-
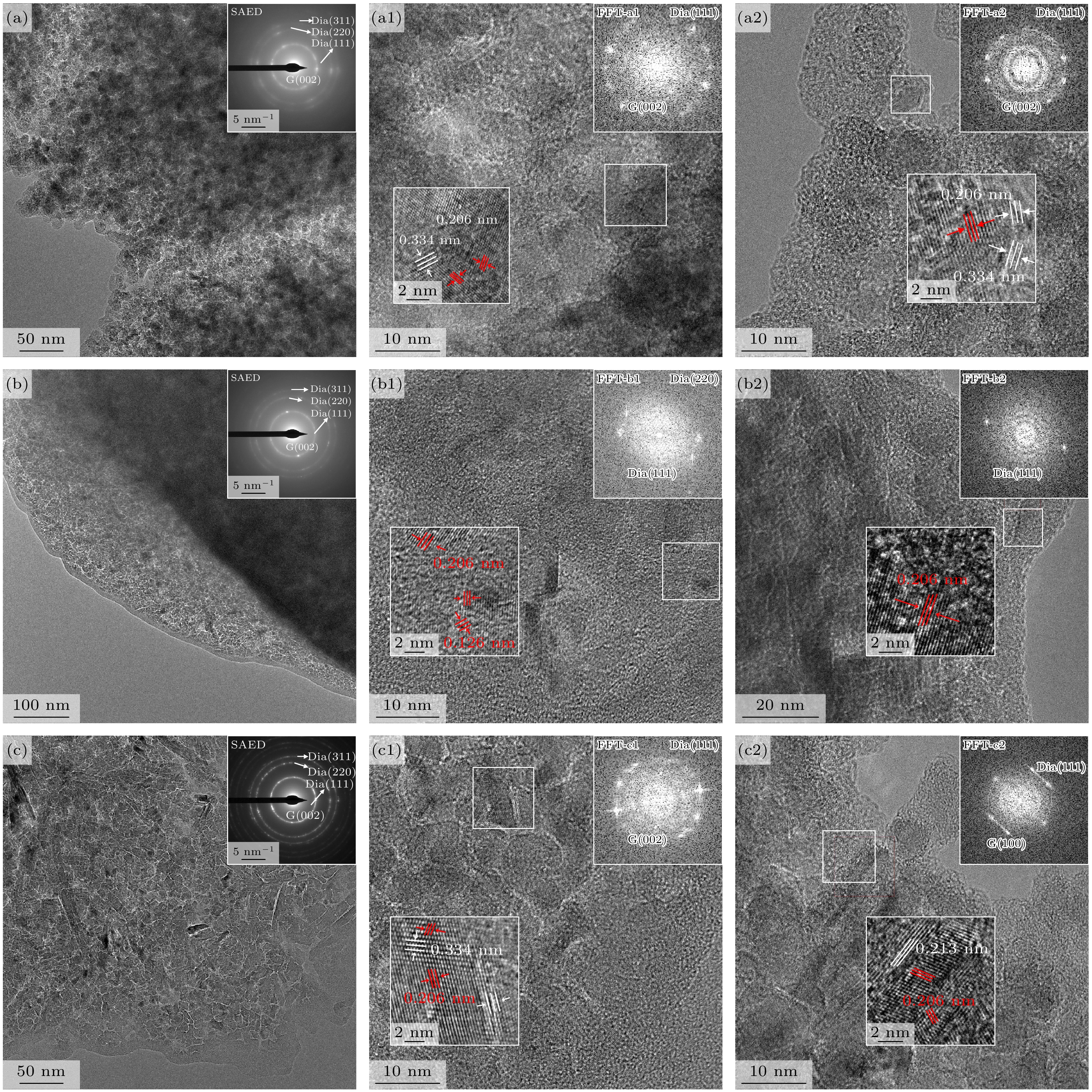
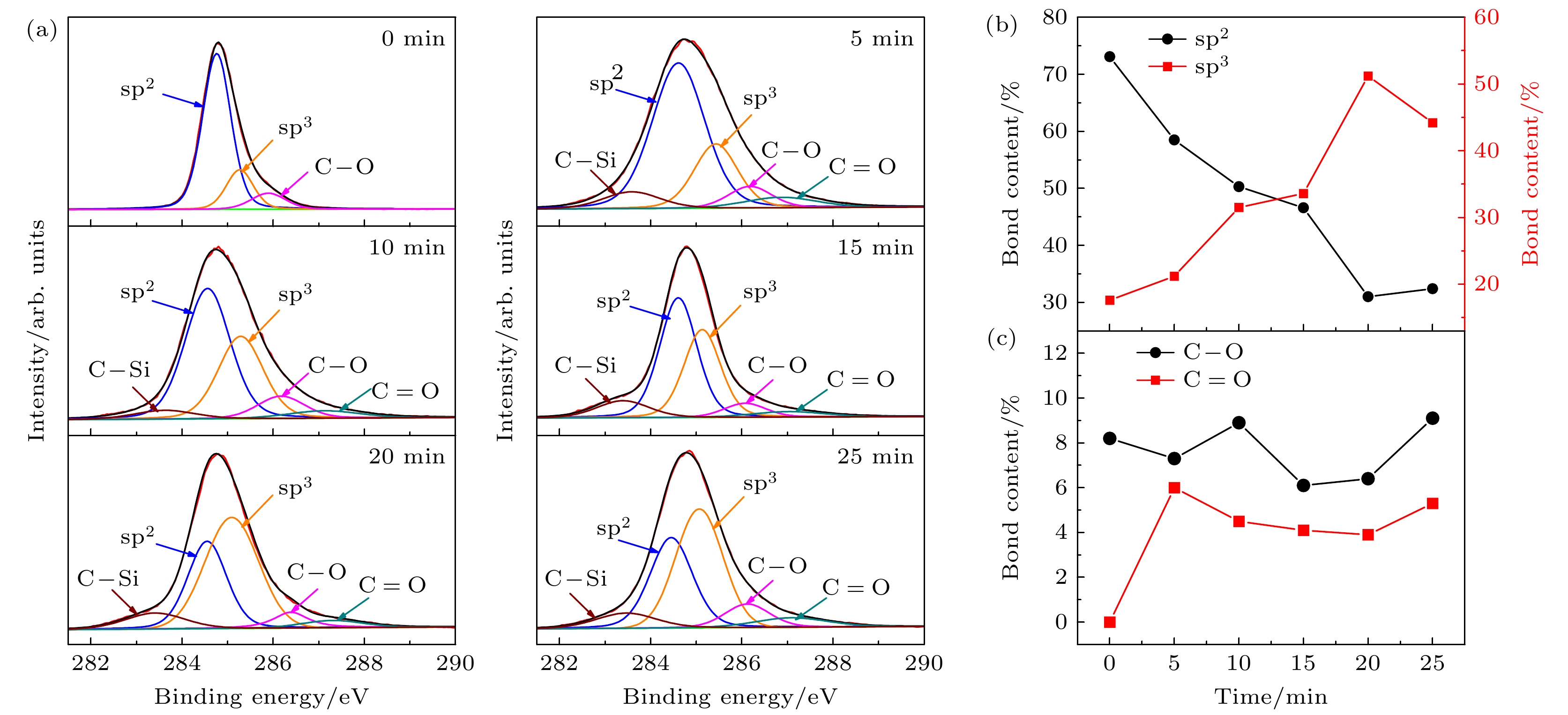
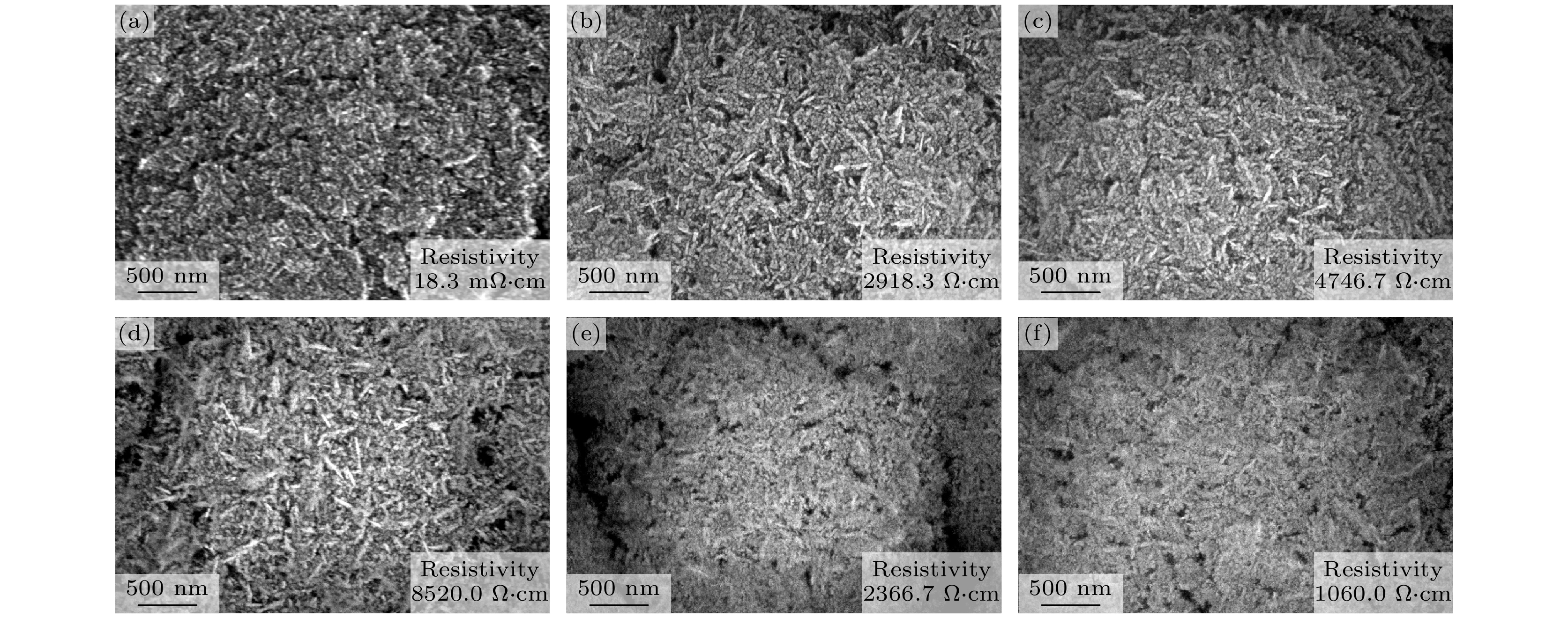
The diamond/graphene composite electrode has garnered significant attention due to its ability to synergistically combine the low background current and broad potential window of the diamond component with the high electrochemical activity of the graphitic component. In this study, argon-oxygen plasma etching is employed to treat nanodiamond/graphite composite films, and the surface structure of the few-layer graphene-coated nanodiamond is obtained by adjusting the etching time to control the number of graphite layers on the surface of the film, and then the surface layer of the few-layer graphene-coated nanodiamond and the bottom layer with good conductivity with more graphite components are constructed to form a double-layer structure. The experimental findings demonstrate that when the argon/oxygen plasma treatment time reaches 5 min, the graphite components on the surface layer of the film are etched into a structure of small-layer graphite coated nanodiamond, which increases the resistivity (2918.3 Ω·cm) and potential window (3.43 V). In addition, the surface state is changed from hydrogen termination to oxygen termination, so that the diamond grain has a positron affinity potential, and the electrochemical active area increases from 387 to 2893 μC/cm2. As the treatment time continues to extend to 20 min, the number of graphite layers on the surface of the film decreases, the diamond phase content increases, the resistivity of the film increases, and the electrochemically active area decreases. When the etching time reaches 25 min, the graphite layer under the composite film is exposed, and the graphite on the surface of the diamond is transformed into few-layer graphene, forming a double-layer structure of the top layer of few-layer graphene-coated diamond and the bottom layer of graphite, which synergistically improves the electrochemical activity (775 μC/cm2), reduces the resistivity of the composite film (1060.0 Ω·cm) and broadens the potential window (3.50 V). This work provides a novel plasma-etching strategy for fabricating diamond/graphene hybrid electrodes, and new insights into using the complementary advantages of these carbon allotropes for advanced electrochemical applications.
-
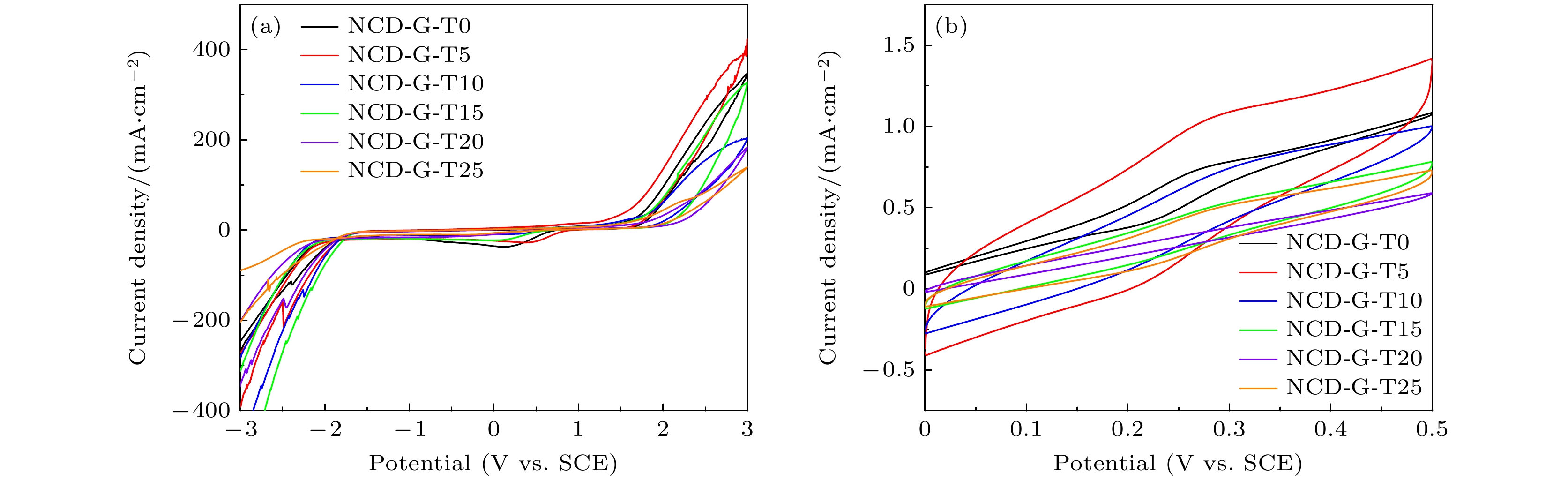
图 2 氩/氧等离子体处理不同时间后NCD-G薄膜电极在不同溶液中的循环伏安i-E曲线 (a) 1 mol/L KCl溶液; (b) 0.001 mol/L [Fe(CN)6]3-/4-和1 mol/L KCl溶液
Figure 2. Cyclic voltammetry i-E curves of NCD-G thin film electrode in different solutions after argon/oxygen plasma treatment for different time: (a) 1 mol/L KCl solution; (b) 0.001 mol/L [Fe(CN)6]3-/4- and 1 mol/L KCl solutions.
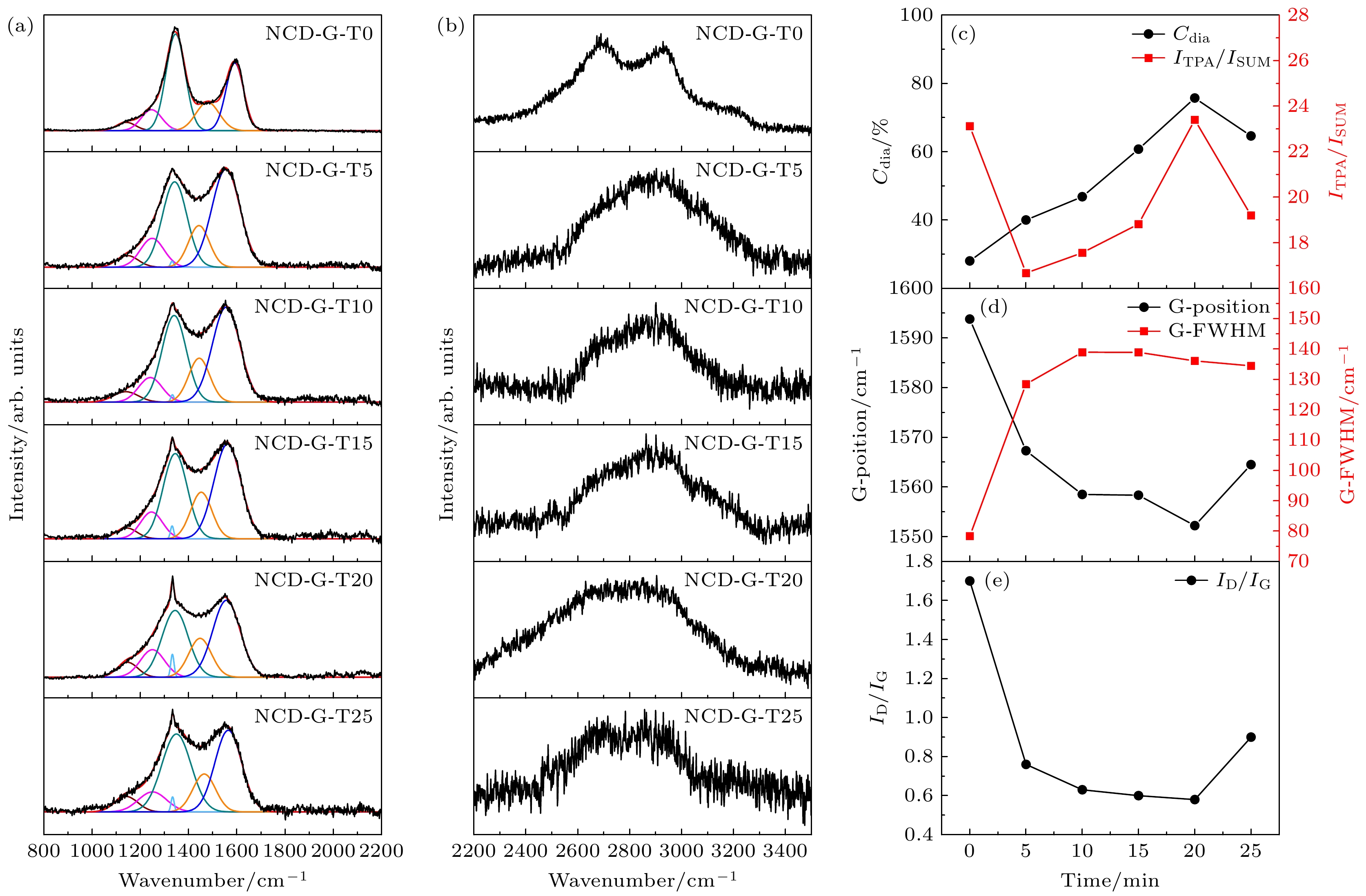
图 3 (a) NCD-G薄膜在800—2200 cm–1范围的可见光Raman光谱及其拟合结果; (b) 2200—3500 cm–1范围Raman谱图; (c)—(e) 800—2200 cm–1可见光Raman光谱拟合后部分典型参数随着氩/氧混合等离子体处理时间的变化图
Figure 3. (a) Visible Raman spectra of NCD-G thin films in the range of 800–2200 cm–1 and their fitting results; (b) Raman spectra in the range of 2200–3500 cm–1; (c)–(e) some typical parameters of 800–2200 cm–1 visible Raman spectra with the treatment time of argon/oxygen mixed plasma.
表 1 氩/氧等离子体处理不同时间后NCD-G薄膜电极在1 mol/L KCl及0.001 mol/L Fe(CN)6]3-/4-溶液中的电化学性能参数
Table 1. Electrochemical performance parameters of NCD-G thin film electrodes in 1 mol/L KCl and 0.001 mol/L [Fe(CN)6]3-/4- solution after argon/oxygen plasma treatment for different time.
Sample Potential window/V Background
current/(mA·cm–2)Peak potential
difference ∆Ep/VElectrochemically
active area /(μC·cm–2)NCD-G-T0 3.32 14.41 0.041 387 NCD-G-T5 3.43 18.52 0.061 2893 NCD-G-T10 3.46 1.66 0.111 1272 NCD-G-T15 3.53 5.14 0.112 811 NCD-G-T20 3.56 1.44 — 257 NCD-G-T25 3.50 10.24 0.075 775 -
[1] Kunuku S, Sankaran K J, Tsai C Y, Chang W H, Tai N H, Leou K C, Lin I N 2013 ACS Appl. Mater. Interfaces 5 7439
 Google Scholar
Google Scholar
[2] Cao Y, Legrain D R, Bigorda O R, Park J M, Watanabe K, Taniguchi T, Herrero P J 2020 Nature 583 215
 Google Scholar
Google Scholar
[3] Jiang M Y, Zhang Z Q, Chen C K, Ma W C, Han S J, Li X, Lu S H, Hu X J 2020 Carbon 168 536
 Google Scholar
Google Scholar
[4] Jiang M Y, Ma W C, Han S J, Chen C K, Fan D, Li X, Hu X J 2020 J. Appl. Phys. 127 015301
 Google Scholar
Google Scholar
[5] Lucio A J, Meyler R E P, Edwards M A, Macpherson J V 2020 ACS Sens. 5 789
 Google Scholar
Google Scholar
[6] Németh P, McColl K, Smith R L, Murri M, GarvieL A J, Alvaro M, Pécz B, Jones A P, Corà F, Salzmann C G, McMillan P F 2020 Nano Lett. 20 3611
 Google Scholar
Google Scholar
[7] Pei J X, Yu X, Zhang Z Q, Zhang J, Wei S B, Boukherroub R 2020 Appl. Surf. Sci. 527 146761
 Google Scholar
Google Scholar
[8] Shi D, Huang N, Liu L S, Yang B, Zhai Z F, Wang Y B, Yuan Z Y, Li H, Gai Z G, Jiang X 2020 Appl. Surf. Sci. 512 145652
 Google Scholar
Google Scholar
[9] Xu J, Yokota Y, Wong R A, Kim Y, Einaga Y 2020 J. Am. Chem. Soc. 142 2310
 Google Scholar
Google Scholar
[10] Arenal R, Bruno P, Miller D J, Bleuel M, Lal J, Gruen D M 2007 Phys. Rev. B 75 195431
 Google Scholar
Google Scholar
[11] Sankaran K J, Kurian J, Chen H C, Dong C L, Lee C Y, Tai N H, Lin I N 2012 J. Phys. D: Appl. Phys. 45 365303
 Google Scholar
Google Scholar
[12] Shang N, Papakonstantinou P, Wang P, Zakharov A, Palnitkar U, Lin I N, Chu M, Stamboulis A 2009 ACS Nano 3 1032
 Google Scholar
Google Scholar
[13] Shalini J, Sankaran K J, Dong C L, Lee C Y, Tai N H, Lin I N 2013 Nanoscale 5 1159
 Google Scholar
Google Scholar
[14] Shalini J, Lin Y C, Chang T H, Sankaran K J, Chen H C, Lin I N, Lee C Y, Tai N H 2013 Electrochim. Acta 92 9
 Google Scholar
Google Scholar
[15] Yuan Q L, Liu Y, Ye C, Sun H Y, Dai D, Wei Q P, Lai G S, Wu T Z, Yu A M, Fu L, Chee K W A, Lin C T 2018 Biosens. Bioelectron. 111 117
 Google Scholar
Google Scholar
[16] 蒋梅燕, 王平, 陈爱盛, 陈成克, 李晓, 鲁少华, 胡晓君 2022 71 198101
 Google Scholar
Google Scholar
Jiang M Y, Wang P, Chen A S, Chen C K, Li X, Lu S H, Hu X J 2022 Acta Phys. Sin. 71 198101
 Google Scholar
Google Scholar
[17] 邢丽丹, 谢启明, 李伟善 2020 69 228205
 Google Scholar
Google Scholar
Xing L D, Xie Q M, Li W S 2020 Acta Phys. Sin. 69 228205
 Google Scholar
Google Scholar
[18] Wang P, Yuan X X, Cui Z, Xu C Y, Sun Z L, Li J H, Liu J S, Tian Y, Li H D 2021 ACS Omega 6 6326
 Google Scholar
Google Scholar
[19] Cançado L G, Jorio A, Ferreira E H M, Stavale F, Achete C A, Capaz R B, Moutinho M V O, Lombardo A, Kulmala T S, Ferrari A C 2011 Nano Lett. 11 3190
 Google Scholar
Google Scholar
[20] Zhang X Y, Wang Y B, Gai Z G, Zhang M, Liu S S, Guo F X, Yang N J, Jiang X 2022 Carbon 196 602
 Google Scholar
Google Scholar
[21] Tully J J, Braxton E, Cobb S J, Breeze B G, Markham M, Newton M E, Rodriguez P, Macpherson J V 2021 Carbon 185 717
 Google Scholar
Google Scholar
[22] Lu Z G, Huang N, Zhai Z F, Chen B, Liu L S, Song H Z, Yuan Z Y, Zhang C Y, Yang B, Jiang X 2022 J. Mater. Sci. Technol. 105 26
 Google Scholar
Google Scholar
[23] Liu F M, Deng Z J, Miao D T, Chen W P, Wang Y J, Zhou K C, Ma L, Wei Q P 2021 J. Environ. Chem. Eng. 9 106369
 Google Scholar
Google Scholar
[24] Wang P, Wang T Y, Yang M C, Wang Q L, Yuan X X, Cui Z, Gao N, Liu J S, Cheng S H, Jiang Z G, Jin H C, Li H D 2024 Small 20 2402481
 Google Scholar
Google Scholar
Metrics
- Abstract views: 791
- PDF Downloads: 5
- Cited By: 0














 DownLoad:
DownLoad: