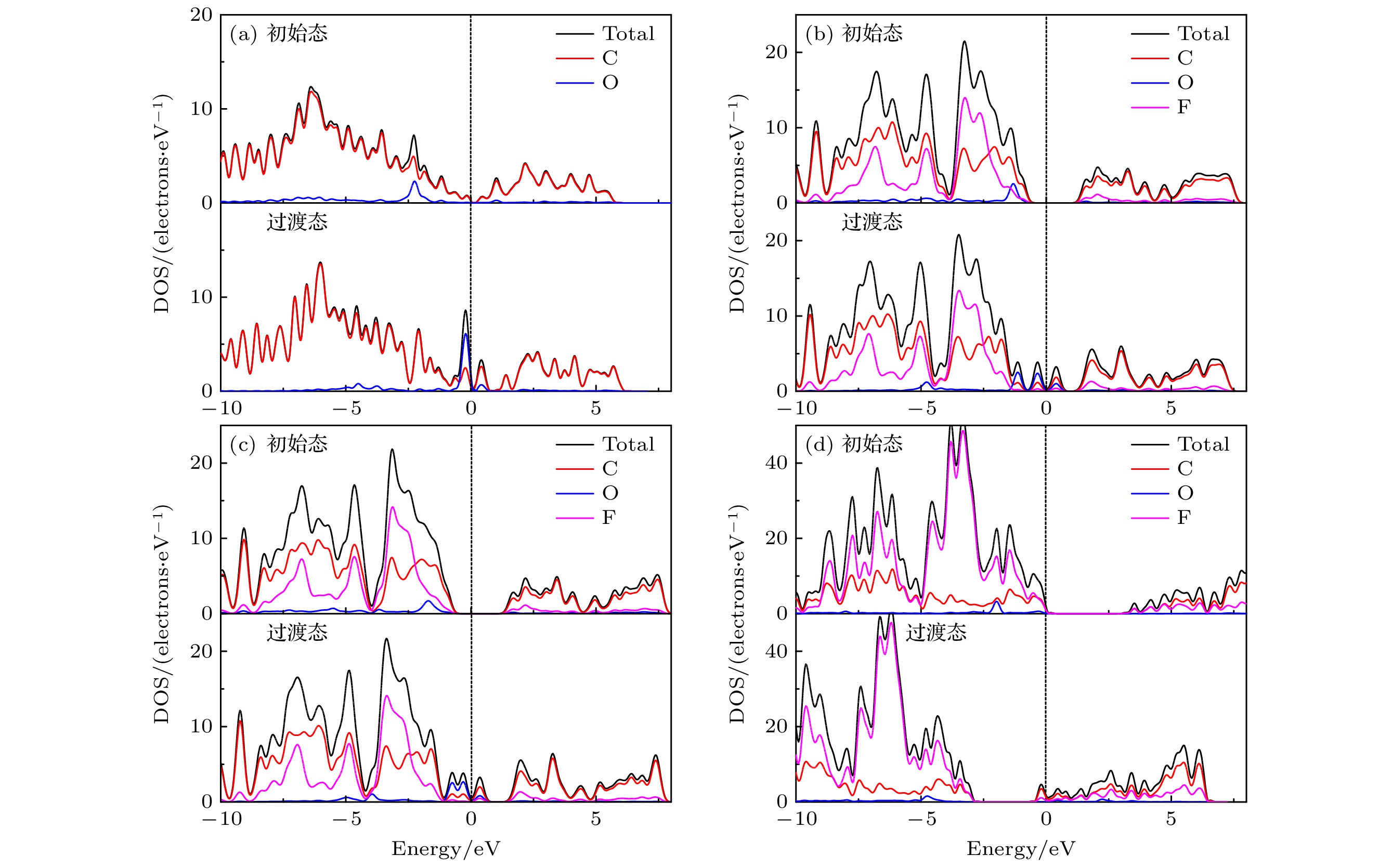
图 9 O原子沿最佳扩散路径扩散的DOS图 (a) G表面初始态和过渡态的DOS图; (b) C4F有F面初始态和过渡态的DOS图; (c) C4F无F面初始态和过渡态的DOS图; (d) CF内初始态和过渡态的DOS图
Figure 9. DOS diagram of O atom diffusion along the optimal diffusion path: (a) DOS diagram of initial state and transition state of G surface; (b) DOS diagram of initial state and transition state on the F adsorbed surface of C4F; (c) DOS diagram of initial state and transition state on the F-free surface of C4F; (d) DOS diagram of initial state and transition state in CF.
表 A1 O原子在C4F不同位置吸附的能量数据
Table A1. Energy data of O atom adsorption at different positions of C4F.
O原子吸附位置 C4F 有F面/eV 无F面/eV 桥位 –325.31 –326.67 顶位 –323.89 –325.23 空心位H1 –321.96 –322.36 空心位H2 –321.92 –323.25 表 A2 O原子在CF内不同位置吸附的能量数据
Table A2. Energy data of O atom adsorption at different positions in CF.
O原子吸附位置 CF内/eV 桥位 –413.70 空心位 –406.10 -
[1] Nine M J, Cole M A, Tran D N H, Losic D 2015 J. Mater. Chem. A Mater. 3 12580
 Google Scholar
Google Scholar
[2] Sun W, Yang Y J, Yang Z Q, Wang L D, Wang J, Xu D K, Liu G C 2021 J. Mater. Sci. Mater. Med. 91 278
[3] Bunch J S, Verbridge S S, Alden J S, van der Zande A M, Parpia J M, Craighead H G, McEuen P L 2008 Nano Lett. 8 2458
 Google Scholar
Google Scholar
[4] Tsetseris L, Pantelides S T 2014 Carbon 67 58
 Google Scholar
Google Scholar
[5] Miao M, Nardelli M B, Wang Q, Liu Y 2013 Phys. Chem. Chem. Phys. 15 16132
 Google Scholar
Google Scholar
[6] Zhang R Y, Yu X, Yang Q W, Cui G, Li Z L 2021 Constr. Build. Mater. 294 123613
 Google Scholar
Google Scholar
[7] Topsakal M, Sahin H, Ciraci S 2012 Phys. Rev. B 85 155445
 Google Scholar
Google Scholar
[8] 赵雯琪, 张岱, 崔明慧, 杜颖, 张树宇, 区琼荣 2021 70 095208
 Google Scholar
Google Scholar
Zhao W Q, Zhang D, Cui M H, Du Y, Zhang S Y, Qu Q R 2021 Acta Phys. Sin. 70 095208
 Google Scholar
Google Scholar
[9] Prasai D, Tuberquia J C, Harl R R, Jennings G K, Rogers B R, Bolotin K I 2012 ACS Nano 6 1102
 Google Scholar
Google Scholar
[10] 郭晓蒙, 青芳竹, 李雪松 2021 70 098102
 Google Scholar
Google Scholar
Guo X M, Qing F Z, Li X S 2021 Acta Phys. Sin. 70 098102
 Google Scholar
Google Scholar
[11] Cui C L, Lim A T O, Huang J X 2017 Nat. Nanotechnol. 12 834
 Google Scholar
Google Scholar
[12] Jihyung L, Diana B 2018 Carbon 126 225
 Google Scholar
Google Scholar
[13] Ding J H, Zhao H R, Zhao X P, Xu BY, Yu H B 2019 J. Mater. Chem. 7 13511
 Google Scholar
Google Scholar
[14] Boukhvalov D W, Kurmaev E Z, Urbańczyk E, Dercz G, Stolarczyk A, Simka W, Kukharenko A I, Zhidkov I S, Slesarev A I, Zatsepin A F, Cholakh S O 2018 Thin Solid Films 665 99
 Google Scholar
Google Scholar
[15] Ankit Y, Rajeev K, Pratap P U, Balaram S 2021 Carbon 173 350
 Google Scholar
Google Scholar
[16] Chauhan D S, Quraishi M A, Ansari K R, Saleh T A 2020 Prog. Org. Coat. 147 105741
 Google Scholar
Google Scholar
[17] 丁锐, 陈思, 吕静, 桂泰江, 王晓, 赵晓栋, 刘杰, 李秉钧, 宋立英, 李伟华 2019 化学学报 77 1140
Ding R, Chen S, Lv J, Gui T J, Wang X, Zhao X D, Liu J, Li B J, Song L Y, Li W H 2019 Acta Chem. Sin. 77 1140
[18] Karolina O, Marek L 2020 Coatings 10 883
 Google Scholar
Google Scholar
[19] Cui G, Bi Z X, Zhang R Y, Liu J G, Yu X, Li Z L 2019 Chem. Eng. J. 373 104
 Google Scholar
Google Scholar
[20] Li Q, Zheng S X, Pu J B, Sun J H, Huang L F, Wang L P, Xue Q J. 2019 Phys. Chem. Chem. Phys. 21 12121
 Google Scholar
Google Scholar
[21] Sadeghi A, Neek Amal M, Berdiyorov G R, Peeters F M 2015 Phys. Rev. B 91 014304
 Google Scholar
Google Scholar
[22] Shen L, Li Y, Zhao W J, Wang K, Ci X J, Wu Y M, Liu G, Liu C, Fang Z W 2020 J. Mater. Sci. Technol. 44 121
 Google Scholar
Google Scholar
[23] 窦宝捷, 付英奎, 高秀磊, 张颖君, 林修洲, 王兆华, 马兵, 方治文 2020 表面技术 49 241
Dou B J, Fu Y K, Gao X L, Zhang Y J, Lin X Z, Wang Z H, Ma B, Fang Z W 2020 Surf. Tech. 49 241
[24] Wu Y M, Jiang F W, Qiang Y J, Zhao W J 2021 Carbon 176 39
 Google Scholar
Google Scholar
[25] Yao W J, Zhou S G, Wang Z X, Lu Z B, Hou C J 2020 Appl. Surf. Sci. 499 143962
 Google Scholar
Google Scholar
[26] Widjaja H, Jiang Z T, Altarawneh M, Yin C Y, Goh B M, Mondinos N, Amri A, Dlugogorski B Z 2016 Appl. Surf. Sci. 373 65
 Google Scholar
Google Scholar
[27] Robinson J T, Burgess J S, Junkermeier C E, Badescu S C, Reinecke T L, Perkins F K, Zalalutdniov M K, Baldwin J W, Culbertson J C, Sheehan P E, Snow E S 2010 Nano Lett. 10 3001
 Google Scholar
Google Scholar
[28] Zbořil R, Karlický F, Bourlinos A B, Steriotis T A, Stubos A K, Georgakilas V, Šafářová K, Jančík D, Trapalis C, Otyepka M 2010 Small 6 2885
 Google Scholar
Google Scholar
[29] Samarakoon D K, Chen Z F, Nicolas C, Wang X Q 2011 Small 7 965
 Google Scholar
Google Scholar
[30] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[31] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[32] Perdew J P, Wang Y 1992 Phys. Rev. B 45 13244
 Google Scholar
Google Scholar
[33] Lee K, Murray É D, Kong L, Lundqvist B I, Langreth D C 2010 Phys. Rev. B 82 081101
 Google Scholar
Google Scholar
[34] Henkelman G, Jónsson H 2000 J. Chem. Phys. 113 9978
 Google Scholar
Google Scholar
[35] 吴迪, 杨永, 章小峰, 黄贞益, 王昭东 2022 71 086301
 Google Scholar
Google Scholar
Wu D, Yang Y, Zhang X F, Huang Z Y, Wang Z D 2022 Acta Phys. Sin. 71 086301
 Google Scholar
Google Scholar
[36] Chronopoulos D D, Bakandritsos A, Pykal M, Zbořil R, Otyepka M 2017 Appl. Mater. Today 9 60
[37] Li Y F, Pantoja B A, Chen Z F. 2014 J. Chem. Theory. Comput. 10 1265
 Google Scholar
Google Scholar
[38] 余金中, 王杏华 2001 物理 3 169
 Google Scholar
Google Scholar
Yu J Z, Wang X H 2001 Physics 3 169
 Google Scholar
Google Scholar
[39] Deringer V L, Tchougréeff A L, Dronskowski R 2011 J. Phys. Chem. A 115 5461
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 7708
- PDF Downloads: 105
- Cited By: 0















 DownLoad:
DownLoad: