-
Transition metal oxides have been a research hotspot for basic scientific research and frontier applications. Owing to the presence of d
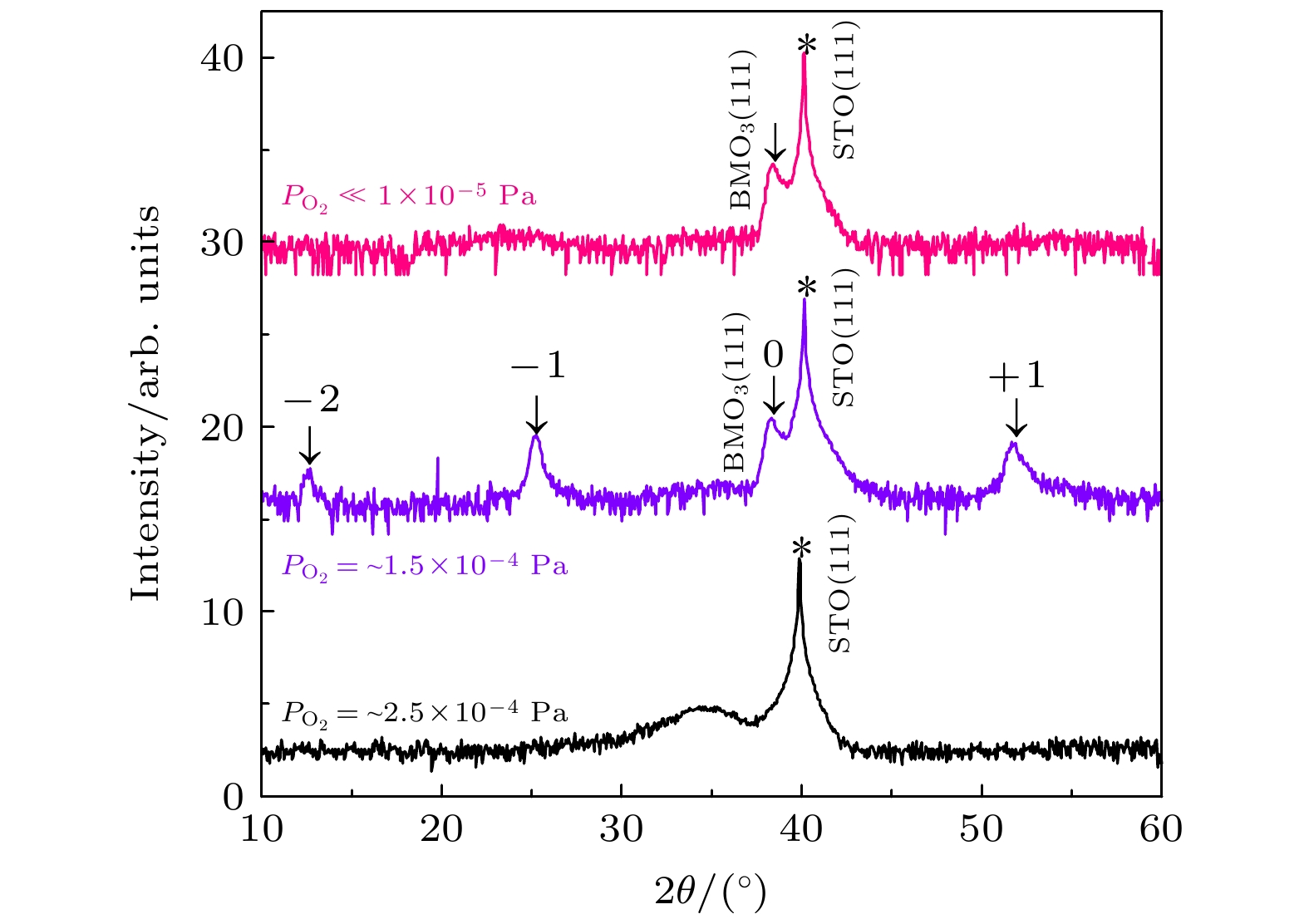
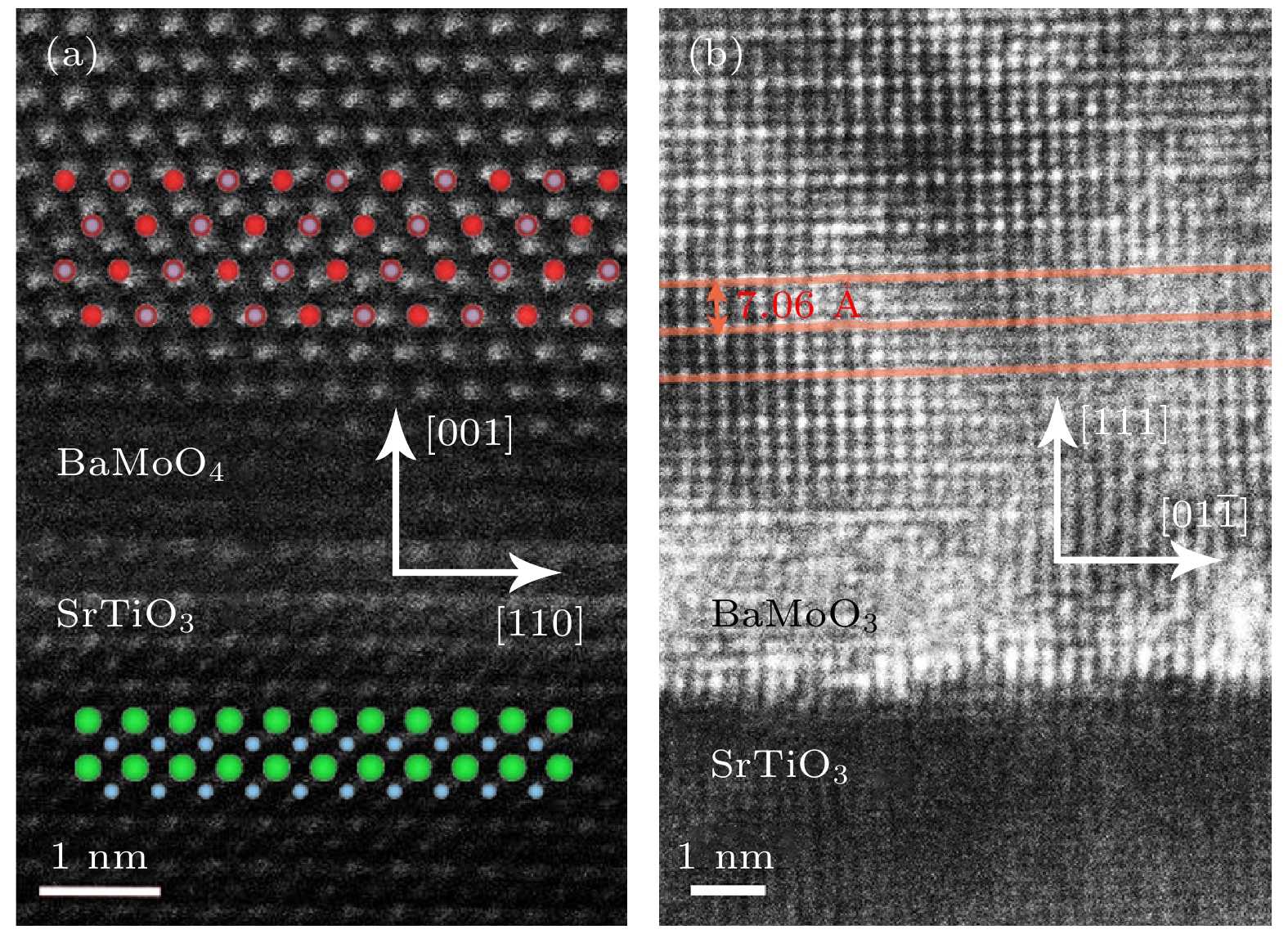
electrons and strong electron correlation, a wealth of physical phenomena emerges in the transition metal oxide family. In particular, extremely fruitful research progress is achieved in a 3d orbital elemental system. In comparison, the 4d transition metal oxides need more attention. Molybdate has excellent optical and electrical properties. Among AMoO3 (A = Ca, Sr, Ba), only BaMoO3 has not been reported for epitaxial films to date. In this work, high-quality epitaxial films of BaMoO3 and BaMoO4 are prepared by using the pulsed laser deposition. We conduct the oxygen partial pressure modulation experiments and the results show that the growth of BaMoO3 is sensitive to oxygen partial pressure. Also, BaMoO3 has a geometrically similar lattice structure to BaMoO4, and there exists epitaxial competition between BaMoO3 and BaMoO4. These two points make the preparation of epitaxial BaMoO3 films more challenging. The key to the preparation of epitaxial BaMoO3 thin films is the reduced laser target material, high vacuum environment, and ultra-low oxygen partial pressure. The epitaxy competition can be avoided by using the SrTiO3 (111) substrate. We conduct oxygen partial pressure modulation experiments on a narrow scale and reveal a self-assembled superlattice of epitaxial BaMoO3 film on a SrTiO3(111) substrate. Both the satellite peaks in the XRD pattern and the HRTEM results indicate the superlattice period of about 7.04 Å. The oxygen partial pressure is the only parameter that regulates this phenomenon, so we presume that the essence of the self-assembled superlattice is periodic oxygen-induced lattice defects. Finally, electrical transport characterization experiments are conducted on representative BaMoO3 films. The $\rho \text{-} T$ curve measurements and fitting results show that the epitaxial BaMoO3 films on SrTiO3(001) substrates have better conductivities. The electrical transport properties of BaMoO3 films grown on SrTiO3(111) substrates are dominated by electron-phonon scattering, and BaMoO3 films grown on SrTiO3(001) substrate have stronger electron-electron scattering interactions. The resistivity of the self-assembled superlattice BaMoO3 films is relatively high and electron-electron scattering plays an important role in determining the electrical transport property.-
Keywords:
- pulsed laser deposition /
- BaMoO3 /
- BaMoO4 /
- epitaxial thin films
[1] Cox P A 2010 Transition Metal Oxides: an Introduction to Their Electronic Structure and Properties (Vol. 27) (Oxford: Oxford University Press) pp1–35
[2] Maeno Y, Hashimoto H, Yoshida K, Nishizaki S, Fujita T, Bednorz J, Lichtenberg F 1994 Nature 372 532
 Google Scholar
Google Scholar
[3] Nakatsuji S, Ikeda S I, Maeno Y 1997 J. Phys. Soc. Jpn. 66 1868
 Google Scholar
Google Scholar
[4] Zhang J, McLeod A S, Han Q, Chen X, Bechtel H A, Yao Z, Corder S G, Ciavatti T, Tao T H, Aronson M 2019 Phys. Rev. X 9 011032
[5] Nobukane H, Yanagihara K, Kunisada Y, Ogasawara Y, Isono K, Nomura K, Tanahashi K, Nomura T, Akiyama T, Tanda S 2020 Sci. Rep. 10 3462
[6] He T, Cava R 2001 Phys. Rev. B 63 172403
 Google Scholar
Google Scholar
[7] Radetinac A, Mani A, Melnyk S, Nikfalazar M, Ziegler J, Zheng Y, Jakoby R, Alff L, Komissinskiy P 2014 Appl. Phys. Lett. 105 114108
[8] Mizoguchi H, Fukumi K, Kitamura N, Takeuchi T, Hayakawa J, Yamanaka H, Yanagi H, Hosono H, Kawazoe H 1999 J. Appl. Phys. 85 6502
[9] Nagai I, Shirakawa N, Ikeda S I, Iwasaki R, Nishimura H, Kosaka M 2005 Appl. Phys. Lett. 87 024105
 Google Scholar
Google Scholar
[10] Radetinac A, Takahashi K S, Alff L, Kawasaki M, Tokura Y 2010 Appl. Phys. Express 3 073003
[11] Ha Y, Lee S 2020 Adv. Funct. Mater. 30 2001489
 Google Scholar
Google Scholar
[12] Hayashi S, Aoki R, Nakamura T 1979 Mater. Res. Bull. 14 409
[13] Kurosaki K, Oyama T, Muta H, Uno M, Yamanaka S 2004 J. Alloys Compd. 372 65
 Google Scholar
Google Scholar
[14] Ma T, Jacobs R, Booske J, Morgan D 2021 J. Mater. Chem. C 9 12778
[15] Panchal V, Garg N, Sharma S M 2006 J. Phys. Condens. Matter 18 3917
 Google Scholar
Google Scholar
[16] Guo D, Yang Q, Hua H, Hu C 2014 J. Phys. Chem. C 118 13826
 Google Scholar
Google Scholar
[17] Ray S K, Kshetri Y K, Lee S W 2019 Nanotechnology 30 454002
 Google Scholar
Google Scholar
[18] Marques A P A, Picon F C, Melo D, Pizani P S, Leite E R, Varela J A, Longo E 2008 J. Fluoresc. 18 51
 Google Scholar
Google Scholar
[19] Kamata K, Nakamura T, Sata T 1975 Mater. Res. Bull. 10 373
[20] Hegde M 2001 J. Chem. Sci. 113 445
 Google Scholar
Google Scholar
[21] Nassif V, Carbonio R E, Alonso J A 1999 J. Solid State Chem. 146 266
[22] Ting X W, Chun Y, Ping H, Guo P Z, Yan R L 2009 Chin. J. Inorg. Chem. 25 1414
-
图 1 (a) 氧分压调制样品的沿着STO[00L]
$\theta\text{—}2\theta$ 联动扫描XRD结果; (b) 沿界面法向观察的STO (001)上外延生长BaMoO3薄膜示意图; (c) STO (001)上外延生长BaMoO3薄膜的立体示意图; (d) 沿界面法向观察的STO (001)上外延生长BaMoO4薄膜示意图; (e) STO (001)上外延生长BaMoO4薄膜的立体示意图Figure 1. (a) Out of plane
$\theta-2\theta$ scan along STO [00L] of samples which were prepared at different oxygen partial pressure; (b) schematic diagram of epitaxial BaMoO3 film on STO (001) substrate observed along normal direction of the heterointerface; (c) three-dimensional schematic diagram of epitaxial BaMoO3 film on STO (001) substrate; (d) schematic diagram of epitaxial BaMoO4 film on STO (001) substrate observed along normal direction of heterointerface; (e) three-dimensional schematic diagram of epitaxial BaMoO4 film on STO (001) substrate.图 2 (a) 高质量BaMoO3和 (b) BaMoO4外延膜沿STO [00L]
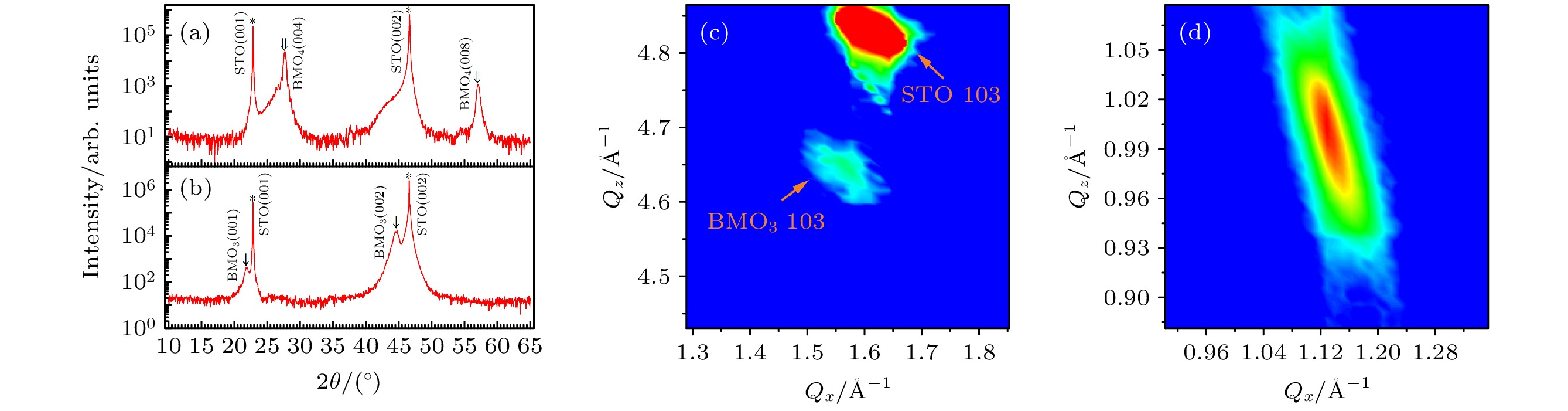
$\theta\text{—}2\theta$ 联动扫描XRD结果; (c) BaMoO3在STO (103)倒易点附近的RSM图谱; (d) BaMoO4 (112)倒易点附近的RSM图谱Figure 2. Out of plane
$\theta-2\theta$ scan along STO [00L] of high quality (a) BaMoO3 and (b) BaMoO4 films; (c) reciprocal space mapping of BaMoO3 near the STO (103); (d) reciprocal space mapping of BaMoO4 (112)图 5 BaMoO3薄膜的
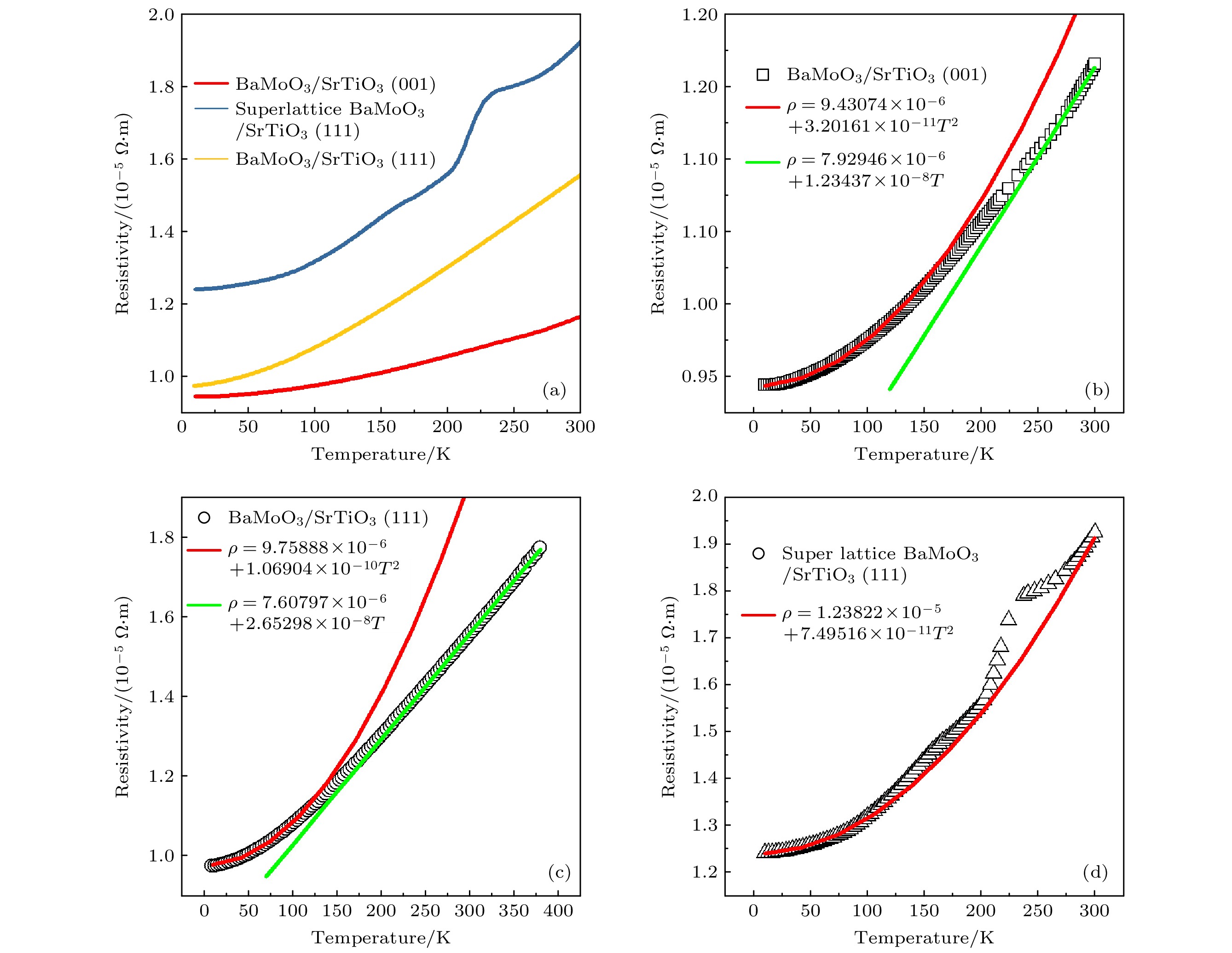
$\rho \text{-}T$ 曲线测量与拟合结果 (a) BaMoO3/STO(001), BaMoO3/STO(111)和自组装超晶格BaMoO3/STO(111)的$\rho \text{-} T$ 曲线测量结果; (b) BaMoO3/STO(001), (c) BaMoO3/STO(111)和(d)自组装超晶格BaMoO3/STO(111)的$\rho \text{-} T$ 曲线拟合结果Figure 5.
$\rho \text{-} T$ curve measurement and fitting results of BaMoO3 films. (a)$\rho \text{-} T$ curve measurement results of BaMoO3/STO (001), BaMoO3/STO (111), and self-assembled superlattice BaMoO3/STO(111);$\rho \text{-} T$ curve fitting results of (b) BaMoO3/STO (001), (c) BaMoO3/STO (111), and (d) self-assembled superlattice BaMoO3/STO(111)表 1 外延BaMoO3和BaMoO4最优生长条件
Table 1. Optimal growth conditions for epitaxial BaMoO3 and BaMoO4 thin film.
主要参数 BaMoO3薄膜 BaMoO4薄膜 衬底温度/℃ 650 600 激光波长/nm 248 248 聚焦后激光能量密度/(J·cm–2) 1.2 1.5 激光频率/Hz 2 2 靶材衬底距离/mm 65 65 氧分压/Pa $\ll 3\times 10^{-5}$ $2.2\times 10^{-3}$ -
[1] Cox P A 2010 Transition Metal Oxides: an Introduction to Their Electronic Structure and Properties (Vol. 27) (Oxford: Oxford University Press) pp1–35
[2] Maeno Y, Hashimoto H, Yoshida K, Nishizaki S, Fujita T, Bednorz J, Lichtenberg F 1994 Nature 372 532
 Google Scholar
Google Scholar
[3] Nakatsuji S, Ikeda S I, Maeno Y 1997 J. Phys. Soc. Jpn. 66 1868
 Google Scholar
Google Scholar
[4] Zhang J, McLeod A S, Han Q, Chen X, Bechtel H A, Yao Z, Corder S G, Ciavatti T, Tao T H, Aronson M 2019 Phys. Rev. X 9 011032
[5] Nobukane H, Yanagihara K, Kunisada Y, Ogasawara Y, Isono K, Nomura K, Tanahashi K, Nomura T, Akiyama T, Tanda S 2020 Sci. Rep. 10 3462
[6] He T, Cava R 2001 Phys. Rev. B 63 172403
 Google Scholar
Google Scholar
[7] Radetinac A, Mani A, Melnyk S, Nikfalazar M, Ziegler J, Zheng Y, Jakoby R, Alff L, Komissinskiy P 2014 Appl. Phys. Lett. 105 114108
[8] Mizoguchi H, Fukumi K, Kitamura N, Takeuchi T, Hayakawa J, Yamanaka H, Yanagi H, Hosono H, Kawazoe H 1999 J. Appl. Phys. 85 6502
[9] Nagai I, Shirakawa N, Ikeda S I, Iwasaki R, Nishimura H, Kosaka M 2005 Appl. Phys. Lett. 87 024105
 Google Scholar
Google Scholar
[10] Radetinac A, Takahashi K S, Alff L, Kawasaki M, Tokura Y 2010 Appl. Phys. Express 3 073003
[11] Ha Y, Lee S 2020 Adv. Funct. Mater. 30 2001489
 Google Scholar
Google Scholar
[12] Hayashi S, Aoki R, Nakamura T 1979 Mater. Res. Bull. 14 409
[13] Kurosaki K, Oyama T, Muta H, Uno M, Yamanaka S 2004 J. Alloys Compd. 372 65
 Google Scholar
Google Scholar
[14] Ma T, Jacobs R, Booske J, Morgan D 2021 J. Mater. Chem. C 9 12778
[15] Panchal V, Garg N, Sharma S M 2006 J. Phys. Condens. Matter 18 3917
 Google Scholar
Google Scholar
[16] Guo D, Yang Q, Hua H, Hu C 2014 J. Phys. Chem. C 118 13826
 Google Scholar
Google Scholar
[17] Ray S K, Kshetri Y K, Lee S W 2019 Nanotechnology 30 454002
 Google Scholar
Google Scholar
[18] Marques A P A, Picon F C, Melo D, Pizani P S, Leite E R, Varela J A, Longo E 2008 J. Fluoresc. 18 51
 Google Scholar
Google Scholar
[19] Kamata K, Nakamura T, Sata T 1975 Mater. Res. Bull. 10 373
[20] Hegde M 2001 J. Chem. Sci. 113 445
 Google Scholar
Google Scholar
[21] Nassif V, Carbonio R E, Alonso J A 1999 J. Solid State Chem. 146 266
[22] Ting X W, Chun Y, Ping H, Guo P Z, Yan R L 2009 Chin. J. Inorg. Chem. 25 1414
Catalog
Metrics
- Abstract views: 5916
- PDF Downloads: 97
- Cited By: 0


















 DownLoad:
DownLoad: