-
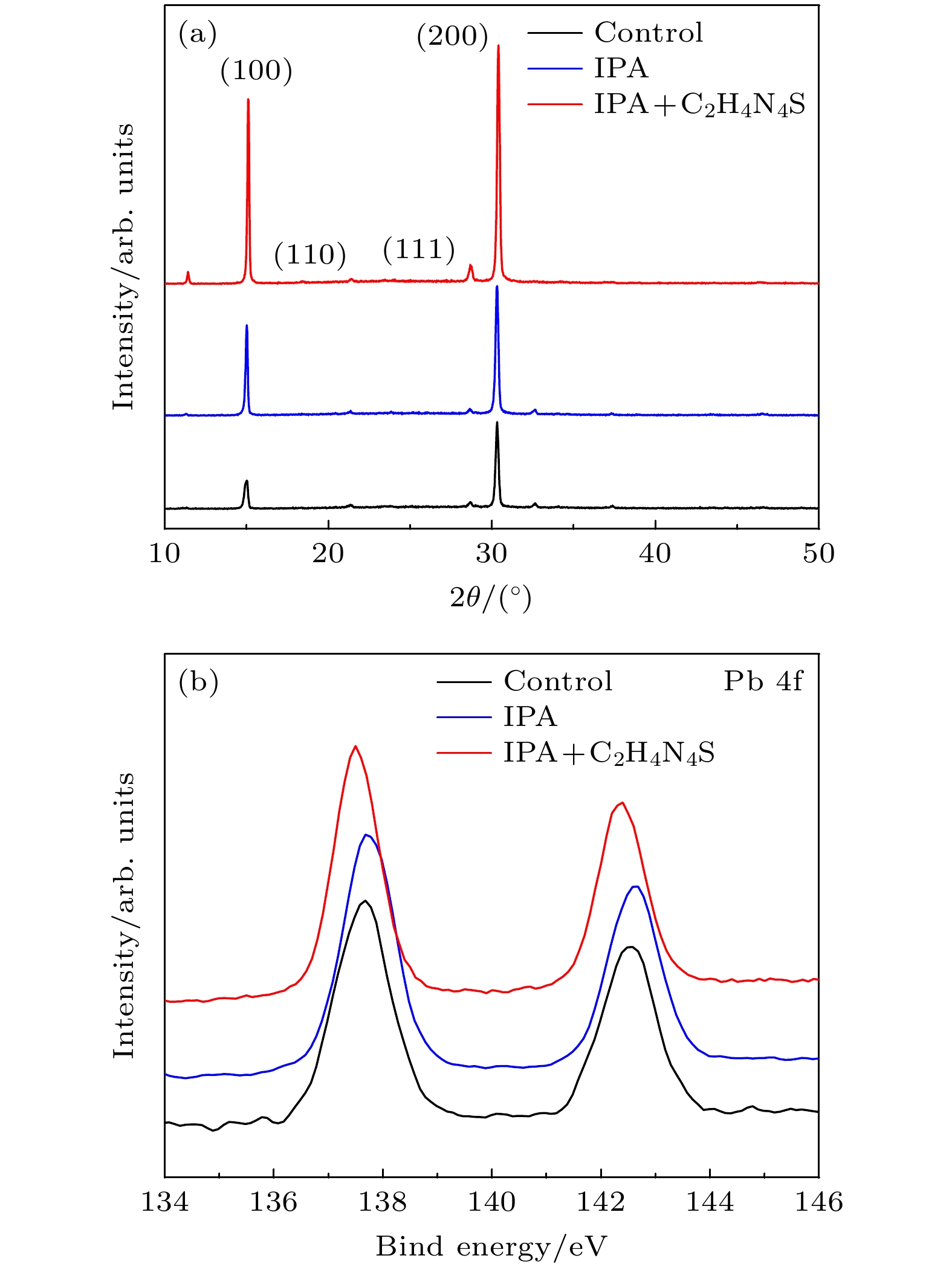
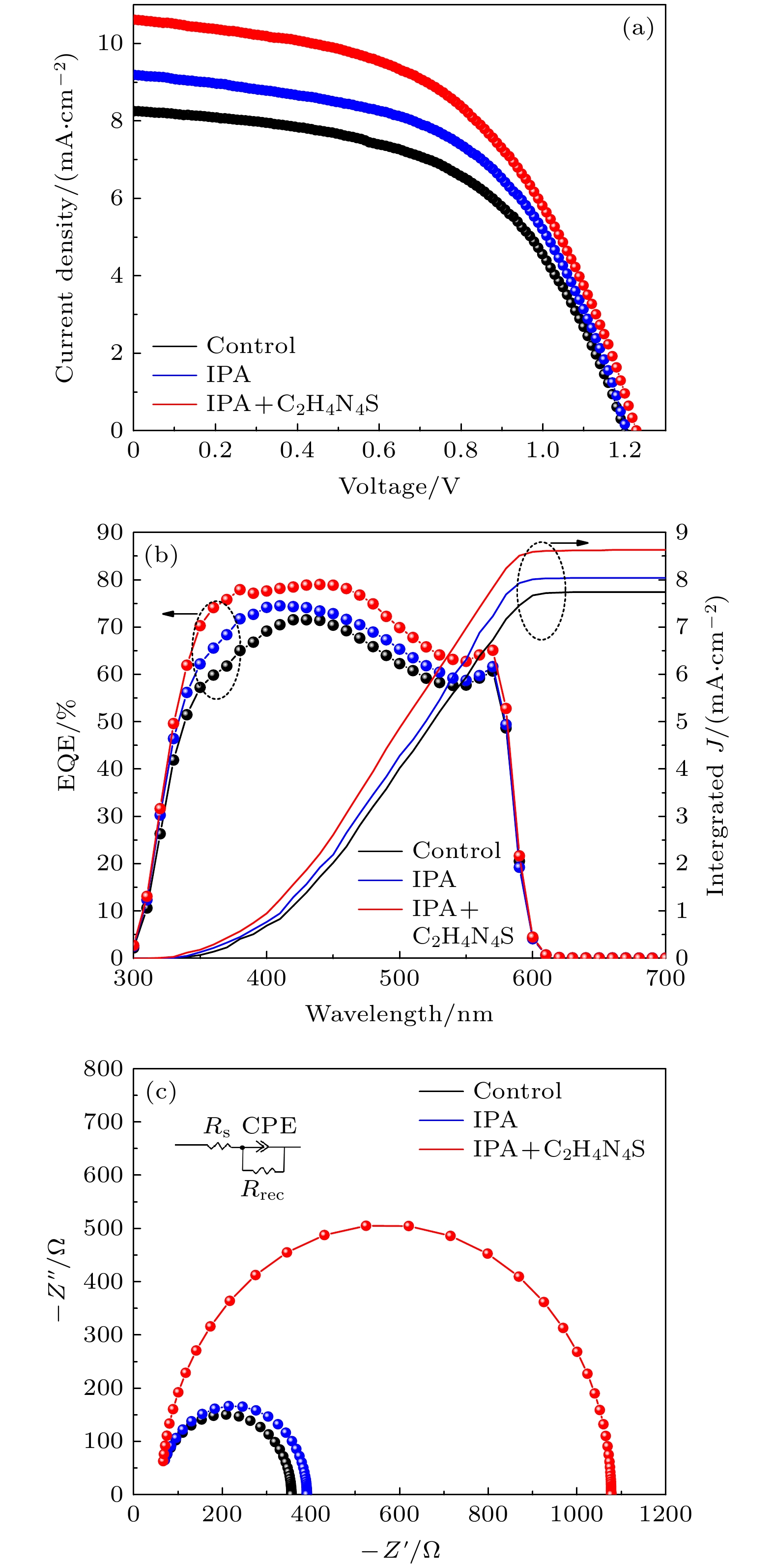
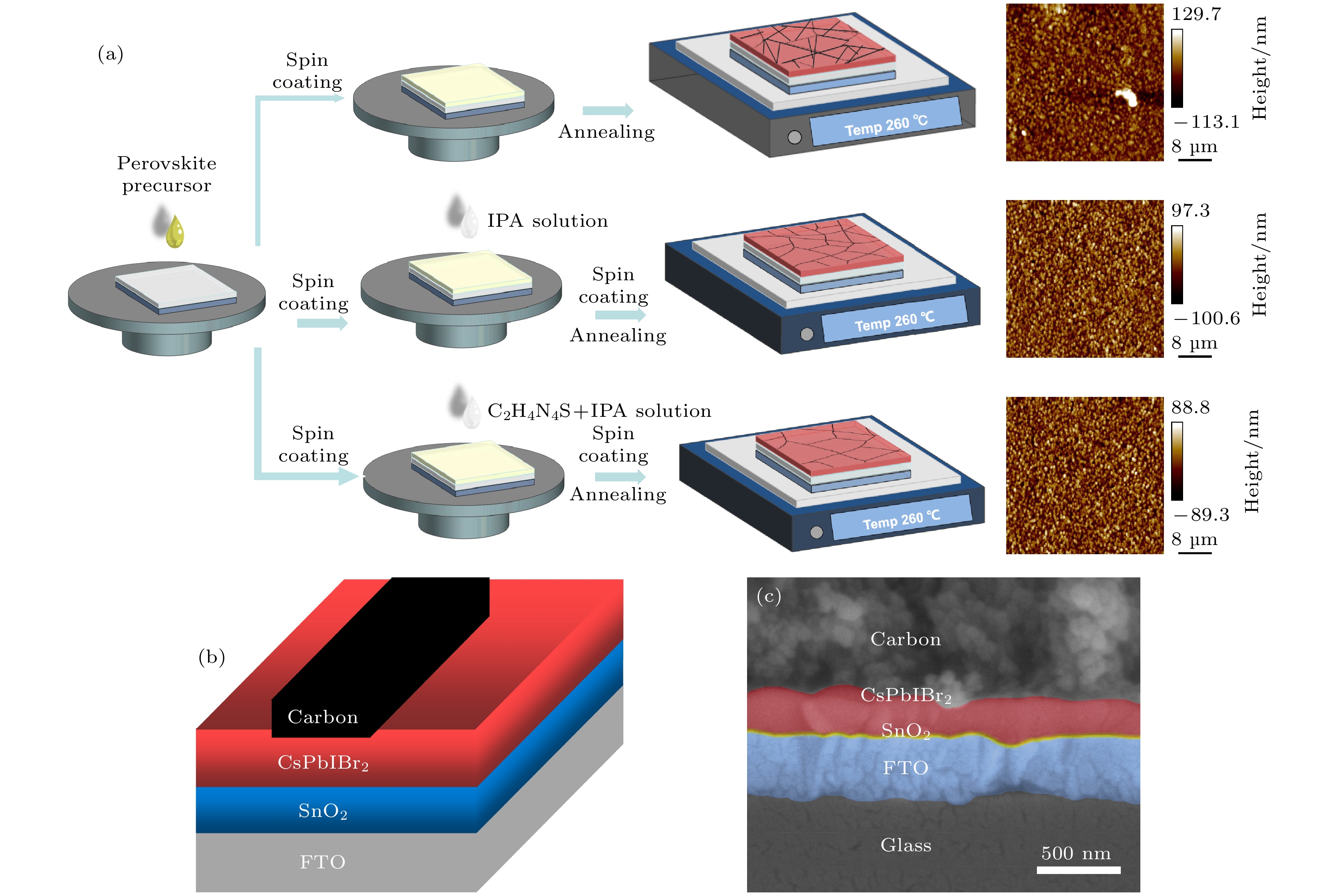
All-inorganic perovskite has attracted extensive attention due to its photovoltaic properties and stability. Typically, the α-phase CsPbI3 has an ideal bandgap of 1.73 eV suitable for the construction of high performance inorganic PSCs. But it suffers phase instability under ambient condition because of the unsatisfactory tolerance factor. By incorporating Br atoms into the perovskite structure, can greatly enhance the phase stability can be greatly enhanced. For example, CsPbBr3 shows an excellent ambient stability and a wide bandgap of 2.3 eV that results in a limited light absorbtion. With the consideration from the unified perspective of the bandgap and the ambient phase stability, CsPbIBr2 has a relatively appropriate bandgap (2.05 eV) and higher stability than CsPbI3 and CsPbI2Br, which is made a good option for stable and efficient PSCs. However, there exist numerous defects on the CsPbIBr2 film prepared by conventional one-step deposition method, which seriously affect the photoelectric conversion efficiency (PCE) of perovskite solar cells (PSCs). Considering the short dripping time and poor reproducibility of conventional anti-solvent technology, a precursor film preparation process is proposed to fabricate efficient and stable carbon-based CsPbIBr2 perovskite solar cells. Using isopropyl alcohol (IPA) as the anti-solvent, the nucleation position of perovskite can be adjusted by regulating the evaporation rate of DMSO in the precursor film. In addition, guanidine thiocyanate (C2H4N4S) is added into IPA solution as a passivator to regulate the nucleation and crystallization process of perovskite. The carboxylic acid group of C2H4N4S can crosslink to Pb2+ of CsPbIBr2 via a chelating interaction, resulting in the easier decomposition of the CsI-DMSO-PbBr2 intermediate phase in the spin-coating process of the precursor film. The amino group of C2H4N4S can also promote the crystallization and suppress the ion migration of the perovskite film through hydrogen bonds. The result shows that the compactness of the optimized CsPbIBr2 film is significantly improved and the average grain size is about 800nm. The crystallinity and grain orientation are improved, and thus achieving better carrier separation and transport efficiency. The highest PCE of carbon-based CsPbIBr2 PSC is obviously improved from 5.29% to 6.71%, i.e. increased by almost 21.16% compared with the control sample. Furthermore, the PSCs with precursor film preparation process possesses better long-term stability. The results obtained in this paper demonstrate that the new preparation technology can improve the quality of inorganic perovskite films in pure DMSO solvent system.
-
Keywords:
- CsPbIBr2 perovskite solar cell /
- precursor film preparation process /
- C2H4N4S /
- IPA
[1] Han S, Zhang H, Wang R, He Q 2021 Mat. Sci. Semicon. Proc. 127 105666
 Google Scholar
Google Scholar
[2] Boyd C C, Cheacharoen R, Leijtens T, McGehee M D 2019 Chem. Rev. 119 3418
 Google Scholar
Google Scholar
[3] https://www.nrel.gov/pv/cell-efficiency.html (8 6 2021).
[4] Bisquert J, Juarez-Perez EJ. 2019 J. Phys. Chem. Lett. 10: 5889.
[5] Chen W, Chen H, Xu G, Xue R, Wang S, Li Y, Li Y 2019 Joule 3 191
 Google Scholar
Google Scholar
[6] Mariotti S, Hutter O. S, Phillips L J, Yates P J, Kundu B, Durose K 2018 ACS Appl. Mater. Inter. 10 3750
 Google Scholar
Google Scholar
[7] Subhani W S, Wang K, Du M, Liu S F 2019 Nano Energy 61 165
 Google Scholar
Google Scholar
[8] Meng F, Liu A, Gao L, Cao J, Yan Y, Wang N, Fan M, Wei G, Ma T 2019 J. Mater. Chem. A. 7 8690
 Google Scholar
Google Scholar
[9] Zhang Z, He F, Zhu W, Chen D, Chai W, Chen D, Xi H, Zhang J, Zhang C, Hao Y 2020 Sustain. Energ. Fuels. 4 4506
 Google Scholar
Google Scholar
[10] Yang B, Wang M, Hu X, Zhou T, Zang Z 2019 Nano Energy 57 718
 Google Scholar
Google Scholar
[11] Duan J, Zhao Y, He B, Tang Q 2018 Angew. Chem. 130 3849
 Google Scholar
Google Scholar
[12] Ouedraogo N A N, Chen Y, Xiao Y Y, Meng Q, Han C B, Yan H, Zhang Y 2020 Nano Energy 67 104249
 Google Scholar
Google Scholar
[13] Steele J A, Lai M, Zhang Y, Lin Z, Hofkens J, Roeffaers B J M, Yang P. 2020 Acc. Mater. Res. 1 3
 Google Scholar
Google Scholar
[14] Duan J, Xu H, Sha W, Zhao Y, Wang Y, Yang X, Tang Q 2019 J. Mater. Chem. A. 7 21036
 Google Scholar
Google Scholar
[15] Li Z, Zhou F, Wang Q, Ding L, Jin Z 2020 Nano Energy 71 104634
 Google Scholar
Google Scholar
[16] Faheem M B, Khan B, Feng C, Farooq M U, Raziq F, Xiao Y, Li Y 2019 ACS. Energy. Lett. 5 290
[17] Wan X, Yu Z, Tian W, Huang F, Jin S, Yang X, Cheng Y, Hagfeldt A, Sun L 2020 J. Energy. Chem. 46 8
 Google Scholar
Google Scholar
[18] Guo Y, Yin X, Liu J, Wen S, Wu Y, Que W. 2019 Sol. RRL. 3 1900135
 Google Scholar
Google Scholar
[19] Yin X, Guo Y, Liu J, Que W, Ma F, Xu K 2020 J Phys. Chem. Lett. 11 7035
 Google Scholar
Google Scholar
[20] Ma Q, Huang S, Wen X, Green M A, Ho-Baillie A W Y 2016 Adv. Energy Mater. 6 1502202
 Google Scholar
Google Scholar
[21] Zhu W, Zhang Z, Chen D, Chai W, Chen D, Zhang J, Zhang C, Hao Y 2020 Nano-Micro Lett. 12 1
 Google Scholar
Google Scholar
[22] Yu B, Zhang H, Wu J, Li Y, Li H, Li Y, Shi J, Wu H, Li D, Luo Y 2018 J. Mater. Chem. A. 6 19810.
[23] Han S, Zhang H, Wang R, He Q 2021 Mat Sci. Semicon. Proc. 131 105847
 Google Scholar
Google Scholar
[24] Subhani W S, Wang K, Du M, Wang X, Liu S 2019 Adv. Energy. Mater. 9 1803785
 Google Scholar
Google Scholar
[25] Li N, Zhu Z, Li J, Jen A K Y 2018 Adv. Energy. Mater. 8 1800525
 Google Scholar
Google Scholar
[26] Luo J, Qiu R Z, Yang Z S, Wang Y X, Zhang Q F 2018 RSC. Adv. 8 724
 Google Scholar
Google Scholar
[27] Song S, Hörantner M T, Choi K, Snaith H J, Park T 2017 J. Mater. Chem. A. 5 3812
 Google Scholar
Google Scholar
[28] Wang Y, Wang K, Subhani W S, Zhang C, Jiang X, Wang S, Bao H, Liu L, Wan L, Liu S 2020 Small 16 1907283
 Google Scholar
Google Scholar
[29] Zhu W, Chai W, Deng M, Chen D, Chen D, Zhang J, Zhang C, Hao Y 2020 Electrochim. Acta. 330 135325
 Google Scholar
Google Scholar
[30] Zhang Q, Zhu W, Chen D, Zhang Z, Lin Z, Chang J, Zhang J, Zhang C, Hao Y 2019 ACS Appl. Mater. Inter. 11 2997
 Google Scholar
Google Scholar
[31] Zhu W, Zhang Q, Zhang C, Zhang Z, Chen D, Lin Z, Chang J 2018 ACS Appl Energy. Mater. 1 4991
 Google Scholar
Google Scholar
[32] Zhang B, Bi W, Wu Y, Chen C, Li H, Song Z, Dail Q, Xu L, Song H 2019 ACS Appl. Mater. Inter. 11 33868
 Google Scholar
Google Scholar
[33] Bian J, Wu Y, Bi W, Liu L, Su X, Zhang B 2020 Energ Fuel. 34 11472
 Google Scholar
Google Scholar
[34] Lu H, Liu Y, Ahlawat P, Mishra A, Tress W, Eickemeyer F, Yang Y, Fu F, Wang Z, Avalos C, Carlsen B, Agarwalla A, Zhang X, Li X, Zhan Y, Zakeeruddin S, Emsley L, Rothlisberger U, Zheng L, Hagfeldt A, Grätzel M 2020 Science. 370 6512
[35] Abdelsamie M, Li T, Babbe F, Xu J, Han Q, Blum V, M Sutter-Fella C, B Mitzi D, Toney M F 2021 ACS Appl. Mater. Inter. 13 13212
 Google Scholar
Google Scholar
-
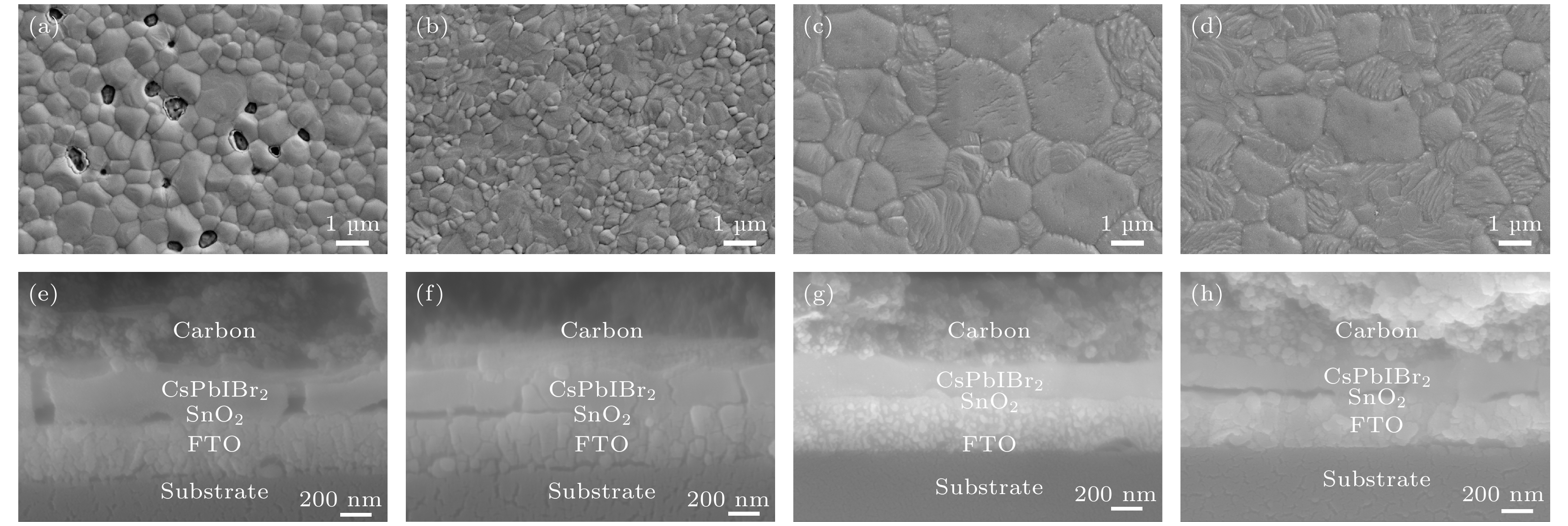
图 3 不同制备工艺下CsPbIBr2薄膜表面SEM图 (a) 传统方法; (b) IPA处理; (c) IPA处理、C2H4N4S钝化(0.4 mg/ml); (d) IPA处理、C2H4N4S钝化(0.8 mg/ml); 对应器件截面SEM图 (e−h)
Figure 3. SEM images of CsPbIBr2 film under different preparation processes: (a) Conventional method; (b) adding IPA solution; (c) adding IPA solution with 0.4 mg/ml of C2H4N4S; (d) adding IPA solution with 0.8 mg/ml of C2H4N4S; (e−h) corresponding crosssectional SEM images.
表 1 不同C2H4N4S浓度下钙钛矿电池性能指标
Table 1. Photovoltaic Parameters of PSCs based on different concentration of C2H4N4S
Perovskite Jsc/mA cm–2 Voc/V FF/% PCE/% Control 8.25 1.19 53.52 5.29 IPA 9.19 1.21 53.77 5.95 IPA+0.2 mg/mL C2H4N4S 10.32 1.21 50.10 6.24 IPA+0.4 mg/mL C2H4N4S 10.61 1.23 52.40 6.71 IPA+0.6 mg/mL C2H4N4S 10.89 1.19 50.12 6.38 IPA+0.8 mg/mL C2H4N4S 10.30 1.19 49.42 6.07 注: FF即填充因子(fill factor); PCE即光电转换效率(photoelectric conversion efficiency). -
[1] Han S, Zhang H, Wang R, He Q 2021 Mat. Sci. Semicon. Proc. 127 105666
 Google Scholar
Google Scholar
[2] Boyd C C, Cheacharoen R, Leijtens T, McGehee M D 2019 Chem. Rev. 119 3418
 Google Scholar
Google Scholar
[3] https://www.nrel.gov/pv/cell-efficiency.html (8 6 2021).
[4] Bisquert J, Juarez-Perez EJ. 2019 J. Phys. Chem. Lett. 10: 5889.
[5] Chen W, Chen H, Xu G, Xue R, Wang S, Li Y, Li Y 2019 Joule 3 191
 Google Scholar
Google Scholar
[6] Mariotti S, Hutter O. S, Phillips L J, Yates P J, Kundu B, Durose K 2018 ACS Appl. Mater. Inter. 10 3750
 Google Scholar
Google Scholar
[7] Subhani W S, Wang K, Du M, Liu S F 2019 Nano Energy 61 165
 Google Scholar
Google Scholar
[8] Meng F, Liu A, Gao L, Cao J, Yan Y, Wang N, Fan M, Wei G, Ma T 2019 J. Mater. Chem. A. 7 8690
 Google Scholar
Google Scholar
[9] Zhang Z, He F, Zhu W, Chen D, Chai W, Chen D, Xi H, Zhang J, Zhang C, Hao Y 2020 Sustain. Energ. Fuels. 4 4506
 Google Scholar
Google Scholar
[10] Yang B, Wang M, Hu X, Zhou T, Zang Z 2019 Nano Energy 57 718
 Google Scholar
Google Scholar
[11] Duan J, Zhao Y, He B, Tang Q 2018 Angew. Chem. 130 3849
 Google Scholar
Google Scholar
[12] Ouedraogo N A N, Chen Y, Xiao Y Y, Meng Q, Han C B, Yan H, Zhang Y 2020 Nano Energy 67 104249
 Google Scholar
Google Scholar
[13] Steele J A, Lai M, Zhang Y, Lin Z, Hofkens J, Roeffaers B J M, Yang P. 2020 Acc. Mater. Res. 1 3
 Google Scholar
Google Scholar
[14] Duan J, Xu H, Sha W, Zhao Y, Wang Y, Yang X, Tang Q 2019 J. Mater. Chem. A. 7 21036
 Google Scholar
Google Scholar
[15] Li Z, Zhou F, Wang Q, Ding L, Jin Z 2020 Nano Energy 71 104634
 Google Scholar
Google Scholar
[16] Faheem M B, Khan B, Feng C, Farooq M U, Raziq F, Xiao Y, Li Y 2019 ACS. Energy. Lett. 5 290
[17] Wan X, Yu Z, Tian W, Huang F, Jin S, Yang X, Cheng Y, Hagfeldt A, Sun L 2020 J. Energy. Chem. 46 8
 Google Scholar
Google Scholar
[18] Guo Y, Yin X, Liu J, Wen S, Wu Y, Que W. 2019 Sol. RRL. 3 1900135
 Google Scholar
Google Scholar
[19] Yin X, Guo Y, Liu J, Que W, Ma F, Xu K 2020 J Phys. Chem. Lett. 11 7035
 Google Scholar
Google Scholar
[20] Ma Q, Huang S, Wen X, Green M A, Ho-Baillie A W Y 2016 Adv. Energy Mater. 6 1502202
 Google Scholar
Google Scholar
[21] Zhu W, Zhang Z, Chen D, Chai W, Chen D, Zhang J, Zhang C, Hao Y 2020 Nano-Micro Lett. 12 1
 Google Scholar
Google Scholar
[22] Yu B, Zhang H, Wu J, Li Y, Li H, Li Y, Shi J, Wu H, Li D, Luo Y 2018 J. Mater. Chem. A. 6 19810.
[23] Han S, Zhang H, Wang R, He Q 2021 Mat Sci. Semicon. Proc. 131 105847
 Google Scholar
Google Scholar
[24] Subhani W S, Wang K, Du M, Wang X, Liu S 2019 Adv. Energy. Mater. 9 1803785
 Google Scholar
Google Scholar
[25] Li N, Zhu Z, Li J, Jen A K Y 2018 Adv. Energy. Mater. 8 1800525
 Google Scholar
Google Scholar
[26] Luo J, Qiu R Z, Yang Z S, Wang Y X, Zhang Q F 2018 RSC. Adv. 8 724
 Google Scholar
Google Scholar
[27] Song S, Hörantner M T, Choi K, Snaith H J, Park T 2017 J. Mater. Chem. A. 5 3812
 Google Scholar
Google Scholar
[28] Wang Y, Wang K, Subhani W S, Zhang C, Jiang X, Wang S, Bao H, Liu L, Wan L, Liu S 2020 Small 16 1907283
 Google Scholar
Google Scholar
[29] Zhu W, Chai W, Deng M, Chen D, Chen D, Zhang J, Zhang C, Hao Y 2020 Electrochim. Acta. 330 135325
 Google Scholar
Google Scholar
[30] Zhang Q, Zhu W, Chen D, Zhang Z, Lin Z, Chang J, Zhang J, Zhang C, Hao Y 2019 ACS Appl. Mater. Inter. 11 2997
 Google Scholar
Google Scholar
[31] Zhu W, Zhang Q, Zhang C, Zhang Z, Chen D, Lin Z, Chang J 2018 ACS Appl Energy. Mater. 1 4991
 Google Scholar
Google Scholar
[32] Zhang B, Bi W, Wu Y, Chen C, Li H, Song Z, Dail Q, Xu L, Song H 2019 ACS Appl. Mater. Inter. 11 33868
 Google Scholar
Google Scholar
[33] Bian J, Wu Y, Bi W, Liu L, Su X, Zhang B 2020 Energ Fuel. 34 11472
 Google Scholar
Google Scholar
[34] Lu H, Liu Y, Ahlawat P, Mishra A, Tress W, Eickemeyer F, Yang Y, Fu F, Wang Z, Avalos C, Carlsen B, Agarwalla A, Zhang X, Li X, Zhan Y, Zakeeruddin S, Emsley L, Rothlisberger U, Zheng L, Hagfeldt A, Grätzel M 2020 Science. 370 6512
[35] Abdelsamie M, Li T, Babbe F, Xu J, Han Q, Blum V, M Sutter-Fella C, B Mitzi D, Toney M F 2021 ACS Appl. Mater. Inter. 13 13212
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 8162
- PDF Downloads: 118
- Cited By: 0














 DownLoad:
DownLoad: