-
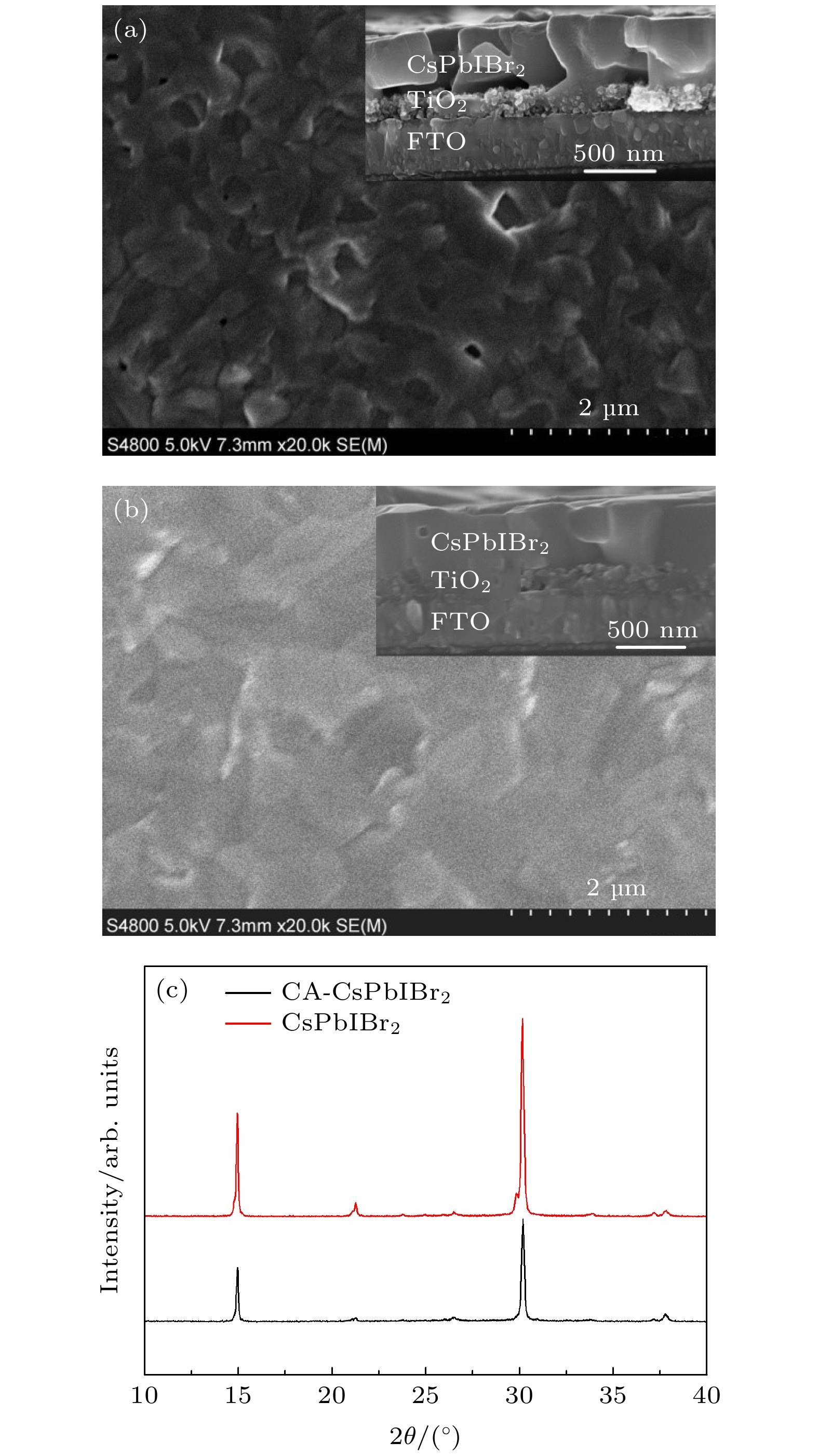
CsPbIBr2 perovskite has been considered as a promising candidate for the light-harvesting material of perovskite solar cells (PSCs) due to its acceptable band gap and high stability. Nevertheless, the efficiency of CsPbIBr2-based PSC still lags behind that of its homologs and is far away from the theoretical value. This can be attributed to the poor quality of CsPbIBr2 perovskite film. Therefore, it is highly desirable to improve the quality of CsPbIBr2 perovskite film for enhancing the photovoltaic performance of CsPbIBr2 PSCs. In this work, cellulose acetate (CA) is used as a polymer additive that is introduced into CsPbIBr2 precursor solution for improving the quality of CsPbIBr2 perovskite film via controlling crystallization process. The interaction between the C=O group of CA and Pb2+ in the precursor solution and the enhanced viscosity of precursor solution induced by CA addition reduce the crystallization rate of CsPbIBr2 perovskite. As a result, a compact CsPbIBr2 perovskite film with high crystallinity, large grain size, and low density of defect is prepared. The remarkably improved quality of CsPbIBr2 perovskite film upon CA addition can be attributed to the relatively slow crystallization rate. The slow crystallization rate allows the perovskite film to have enough time to form perfect perovskite crystal structure with large-size crystal grain and low density of defects. Furthermore, the oxygen functional groups of CA can passivate the undercoordinated Pb2+, which effectively suppresses the defects and traps induced by Pb2+ in CsPbIBr2 perovskite film. The stability of CsPbIBr2 perovskite film is also greatly improved by CA addition. The added CA does not participate into the CsPbIBr2 perovskite crystal but distributes at the grain boundaries and, or, interfaces area and forms a moisture barrier around perovskite grains, which obviously enhances the stability of CsPbIBr2 perovskite film in the ambient air. The carbon-based CsPbIBr2 perovskite solar cells with a configuration of FTO/TiO2/perovskite film/ carbon are fabricated by using the carbon layer as both the hole-transport layer and the back electrode. Under the illumination of 100 mW/cm2, the PSC based on CA-CsPbIBr2 perovskite film delivers a high conversion efficiency of 7.52%, which is increased by 40% compared with the efficiency of the device based on the pure CsPbIBr2 perovskite film. In addition, the PSC based on CA-CsPbIBr2 perovskite film shows a hysteresis index (HI) of 7%, while the device based on pure CsPbIBr2 film displays a higher HI of 22%. This result demonstrates that the CA addition can effectively suppress the hysteresis effect of inorganic PSCs. The stability of the PSC based on CA-CsPbIBr2 perovskite film is investigated by tracking the variation of the efficiency with time in the ambient condition. The fabricated PSCs without any encapsulation are stored in the air. The photovoltaic performance is measured once a day. The efficiency of the PSC based on CA-CsPbIBr2 perovskite remains more than 90% of its initial value after being stored in the air for 800 h, showing an excellent long-term stability. Therefore, this work provides a facile and effective method of improving the quality of CsPbIBr2 perovskite films, which is expected to be helpful in developing high-efficiency and stable carbon-based inorganic PSCs. -
Keywords:
- cellulose acetate /
- CsPbIBr2 perovskite /
- stability /
- perovskite solar cells
[1] Kojima A, Teshima K, Shirai Y, Miyasaka T 2009 J. Am. Chem. Soc. 131 6050
 Google Scholar
Google Scholar
[2] Ono L K, Qi Y 2016 J. Phys. Chem. Lett. 7 4764
 Google Scholar
Google Scholar
[3] 姚鑫, 丁艳丽, 张晓丹, 赵颖 2015 64 038805
 Google Scholar
Google Scholar
Yao X, Ding Y L, Zhang X D, Zhao Y 2015 Acta Phys. Sin. 64 038805
 Google Scholar
Google Scholar
[4] Deng X, Xie L, Wang S 2020 Chem. Eng. J. 398 125594
 Google Scholar
Google Scholar
[5] Huang Y, Liu T, Liang C 2020 Adv. Funct. Mater. 30 2000863
 Google Scholar
Google Scholar
[6] Jia X, Zho C, Tao S 2019 Sci. Bull. 64 1532
 Google Scholar
Google Scholar
[7] Tai Q, Tang K, Yan F 2019 Energy Environ. Sci. 12 2375
 Google Scholar
Google Scholar
[8] Li B, Fu L, Li S, Pan L, Wang L, Yin L 2019 J. Mater. Chem. A 7 20494
 Google Scholar
Google Scholar
[9] Wang G, Lei M, Liu J, Zhang W, He Q 2020 Solar RRL 4 2000528
 Google Scholar
Google Scholar
[10] Duan J, Xu H, Sha W, Tang Q 2019 J. Mater. Chem. A 7 21036
 Google Scholar
Google Scholar
[11] Wang Y, Liu X, Zhang T, Wang X, J. Shi, Zhao Y 2019 Angew. Chem. Int. Ed. 58 16691
 Google Scholar
Google Scholar
[12] Li Z, Zhou F, Wang Q 2020 Nano Energy 71 104634
 Google Scholar
Google Scholar
[13] Duan J, Zhao Y, He B, Tang Q 2018 Angew. Chem. Int. Ed. 57 3787
 Google Scholar
Google Scholar
[14] Chang X, Li W, Zhu L 2016 ACS Appl. Mater. Interfaces 8 33649
 Google Scholar
Google Scholar
[15] Liu X, Tan X, Liu Z, Sun B, Tan Z, Liao G 2019 Nano Energy 56 184
 Google Scholar
Google Scholar
[16] Guo Y, Yin X, Liu J 2019 Solar RRL 3 1900135
 Google Scholar
Google Scholar
[17] Guo Z, Teo S, Xu Z, S. Hayase, Ma T 2019 J. Mater. Chem. A 7 1227
 Google Scholar
Google Scholar
[18] Ma Q, Huang S, Wen X 2016 Adv. Energy Mater. 6 1502202
 Google Scholar
Google Scholar
[19] Subhani W, Wang K, Du M 2019 Adv. Energy Mater. 9 1803785
 Google Scholar
Google Scholar
[20] Ren Y, Hao Y, Zhang N, Cai M, Dai S 2020 Chem. Eng. J. 392 123805
 Google Scholar
Google Scholar
[21] Li B, Zhang Y, Fu L 2018 Nat. Commun. 9 1076
 Google Scholar
Google Scholar
[22] Peng H, Cai M, Zhou J, Ding X, Pan J, Dai S 2020 Solar RRL 4 2000216
 Google Scholar
Google Scholar
[23] Xu W, Zhu T, Wu H 2020 ACS Appl. Mater. Interfaces 12 45045
 Google Scholar
Google Scholar
[24] Zhao Y C, Wei J, Li H, Yan Y, Zhou W, Yu D, Zhao Q 2016 Nat. Commun. 7 10228
 Google Scholar
Google Scholar
[25] Wu W, Zhong J, Liao J 2020 Nano Energy 75 104929
 Google Scholar
Google Scholar
[26] Yang J, Liu C, Cai C 2019 Adv. Energy Mater. 9 1900198
 Google Scholar
Google Scholar
[27] Yin G, Zhao H, Jiang H, Liu Z, Liu S 2018 Adv. Funct Mater. 28 1803269
 Google Scholar
Google Scholar
[28] Du J, Duan J, Yang X, Duan Y, Zhou Q, Tang Q 2021 Angew. Chem. Int. Ed. 60 1
 Google Scholar
Google Scholar
[29] Wang Z, BaranwalA K, Kamarudin M A, Ng C, Pandey M, Ma T, Hayase S 2019 Nano Energy 59 258
 Google Scholar
Google Scholar
-
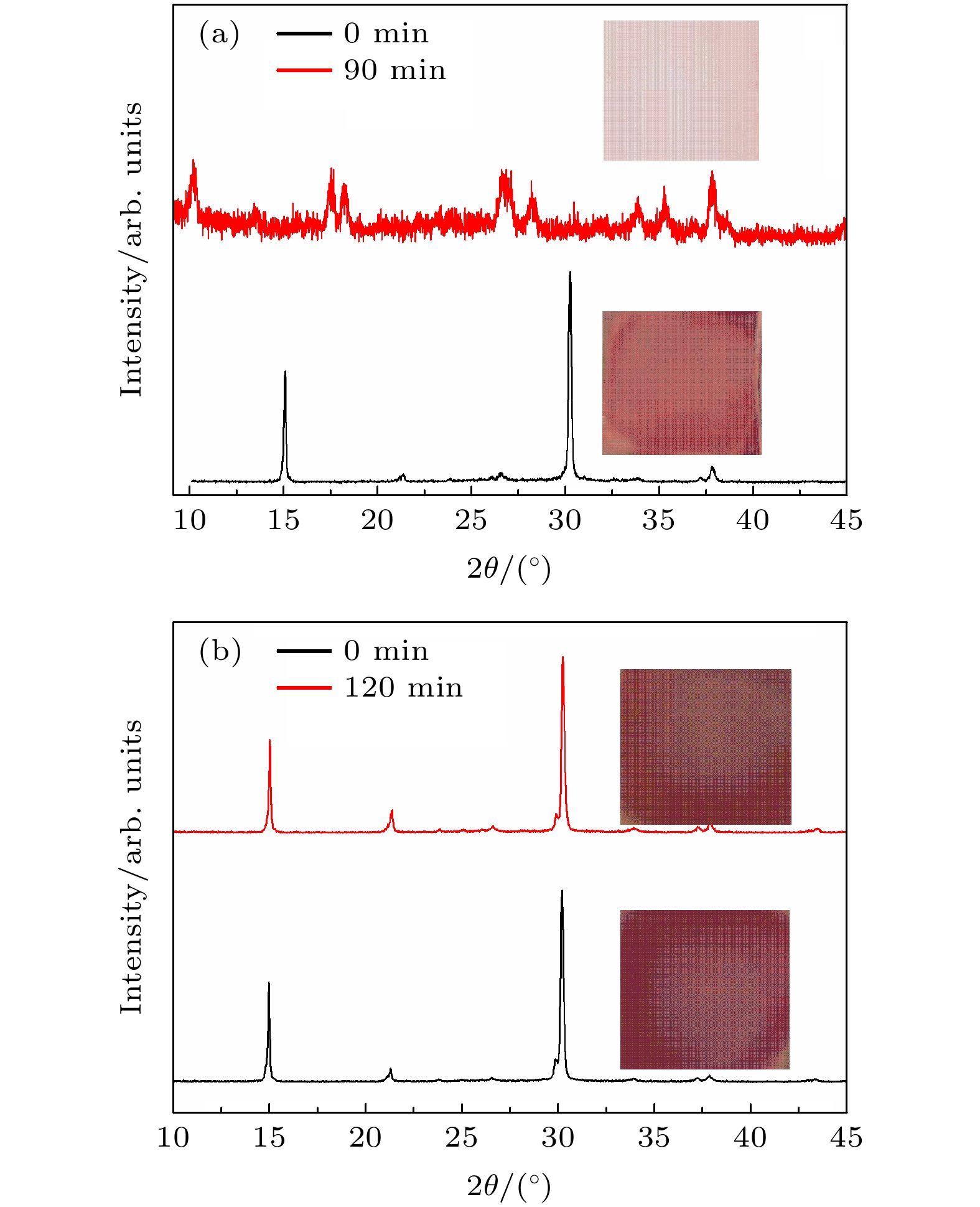
图 4 贮存在温度为30 ℃、相对湿度为85%的空气环境中不同时间点的(a) CsPbIBr2和(b) CA-CsPbIBr2钙钛矿膜的XRD曲线(插图为对应的CsPbIBr2和CA- CsPbIBr2照片)
Figure 4. XRD curves of (a) CsPbIBr2 and (b) CA-CsPbIBr2 perovskite films stored in the air with a temperature of 30 ℃ and a relative humidity of 85% (the insets are the photos of CsPbIBr2 and CA-CsPbIBr2 perovskite films).
图 7 (a) CsPbIBr2和CA-CsPbIBr2钙钛矿太阳能电池在正向和反向扫描条件下测得的光电流密度-电压(J-V)曲线; (b) CsPbIBr2和CA-CsPbIBr2钙钛矿太阳能电池Nyquist曲线; (c) 空气环境中, 未密封CsPbIBr2和CA-CsPbIBr2钙钛矿太阳能电池的光电转换效率随时间的变化趋势
Figure 7. (a) Photocurrent density-voltage (J-V) curves of perovskite solar cells based on CsPbIBr2 and CA-CsPbIBr2 perovskite films measured under forward and reverse scans; (b) Nyquist plots of CsPbIBr2 and CA-CsPbIBr2 perovskite solar cells; (c) variation of PCE of perovskite solar cells based on CsPbIBr2 and CA-CsPbIBr2 perovskite films stored in ambient air.
表 1 CsPbIBr2和CA-CsPbIBr2钙钛矿太阳能电池界面电荷复合电阻及正向和反向扫描测得的光电参数
Table 1. The recombination resistances (Rrec) and photovoltaic parameters of perovskite solar cells based on CsPbIBr2 and CA-CsPbIBr2 perovskite films measured under forward and reverse scans.
电池 扫描方向 Voc/V Jsc/(mA·cm2) FF PCE/% HIa Rrec/Ω CsPbIBr2 正向 平均 0.96 ± 0.05 9.89 ± 0.39 0.33 ± 0.4 3.13 ± 0.72 22% 1448 最高 1.03 10.35 0.39 4.16 反向 平均 1.01 ± 0.03 10.21 ± 0.42 0.45 ± 0.02 4.64 ± 0.56 最高 1.07 10.62 0.47 5.34 CA
-CsPbIBr2正向 平均 1.02 ± 0.03 10.52 ± 0.37 0.57 ± 0.02 6.12 ± 0.67 7% 2269 最高 1.06 10.91 0.60 6.94 反向 平均 1.05 ± 0.03 10.55 ± 0.27 0.62 ± 0.02 6.87 ± 0.38 最高 1.08 10.88 0.64 7.52 a HI = (PCE反向–PCE正向)/PCE反向 -
[1] Kojima A, Teshima K, Shirai Y, Miyasaka T 2009 J. Am. Chem. Soc. 131 6050
 Google Scholar
Google Scholar
[2] Ono L K, Qi Y 2016 J. Phys. Chem. Lett. 7 4764
 Google Scholar
Google Scholar
[3] 姚鑫, 丁艳丽, 张晓丹, 赵颖 2015 64 038805
 Google Scholar
Google Scholar
Yao X, Ding Y L, Zhang X D, Zhao Y 2015 Acta Phys. Sin. 64 038805
 Google Scholar
Google Scholar
[4] Deng X, Xie L, Wang S 2020 Chem. Eng. J. 398 125594
 Google Scholar
Google Scholar
[5] Huang Y, Liu T, Liang C 2020 Adv. Funct. Mater. 30 2000863
 Google Scholar
Google Scholar
[6] Jia X, Zho C, Tao S 2019 Sci. Bull. 64 1532
 Google Scholar
Google Scholar
[7] Tai Q, Tang K, Yan F 2019 Energy Environ. Sci. 12 2375
 Google Scholar
Google Scholar
[8] Li B, Fu L, Li S, Pan L, Wang L, Yin L 2019 J. Mater. Chem. A 7 20494
 Google Scholar
Google Scholar
[9] Wang G, Lei M, Liu J, Zhang W, He Q 2020 Solar RRL 4 2000528
 Google Scholar
Google Scholar
[10] Duan J, Xu H, Sha W, Tang Q 2019 J. Mater. Chem. A 7 21036
 Google Scholar
Google Scholar
[11] Wang Y, Liu X, Zhang T, Wang X, J. Shi, Zhao Y 2019 Angew. Chem. Int. Ed. 58 16691
 Google Scholar
Google Scholar
[12] Li Z, Zhou F, Wang Q 2020 Nano Energy 71 104634
 Google Scholar
Google Scholar
[13] Duan J, Zhao Y, He B, Tang Q 2018 Angew. Chem. Int. Ed. 57 3787
 Google Scholar
Google Scholar
[14] Chang X, Li W, Zhu L 2016 ACS Appl. Mater. Interfaces 8 33649
 Google Scholar
Google Scholar
[15] Liu X, Tan X, Liu Z, Sun B, Tan Z, Liao G 2019 Nano Energy 56 184
 Google Scholar
Google Scholar
[16] Guo Y, Yin X, Liu J 2019 Solar RRL 3 1900135
 Google Scholar
Google Scholar
[17] Guo Z, Teo S, Xu Z, S. Hayase, Ma T 2019 J. Mater. Chem. A 7 1227
 Google Scholar
Google Scholar
[18] Ma Q, Huang S, Wen X 2016 Adv. Energy Mater. 6 1502202
 Google Scholar
Google Scholar
[19] Subhani W, Wang K, Du M 2019 Adv. Energy Mater. 9 1803785
 Google Scholar
Google Scholar
[20] Ren Y, Hao Y, Zhang N, Cai M, Dai S 2020 Chem. Eng. J. 392 123805
 Google Scholar
Google Scholar
[21] Li B, Zhang Y, Fu L 2018 Nat. Commun. 9 1076
 Google Scholar
Google Scholar
[22] Peng H, Cai M, Zhou J, Ding X, Pan J, Dai S 2020 Solar RRL 4 2000216
 Google Scholar
Google Scholar
[23] Xu W, Zhu T, Wu H 2020 ACS Appl. Mater. Interfaces 12 45045
 Google Scholar
Google Scholar
[24] Zhao Y C, Wei J, Li H, Yan Y, Zhou W, Yu D, Zhao Q 2016 Nat. Commun. 7 10228
 Google Scholar
Google Scholar
[25] Wu W, Zhong J, Liao J 2020 Nano Energy 75 104929
 Google Scholar
Google Scholar
[26] Yang J, Liu C, Cai C 2019 Adv. Energy Mater. 9 1900198
 Google Scholar
Google Scholar
[27] Yin G, Zhao H, Jiang H, Liu Z, Liu S 2018 Adv. Funct Mater. 28 1803269
 Google Scholar
Google Scholar
[28] Du J, Duan J, Yang X, Duan Y, Zhou Q, Tang Q 2021 Angew. Chem. Int. Ed. 60 1
 Google Scholar
Google Scholar
[29] Wang Z, BaranwalA K, Kamarudin M A, Ng C, Pandey M, Ma T, Hayase S 2019 Nano Energy 59 258
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 8854
- PDF Downloads: 172
- Cited By: 0















 DownLoad:
DownLoad: