-
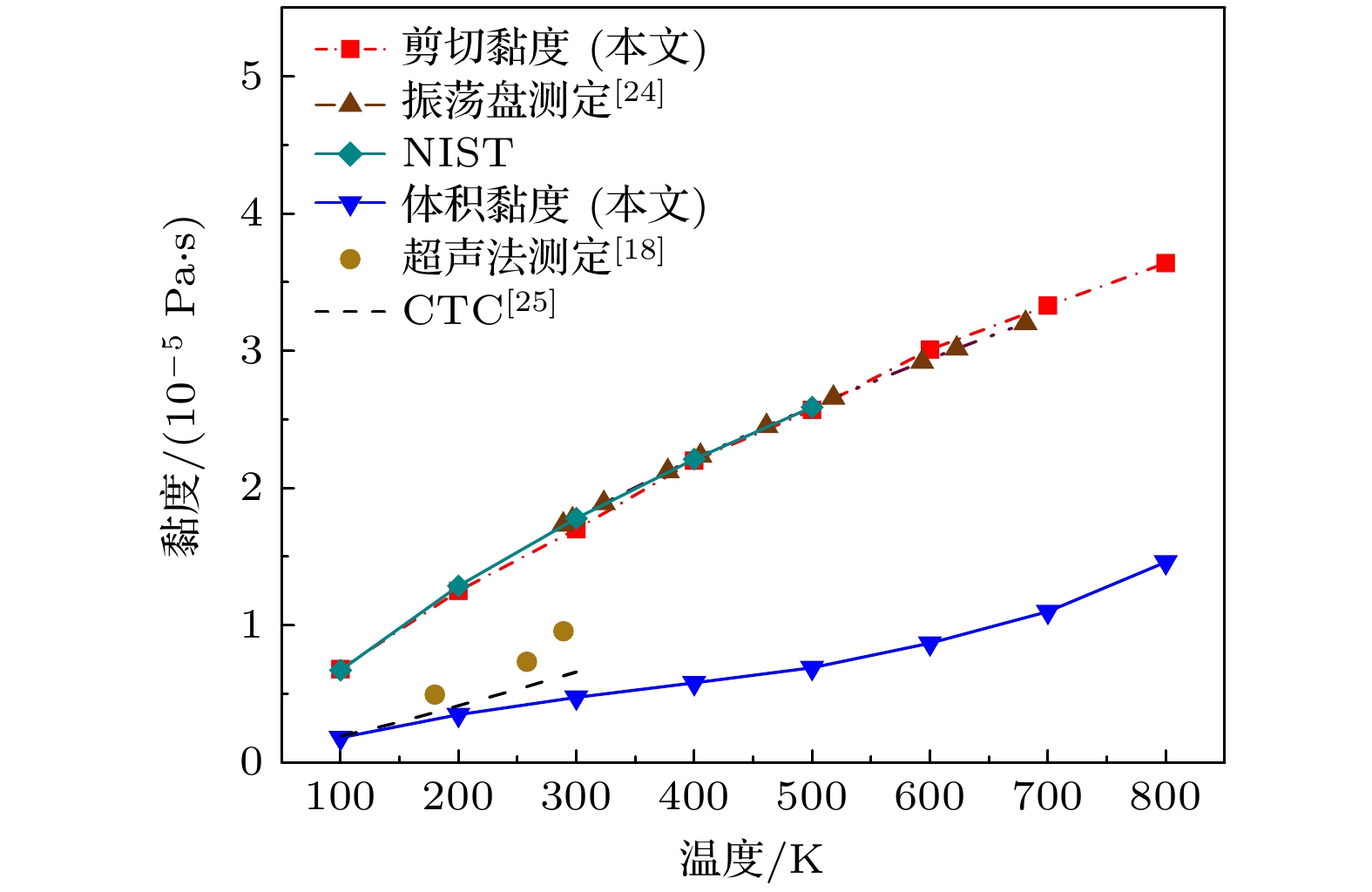
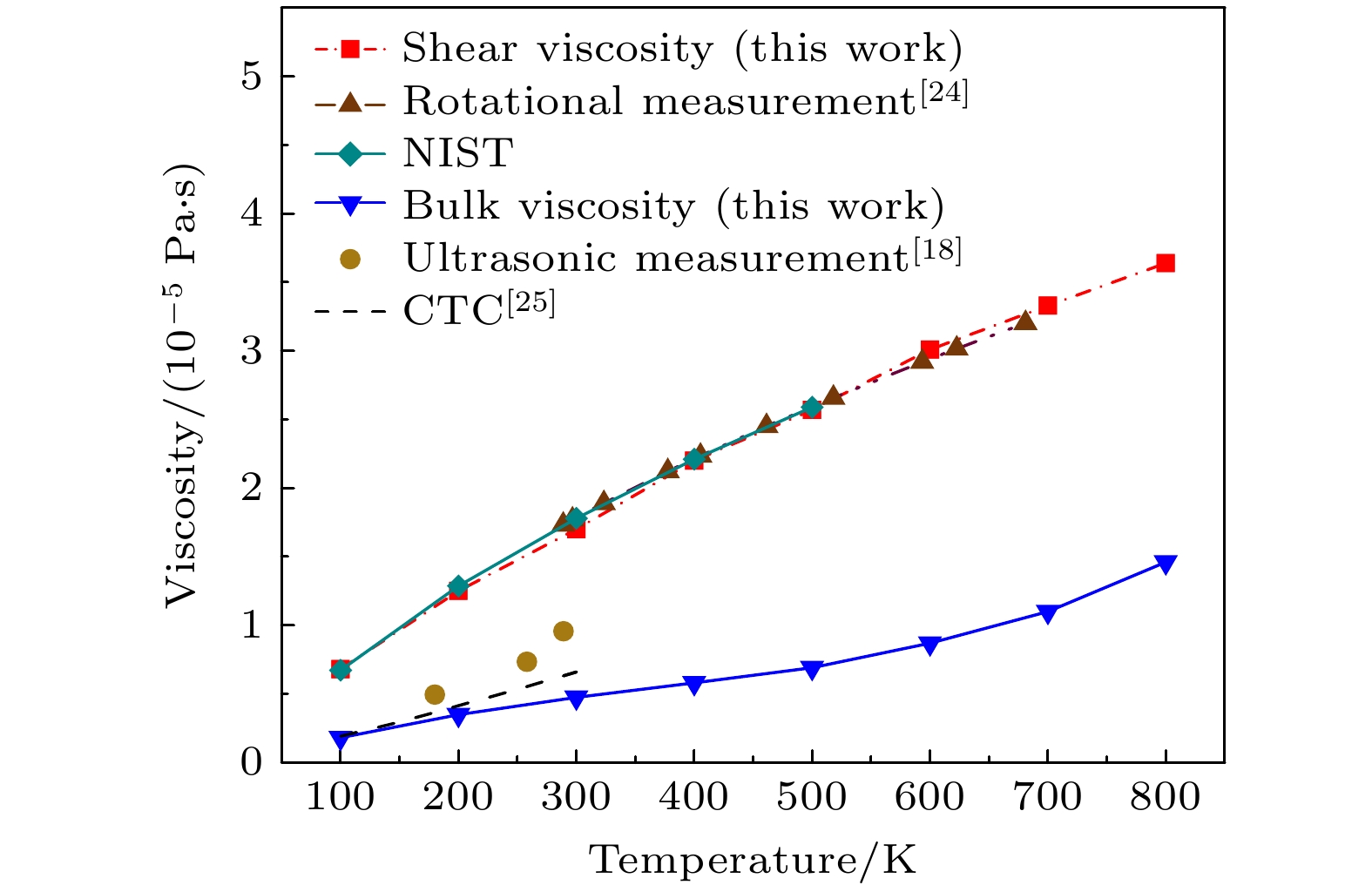
Viscosity is an essential transport property in gas dynamics, especially the bulk viscosity, which exhibits more complex behavior. Carbon monoxide (CO) is a molecule of weak polarity, which exists in many important fields such as combustion and coke metallurgy. In order to effectively uncover the mechanism of the CO viscosity, this study dealt with it from a microscopic view. A transcale model is built which integrates density functional theory (DFT, first-principles) calculations with equilibrium molecular dynamics (EMD) simulations to establish a microscale foundation. Based on that, a fitted high-precision potential function is formed, then by using the Green-Kubo linear response theory, the shear and bulk viscosities of CO are achieved in a medium temperature range of 100–800 K. The MD simulation is implemented with C programming language, and an adaptive time-step algorithm is applied so that the computational efficiency is significantly enhanced. The resulting bulk viscosity exhibits quite obvious sensitivity to the potential function of the molecule system, while the shear viscosity shows little. Unlike the shear viscosity, which appears more linear, the bulk viscosity shows clear nonlinear behavior that changes with temperature. Correspondingly, traditional theoretic models and experimental results from different literature indicate that the bulk viscosity at medium temperatures is overestimated to various degrees. Fitting functions on the shear and bulk viscosities in the defined temperature range are established, respectively. Additionally, the lower system pressure and larger system size in the model effectively reduce statistical pressure fluctuations and improve the convergence of relevant laws. This work elucidates the microscopic mechanism of CO viscosity and provides a high-fidelity theoretical tool for modeling the viscosity of high-temperature nonequilibrium gas flows (e.g. hypersonic boundary layers, and plasma transport).
-
Keywords:
- density functional theory /
- potential function /
- molecular dynamics /
- shear viscosity /
- bulk viscosity /
- nonlinearity
-
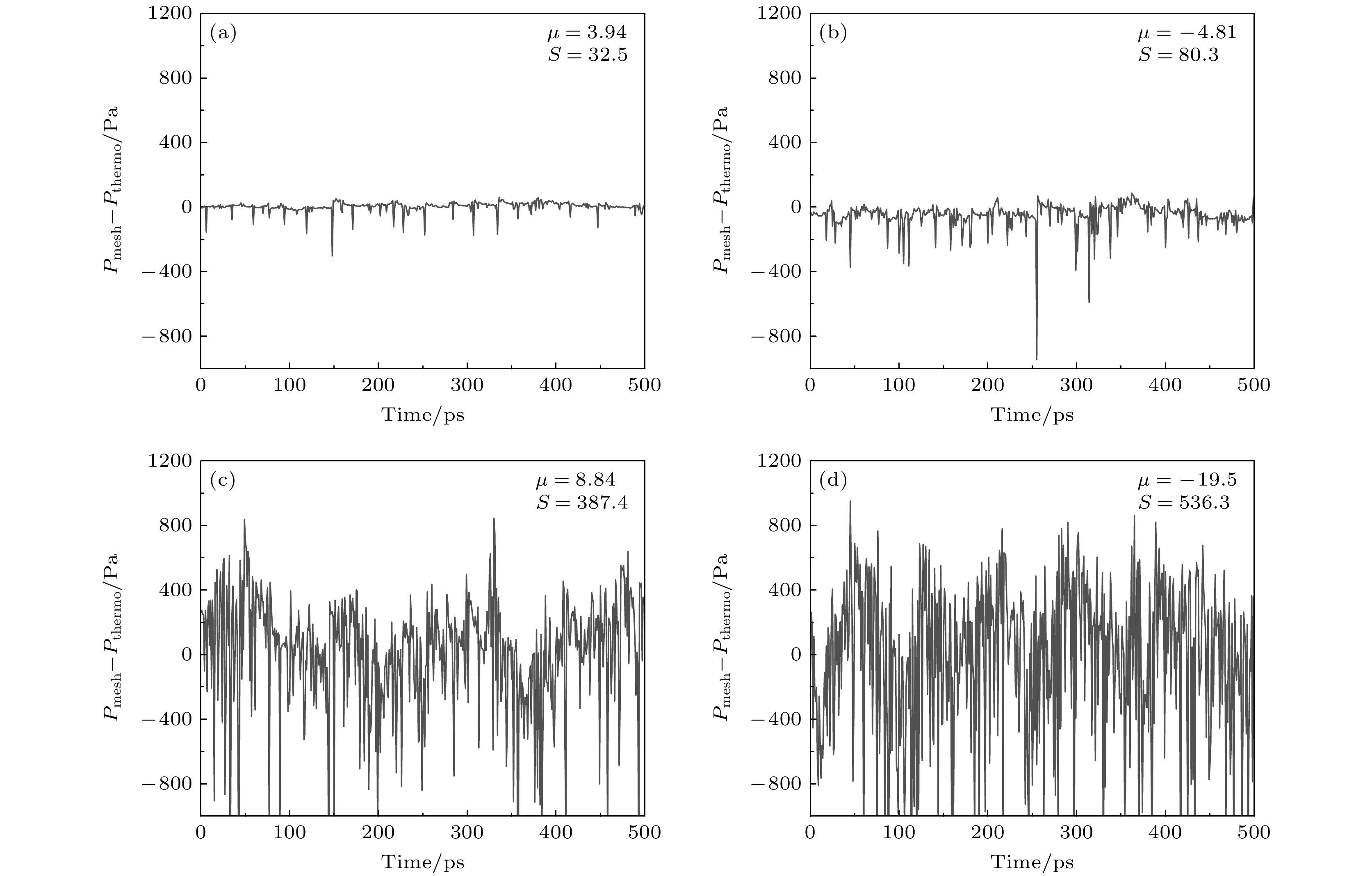
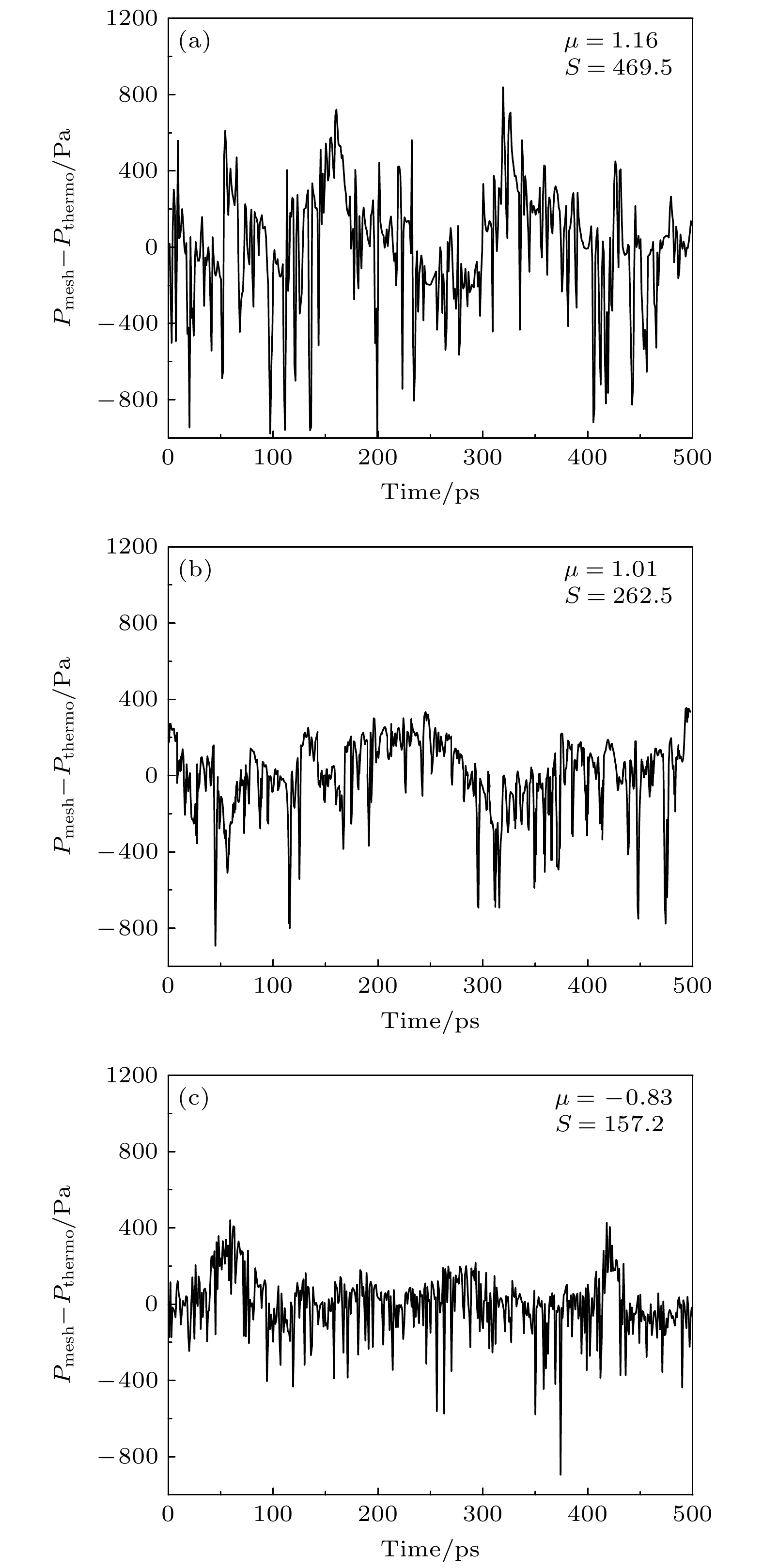
图 5 不同压力下系统平衡后$ {P_{{\text{mech}}}} - {P_{{\text{thermo}}}} $随模拟时间的变化(图中分别给出了其均值μ与b标准差S) (a) p = 0.25 atm; (b) p = 0.5 atm; (c) p = 1.5 atm; (d) p = 2 atm
Figure 5. Variation of $ {P_{{\text{mech}}}} - {P_{{\text{thermo}}}} $ with time after equilibration in systems under different pressures, with the mean value and the standard deviation presented, respectively: (a) p = 0.25 atm; (b) p = 0.5 atm; (c) p = 1.5 atm; (d) p = 2 atm.
图 6 分子数不同的体系平衡后$ {P_{{\text{mech}}}} - {P_{{\text{thermo}}}} $随运行时间的变化(图中分别给出了其均值μ与标准差S) (a) N = 500; (b) N = 1000; (c) N = 1500
Figure 6. Variation of $ {P_{{\text{mech}}}} - {P_{{\text{thermo}}}} $ with time after equilibration in systems with different numbers of molecules, with the mean value and the standard deviation presented, respectively: (a) N = 500; (b) N = 1000; (c) N = 1500.
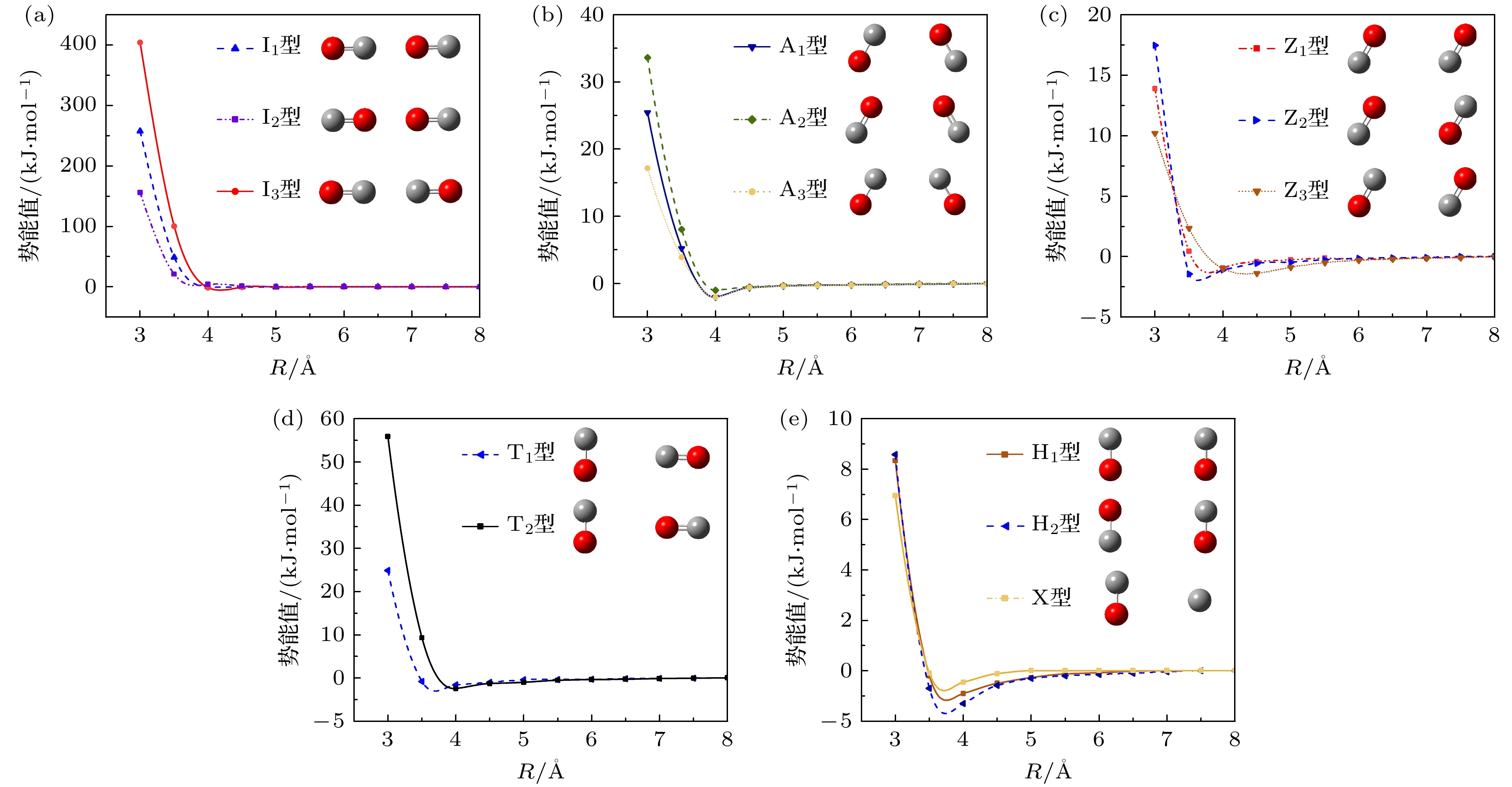
表 1 CO-CO典型构型对应势函数的拟合系数
Table 1. Fitting coefficients of the potential function corresponding to the typical configurations of CO-CO.
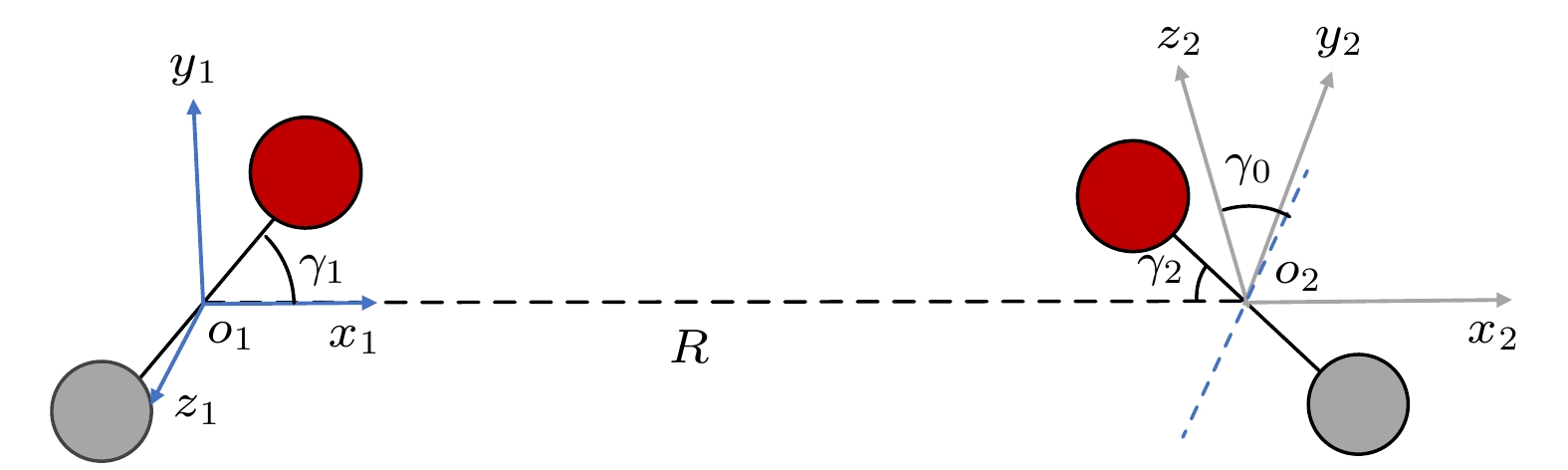
构型 a b c d e f I1型 2 0 0 1.765×107 0 0 I2型 3.66 0 0 3.188×107 0 0 I3型 1.41 0 0 1.228×107 0 0 A1型 0.48 –0.641 1.025 0.51×106 –0.695×104 0 A2型 0.64 –0.846 1.354 0.674×106 –0.919×104 0 A3型 0.37 –0.432 0.691 0.343×106 –0.468×104 0 Z1型 0.8675 –0.553 0.629 2.15×105 –6.688×103 51.29 Z2型 0.913 –0.674 0.785 3.12×105 –7.341×103 62.32 Z3型 0.878 –0.576 0.701 2.98×105 –6.89×103 58.31 T1型 1.653 –1.986 2.99 1.785×106 –3.642×105 196.8 T2型 3.715 –2.34 3.54 1.987×106 –4.031×105 213.4 H1型 1.23 –3.86 7.87 2134 12 0.42 H2型 1.27 –3.98 7.98 2053.4 –6 10.76 X型 1.395 –4.21 8.09 12310 –321.58 13.32 表 2 6种典型构型下LJ势、Morse势与拟合势函数与DFT计算值的误差
Table 2. Errors between the LJ potential, Morse potential, fitted potential, and the values calculated by DFT for six typical configurations.
I1型 A1型 T1型 Z1型 H1型 X型 LJ势 25.2% 22.53% 18.66% 18.20% 14.8% 10.7% Morse势 8.49% 8.51% 9.86% 7.73% 8.6% 6.23% Ef势 2.44% 3.3% 3.9% 1.2% 1.3% 0.35% -
[1] 李志辉, 梁杰, 李中华, 李海燕, 吴俊林, 戴金雯, 唐志共 2018 空气动力学学报 36 826
 Google Scholar
Google Scholar
Li Z H, Liang J, Li Z H, Li H Y, Wu J L, Dai J W, Tang Z G 2018 Acta Aerodyn. Sin. 36 826
 Google Scholar
Google Scholar
[2] 罗仕超, 张志刚, 柳军, 龚红明, 胡守超, 吴里银, 常雨, 庄宇, 李贤, 黄成扬 2023 力学学报 55 2439
 Google Scholar
Google Scholar
Luo S C, Zhang Z G, Liu J, Gong H M, Hu S C, Wu L Y, Chang Y, Zhuang N, Li X, Huang C Y 2023 Chin. J. Theor. Appl. Mech. 55 2439
 Google Scholar
Google Scholar
[3] Xing H W, Chen Z X, Yang L , Su Y, Li Y H, Huhe C 2024 Acta Phys. Sin. 73 [094701(in Chinese)[邢赫威, 陈占秀, 杨历, 苏瑶, 李源华, 呼和仓2024 73 094701]]
Xing H W, Chen Z X, Yang L , Su Y, Li Y H, Huhe C 2024 Acta Phys. Sin. 73
[4] Qian X S 1946 J. Math. Phys. 25 247
 Google Scholar
Google Scholar
[5] 樊菁 2013 力学进展 43 185
 Google Scholar
Google Scholar
Fan J 2013 Adv. Mech. 43 185
 Google Scholar
Google Scholar
[6] Bruno D, Frezzotti A, Ghiroldi G P 2015 Phys. Fluids 27 057101
 Google Scholar
Google Scholar
[7] Tokumasu T, Matsumoto Y 2000 JSME Int J. Ser. B 43 288
 Google Scholar
Google Scholar
[8] 李锦, 耿湘人, 陈坚强, 江定武 2016 应用数学和力学 37 1403
Li J, Geng X R, Chen J J, Jiang D W 2016 Appl. Math. Mech. 37 1403
[9] Valentini P, Zhang C, Schwartzentruber T E 2012 Phys. Fluids 24 106101
 Google Scholar
Google Scholar
[10] Baidakov V, Protsenko S, Kozlova Z 2012 J. Chem. Phys. 137 164507
 Google Scholar
Google Scholar
[11] Baidakov V G, Protsenko S P 2014 J. Chem. Phys. 141 114503
 Google Scholar
Google Scholar
[12] Sharma B, Kumar R 2019 Phys. Rev. E 100 090003
[13] Parker J G, Adams C E, Stavseth R M 1953 J. Acoust. Soc. Am. 25 263
 Google Scholar
Google Scholar
[14] Marcy S J 1990 AIAA J. 28 171
 Google Scholar
Google Scholar
[15] Dukhin A S, Goetz P J 2009 J. Chem. Phys. 130 124519
 Google Scholar
Google Scholar
[16] Gu Z, Ubachs W 2013 Opt. Lett. 38 1110
 Google Scholar
Google Scholar
[17] Carnevale E H, Carey C, Larson G 1967 J. Chem. Phys. 47 2829
 Google Scholar
Google Scholar
[18] 赵宁波, 郑洪涛, 李淑英 2018 哈尔滨工程大学学报 39 60
 Google Scholar
Google Scholar
Zhao N B, Zheng H T, Li S Y 2018 J. Harbin Eng. Univ. 39 60
 Google Scholar
Google Scholar
[19] Kubo R 1985 Pure Appl. Chem. 57 201
 Google Scholar
Google Scholar
[20] Hellmann R 2013 Mol. Phys. 111 387
 Google Scholar
Google Scholar
[21] Cramer M S 2012 Phys. Fluids 24 066102
 Google Scholar
Google Scholar
[22] Lu T, Chen F. 2013 J. Mol. Model 19 5387
 Google Scholar
Google Scholar
[23] KASPER P K 2017 J. Phys. Chem. A 121 9092
 Google Scholar
Google Scholar
[24] Vogel E 2012 Int. J. Thermophys 33 741
 Google Scholar
Google Scholar
[25] Heck E L, Dickinson A S 1995 Physica A 217 107
 Google Scholar
Google Scholar
[26] Carnevale E H, Carey C, Larson G 1967 J. Chem. Phy. 4 2829
Metrics
- Abstract views: 297
- PDF Downloads: 9
- Cited By: 0














 DownLoad:
DownLoad: