-
Recovering the protein activity of p53 mutants through small molecule ligand binding (eg. arsenic) is an important strategy for tumor suppressor therapy. However, the mechanistic basis on the changes of collective dynamics and their roles of p53 protein in functional recovery process has not been fully elucidated. Herein, the normal mode calculations based on all-atom elastic network model are employed to characterize the terahertz low frequency motions of core DNA-binding domain (p53C) which is essential for p53 protein activities in transcriptional transactivation. We find that the lowest-frequency collective vibration mode of the p53C mutant is effectively restored by the binding of arsenic (III) ligand. In R249S mutant, the L1 loop is stabilized through restricting the swing-out movement. The results obtained from atomic backbone fluctuations suggest that the arsenic binding can significantly improve the L1 loop and L2 loop fluctuations. The statistical analysis of low frequency vibration mode reflects that the arsenic-bound R249S mutant has an apparent recovery of frequency shift in the terahertz range. The residue-residue motion correlation also suggests that structural components binding to arsenic are dynamically coupled. In the H2 helix with arsenic-binding residues, the motions of C124, C135, M133 and C141, are correlated with the arsenic recovery. These results provide the terahertz biophysical mechanism for the recovery effect of arsenic (III) on the p53 protein activity and new evidence for the coupling of the low-frequency vibration characteristics of protein structures with its function, thus giving a new physical insight into the p53 related cancer therapies.
-
Keywords:
- p53 protein /
- function recovery /
- terahertz vibration /
- arsenic (III)
[1] Vousden K H, Lane D P 2007 Nat. Rev. Mol. Cell. Bio. 8 275
 Google Scholar
Google Scholar
[2] Zehir A, Benayed R, Shah R H, et al. 2017 Nat. Med. 23 703
 Google Scholar
Google Scholar
[3] Perdrix A, Najem A, Saussez S, et al. 2017 Cancers 9 172
 Google Scholar
Google Scholar
[4] 丁笠, 张新跃 2020 扬州大学学报(农业与生命科学版) 41 57
 Google Scholar
Google Scholar
Ding L, Zhang X Y 2020 J. Yangzhou Univ. (Agric. Life Sci. Ed.) 41 57
 Google Scholar
Google Scholar
[5] Joerger A C, Fersht A R 2008 Annu. Rev. Biochem. 77 557
 Google Scholar
Google Scholar
[6] Canadillas J M P, Tidow H, Freund S M V, et al. 2006 Proc. Natl. Acad. Sci. U. S. A. 103 2109
 Google Scholar
Google Scholar
[7] Viadiu H, Fronza G, Inga A 2014 Subcell. Biochem. 85 119
 Google Scholar
Google Scholar
[8] Duan J, Nilsson L 2006 Biochemistry 45 7483
 Google Scholar
Google Scholar
[9] Kitayner M, Rozenberg H, Kessler N, et al. 2006 Mol. Cell 22 741
 Google Scholar
Google Scholar
[10] Cho Y J, Gorina S, Jeffrey P D, et al. 1994 Science 265 346
 Google Scholar
Google Scholar
[11] Petitjean A, Mathe E, Kato S, et al. 2007 Hum. Mutat. 28 622
 Google Scholar
Google Scholar
[12] Chen S, Wu J L, Liang Y, et al. 2021 Cancer Cell 39 225
 Google Scholar
Google Scholar
[13] Karplus M, McCammon J A 2002 Nat. Struct. Biol. 9 646
 Google Scholar
Google Scholar
[14] Kitao A, Go N 1999 Curr. Opin. Struct. Biol. 9 164
 Google Scholar
Google Scholar
[15] Acbas G, Niessen K A, Snell E H, et al. 2014 Nat. Commun. 5 3076
 Google Scholar
Google Scholar
[16] Niessen K A, Xu M, George D K, et al. 2019 Nat. Commun. 10 1026
 Google Scholar
Google Scholar
[17] Turton D A, Senn H M, Harwood T, et al. 2014 Nat. Commun. 5 3999
 Google Scholar
Google Scholar
[18] Bahar I, Lezon T R, Bakan A, et al. 2010 Chem. Rev. 110 1463
 Google Scholar
Google Scholar
[19] Bahar I, Lezon T R, Yang L-W, et al. 2010 Annu. Rev. Biophys. 39 23
 Google Scholar
Google Scholar
[20] Balog E, Perahia D, Smith J C, et al. 2011 J. Phys. Chem. B 115 6811
 Google Scholar
Google Scholar
[21] Berman H M, Westbrook J, Feng Z, et al. 2000 Nucleic Acids Res. 28 235
 Google Scholar
Google Scholar
[22] Eswar N, Webb B, Marti-Renom M A, et al. 2007 Curr. Protoc. Protein. Sci. 50 2.9.1
 Google Scholar
Google Scholar
[23] Guex N, Peitsch M C 1997 Electrophoresis 18 2714
 Google Scholar
Google Scholar
[24] Yao X Q, Skjærven L, Grant B J 2016 J. Phys. Chem. B 120 8276
 Google Scholar
Google Scholar
[25] Grant B J, Rodrigues A P C, ElSawy K M, et al. 2006 Bioinformatics 22 2695
 Google Scholar
Google Scholar
[26] Brooks B R, Janežič D, Karplus M 1995 J. Comput. Chem. 16 1522
 Google Scholar
Google Scholar
[27] Kaynak B T, Doruker P 2019 J. Chem. Inf. Model. 59 2352
 Google Scholar
Google Scholar
[28] Ichiye T, Karplus M 1991 Proteins 11 205
 Google Scholar
Google Scholar
[29] Tiwari S P, Fuglebakk E, Hollup S M, et al. 2014 BMC Bioinformatics 15 427
 Google Scholar
Google Scholar
[30] Alakent B, Baskan S, Doruker P 2011 J. Comput. Chem. 32 483
 Google Scholar
Google Scholar
-
图 1 p53C蛋白晶体结构 (a)砷(III)结合位点没有砷(III)结合时的局部细节; (b)砷(III)结合位点在砷(III)结合时的局部细节; (c) R249残基与相邻区域相互作用的局部示意图. 颜色标注如下: L1环及相邻区域(残基序列112—141)为黄色, L2环及相邻区域(残基序列163—195)为蓝色, L3环(残基序列236—251)为红色, H2螺旋(残基序列271—286)为紫色, DNA为青色/绿色. 除非注明, 后图颜色标注一致
Figure 1. Structure of p53C: (a) The structure of As3+-binding region in the absence of As3+; (b) the structure of As3+-binding region with As3+; (c) R249 region in detail. It is colored as following: L1 loop and adjacent area (residues 112–141) in yellow, L2 loop and adjacent area (residues 163–195) in blue, L3 loop (residues 236–251) in red, H2 helix (residues 271–286) in purple and DNA in cyan/green. Unless otherwise specified, the colored regions in following figures are consistent with this.
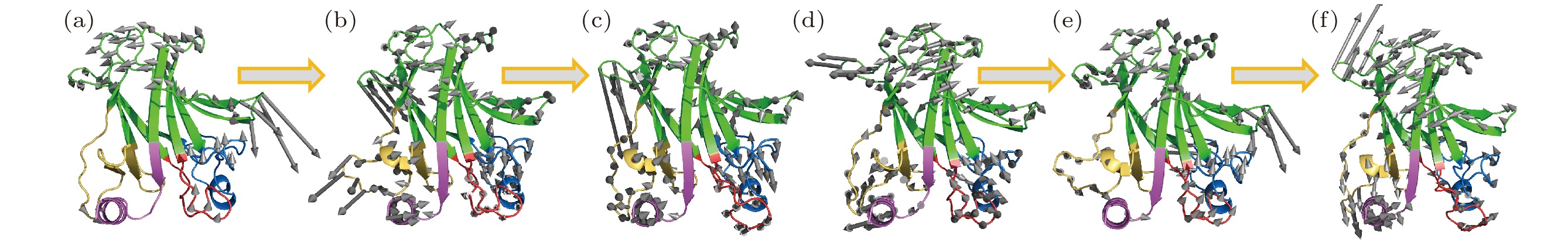
图 2 砷(III)对p53C突变体R249S的低频振动模式恢复 (a) 野生型p53C (wild type,WT) 、(b) R249S突变型p53C (R249S)、(c) 砷(III)结合的R249S突变型p53C的最低频率集体振动模式轨迹; (d)野生型p53C (wild type,WT) 、(e) R249S突变型p53C (R249S)、(f) 砷(III)结合的R249S突变型p53C的次低频率集体振动模式轨迹; 灰色箭头表示对应频率蛋白分子骨架的振动方向, 箭头长度代表相对振幅大小
Figure 2. The trajectory of the lowest-frequency vibrations of (a) wild type (WT), (b) R249S, (c) R249S-As. The trajectory of the second lowest-frequency vibrations of (d) wild type (WT), (e) R249S, (f) R249S-As. Arrows represent the direction of motion of each residue from the initial position, with length of arrows indicating vibration amplitude, relatively.
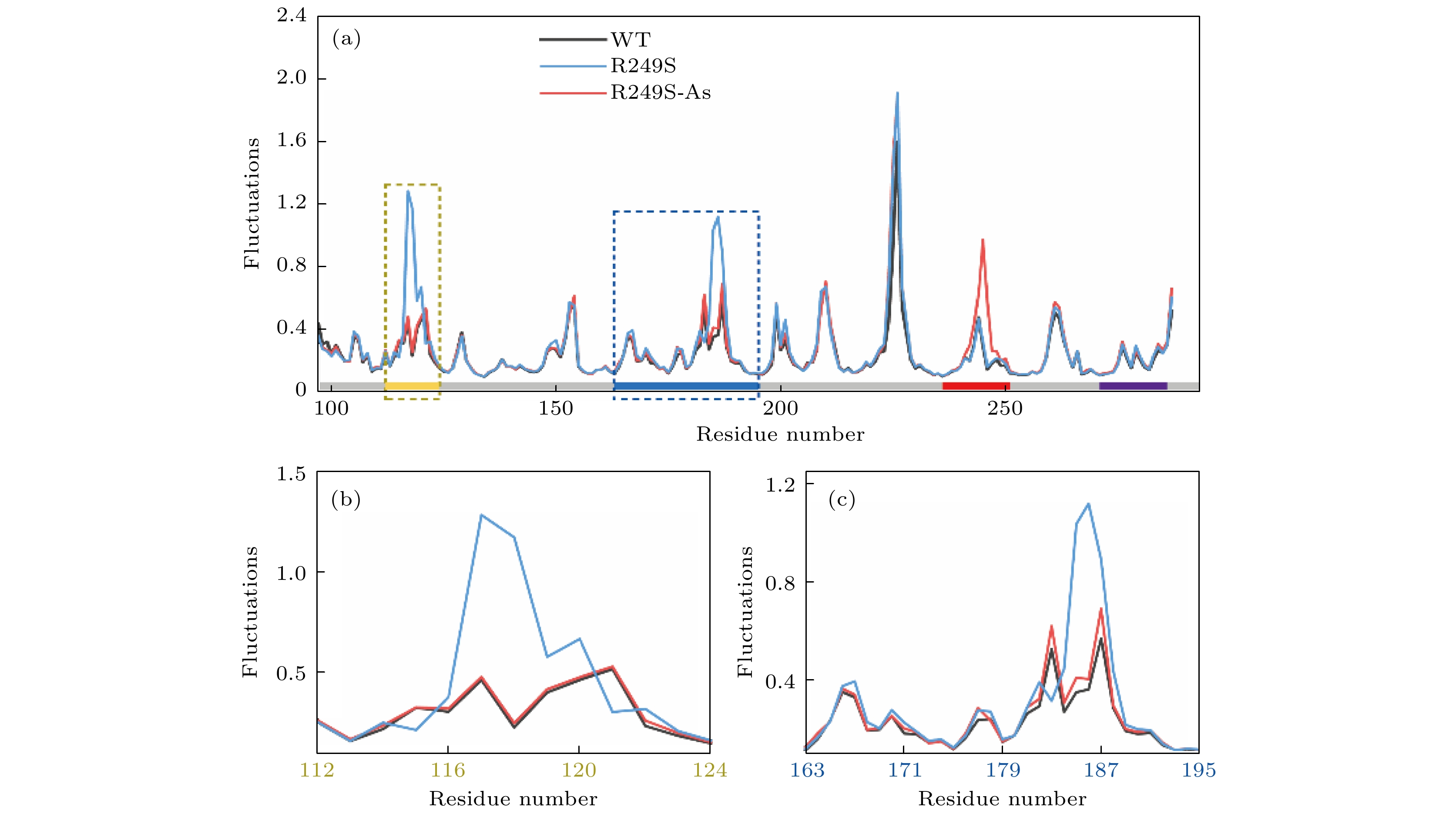
图 3 (a) p53C野生型、R249S突变型和砷(III)结合的R249S突变型p53C的原子波动分析(沿X轴的灰色条代表序列, 其中用四种不同颜色分别标记4个区域); (b) L1环区域(残基序列112—124)的局部原子波动分析; (c) L2环区域(残基序列163—195)的局部原子波动分析
Figure 3. (a) Atomic fluctuation analysis of the DNA binding domain of the WT, R249S, and R249S-As. The gray bar above the X axis represents the sequence, where four regions are marked with four colors. (b) The details of the yellow (L1, residues 112–124) dotted areas. (c) The details of the blue (L2, residues 163–195) dotted areas.
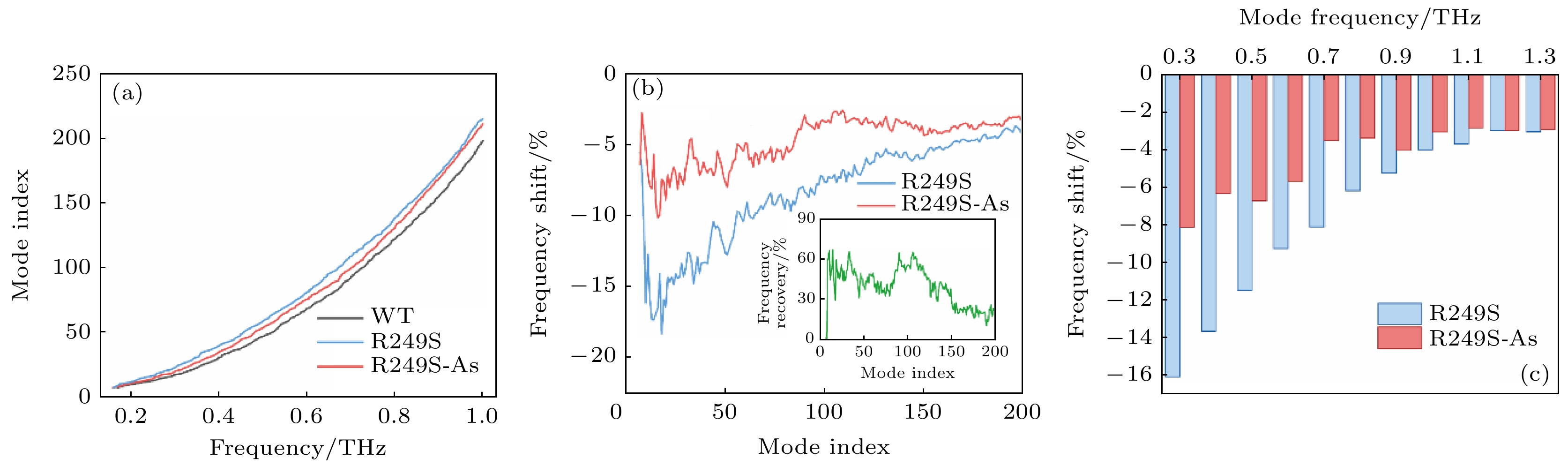
图 4 (a) 0—1.0 THz内的野生型p53C (WT), 突变体(R249S)和砷(III)结合突变体(R249S-As)的振动谱; (b) 突变体(R249S)和砷(III)结合突变体(R249S-As)相对于野生型p53 C的前200个振动模式的太赫兹振动频率偏移百分数, 插图为砷(III)结合后突变体的频率恢复比例; (c) R249S和R249S-As在野生型p53C特定频率处对应振动模式的频率偏移百分数
Figure 4. (a) Mode index spectra of wild-type p53C (WT), mutant (R249S) and As-bound mutant (R249S-As) in 0–1.0 THz; (b) the frequency shift (%) of the R249S and the R249S-As mutant from the WT in first 200 collective vibration modes, and the insert shows the proportion of frequency recovery for the As-bound R249S mutants; (c) the frequency shift (%) of corresponding mode of the R249S and the R249S-As at certain frequencies of WT.
图 5 野生型p53C的动力学相关矩阵. 每个点根据它在X轴和Y轴上的两个残基的Cab值(残基a与b之间的相关性)来着色. Cab值根据所得太赫兹振动模态计算, 其中Cab为1, –1和0, 分别表示完全相关、完全负相关和不相关. 横轴上用绿色虚线标出了4个与DNA作用的残基, 纵轴上用黄色虚线标出了4个与砷(III)结合的残基. 黑色虚线框标出了4个正相关区域, 其中L1与H2相关区域被专门表示在右边, 并与R249S突变体, 砷(III)结合R249S突变体动力学相关矩阵的对应区域比较
Figure 5. Residue-residue motion correlation map of the DNA binding domain of the wild type p53C, where each point is colored according to its Cab (correlation between residues a and b) of the two residues on the X axis and Y axis. The Cab are calculated by all modes, where Cab = 1, –1, 0 means completely correlation, completely anticorrelation and no correlation, respectively. The four DNA-contact residues are marked in dark green dash curves on the X axis, and the four As-bound residues are marked in yellow dash curves on the Y axis. The black dashed boxes mark the four positively correlated regions, where the correlated regions between L1 and H2 are specifically represented on the right and compared with the corresponding regions of the correlation map of the R249S and the As-bound R249S mutant.
-
[1] Vousden K H, Lane D P 2007 Nat. Rev. Mol. Cell. Bio. 8 275
 Google Scholar
Google Scholar
[2] Zehir A, Benayed R, Shah R H, et al. 2017 Nat. Med. 23 703
 Google Scholar
Google Scholar
[3] Perdrix A, Najem A, Saussez S, et al. 2017 Cancers 9 172
 Google Scholar
Google Scholar
[4] 丁笠, 张新跃 2020 扬州大学学报(农业与生命科学版) 41 57
 Google Scholar
Google Scholar
Ding L, Zhang X Y 2020 J. Yangzhou Univ. (Agric. Life Sci. Ed.) 41 57
 Google Scholar
Google Scholar
[5] Joerger A C, Fersht A R 2008 Annu. Rev. Biochem. 77 557
 Google Scholar
Google Scholar
[6] Canadillas J M P, Tidow H, Freund S M V, et al. 2006 Proc. Natl. Acad. Sci. U. S. A. 103 2109
 Google Scholar
Google Scholar
[7] Viadiu H, Fronza G, Inga A 2014 Subcell. Biochem. 85 119
 Google Scholar
Google Scholar
[8] Duan J, Nilsson L 2006 Biochemistry 45 7483
 Google Scholar
Google Scholar
[9] Kitayner M, Rozenberg H, Kessler N, et al. 2006 Mol. Cell 22 741
 Google Scholar
Google Scholar
[10] Cho Y J, Gorina S, Jeffrey P D, et al. 1994 Science 265 346
 Google Scholar
Google Scholar
[11] Petitjean A, Mathe E, Kato S, et al. 2007 Hum. Mutat. 28 622
 Google Scholar
Google Scholar
[12] Chen S, Wu J L, Liang Y, et al. 2021 Cancer Cell 39 225
 Google Scholar
Google Scholar
[13] Karplus M, McCammon J A 2002 Nat. Struct. Biol. 9 646
 Google Scholar
Google Scholar
[14] Kitao A, Go N 1999 Curr. Opin. Struct. Biol. 9 164
 Google Scholar
Google Scholar
[15] Acbas G, Niessen K A, Snell E H, et al. 2014 Nat. Commun. 5 3076
 Google Scholar
Google Scholar
[16] Niessen K A, Xu M, George D K, et al. 2019 Nat. Commun. 10 1026
 Google Scholar
Google Scholar
[17] Turton D A, Senn H M, Harwood T, et al. 2014 Nat. Commun. 5 3999
 Google Scholar
Google Scholar
[18] Bahar I, Lezon T R, Bakan A, et al. 2010 Chem. Rev. 110 1463
 Google Scholar
Google Scholar
[19] Bahar I, Lezon T R, Yang L-W, et al. 2010 Annu. Rev. Biophys. 39 23
 Google Scholar
Google Scholar
[20] Balog E, Perahia D, Smith J C, et al. 2011 J. Phys. Chem. B 115 6811
 Google Scholar
Google Scholar
[21] Berman H M, Westbrook J, Feng Z, et al. 2000 Nucleic Acids Res. 28 235
 Google Scholar
Google Scholar
[22] Eswar N, Webb B, Marti-Renom M A, et al. 2007 Curr. Protoc. Protein. Sci. 50 2.9.1
 Google Scholar
Google Scholar
[23] Guex N, Peitsch M C 1997 Electrophoresis 18 2714
 Google Scholar
Google Scholar
[24] Yao X Q, Skjærven L, Grant B J 2016 J. Phys. Chem. B 120 8276
 Google Scholar
Google Scholar
[25] Grant B J, Rodrigues A P C, ElSawy K M, et al. 2006 Bioinformatics 22 2695
 Google Scholar
Google Scholar
[26] Brooks B R, Janežič D, Karplus M 1995 J. Comput. Chem. 16 1522
 Google Scholar
Google Scholar
[27] Kaynak B T, Doruker P 2019 J. Chem. Inf. Model. 59 2352
 Google Scholar
Google Scholar
[28] Ichiye T, Karplus M 1991 Proteins 11 205
 Google Scholar
Google Scholar
[29] Tiwari S P, Fuglebakk E, Hollup S M, et al. 2014 BMC Bioinformatics 15 427
 Google Scholar
Google Scholar
[30] Alakent B, Baskan S, Doruker P 2011 J. Comput. Chem. 32 483
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 8736
- PDF Downloads: 230
- Cited By: 0















 DownLoad:
DownLoad: