-
All-solid-state sodium batteries are promising candidates in energy storage applications due to their high safety and low cost. A suitable solid electrolyte is a key component for high-performance all-solid-state sodium battery. Current inorganic solid electrolytes mainly include oxide- and sulfide-based electrolytes. However, the oxide-based electrolytes require to be sinetred above 1000 ℃ for high ionic conductivity, and most sulfide-based electrolytes can react with H2O torelease toxic H2S gas. These features will hinder the practical application of all-solid-state sodium batteries. In recent years, novel sodium ionic conductors have appeared successively. Among them, anti-perovskite type of Li/Na ionic conductor has received a lot of attention because of its high ionic conductivity and flexible structure design. Nevertheless, the synthesis of Na-rich anti-perovskite Na3OBrxI1–x (0 < x < 1) is complex, the ionic conductivity at room temperature is relatively low, and its electrochemical properties remain unknown. Here in this work, the phase-pure Na-rich anti-perovskite Na3OBrxI1–x is synthesized by a facile synthesis way. The X-ray diffraction patterns show that the anti-perovskite structure without any impurity phase is obtained. Alternating-current (AC) impedance spectrum is used for measuring ionic conductivity of electrolyte pellets after thermally being treated at around 100 ℃. The Na3OBr0.3I0.7 exhibits an ionic conductivity of 1.47 × 10–3 S/cm at 100 ℃. Unfortunately, the ionic conductivity experiences a sharp drop with the decrease of temperature, which may be related to the change of structural symmetry and Na sites in the structure revealed by solid state 23Na NMR. In particular, the ionic conductivities of Na3OBrxI1–x demonstrate the potential applications at medium temperature (40-80 ℃ in which the ionic conductivity of Na3OBrxI1–x is close to or higher than 10–4 S/cm) for all-solid-state sodium battery. Therefore, the compatibility against Na metal and the electrochemical performance in all-solid-state batteries have been evaluated. Since Na3OBrxI1–x is not “Na-philic”, the resistance in impedance of the Na/Na3OBr0.5I0.5/Na is very high. However, after modifying the interface by ionic liquid, the Na3OBr0.5I0.5 exhibits good compatibility against Na metal and tiny ionic liquid also leads to high initial discharge specific capacity of 190 mAh/g and excellent cycling stability (around 127 mAh/g after 10 cycles) in the TiS2/Na3OBr0.5I0.5/Na-Sn solid-state battery. The capacity decay maybe results from the inferior interfacial contact between the solid electrolyte and the electrode materials because the electrode materials in this system experience large volume change during cycling. The successful operation in solid-state sodium batteries indicates that the Na3OBrxI1–x is feasible to be used as a sodium solid electrolyte, which is of great importance for practical application of Na-rich anti-perovskite solid electrolytes.
-
Keywords:
- solid-state battery /
- sodium ion electrolyte /
- anti-perovskite /
- electrochemical properties
[1] Li M, Lu J, Chen Z, Amine K 2018 Adv. Mater. 30 e1800561
 Google Scholar
Google Scholar
[2] Service R F 2019 Science 366 292
 Google Scholar
Google Scholar
[3] Yabuuchi N, Kubota K, Dahbi M, Komaba S 2014 Chem. Rev. 114 11636
 Google Scholar
Google Scholar
[4] Lee J M, Singh G, Cha W, Kim S, Yi J, Hwang S J, Vinu A 2020 ACS Energy Lett. 5 1939
 Google Scholar
Google Scholar
[5] Yang C, Xin S, Mai L, You Y 2020 Adv. Energy Mater. 10.1002/aenm.202000974
 Google Scholar
Google Scholar
[6] Rajagopalan R, Tang Y, Jia C, Ji X, Wang H 2020 Energy Environ. Sci. 13 1568
 Google Scholar
Google Scholar
[7] Xiao Y H, Wang Y, Bo S H, Kim J C, Miara L J, Ceder G 2020 Nat. Rev. Mater. 5 105
 Google Scholar
Google Scholar
[8] Feng X, Ren D, He X, Ouyang M 2020 Joule 4 743
 Google Scholar
Google Scholar
[9] Chen R, Li Q, Yu X, Chen L, Li H 2019 Chem. Rev. 120 6820
 Google Scholar
Google Scholar
[10] Xu L, Li J, Deng W, Shuai H, Li S, Xu Z, Li J, Hou H, Peng H, Zou G, Ji X 2020 Adv. Energy Mater. 10.1002/aenm. 202000648
 Google Scholar
Google Scholar
[11] Lu Y, Li L, Zhang Q, Niu Z, Chen J 2018 Joule 2 1747
 Google Scholar
Google Scholar
[12] Kamaya N, Homma K, Yamakawa Y, Hirayama M, Kanno R, Yonemura M, Kamiyama T, Kato Y, Hama S, Kawamoto K, Mitsui A 2011 Nat. Mater. 10 682
 Google Scholar
Google Scholar
[13] Kato Y, Hori S, Saito T, Suzuki K, Hirayama M, Mitsui A, Yonemura M, Iba H, Kanno R 2016 Nat. Energy 1 16030
 Google Scholar
Google Scholar
[14] Zhang Z, Sun Y, Duan X, Peng L, Jia H, Zhang Y, Shan B, Xie J 2019 J. Mater. Chem. A 7 2717
 Google Scholar
Google Scholar
[15] Zhou L, Assoud A, Zhang Q, Wu X, Nazar L F 2019 J. Am. Chem. Soc. 141 19002
 Google Scholar
Google Scholar
[16] Wang C, Fu K, Kammampata S P, McOwen D W, Samson A J, Zhang L, Hitz G T, Nolan A M, Wachsman E D, Mo Y, Thangadurai V, Hu L 2020 Chem. Rev. 120 4257
 Google Scholar
Google Scholar
[17] Zhang Z, Zhang J, Jia H, Peng L, An T, Xie J 2020 J. Power Sources 450
 Google Scholar
Google Scholar
[18] Fuchs T, Culver S P, Till P, Zeier W G 2019 ACS Energy Lett. 5 146
 Google Scholar
Google Scholar
[19] Jia H, Sun Y, Zhang Z, Peng L, An T, Xie J 2019 Energy Storage Mater. 23 508
 Google Scholar
Google Scholar
[20] Hayashi A, Masuzawa N, Yubuchi S, Tsuji F, Hotehama C, Sakuda A, Tatsumisago M 2019 Nat. Commun. 10 5266
 Google Scholar
Google Scholar
[21] Jia H, Liang X, An T, Peng L, Feng J, Xie J 2020 Chem. Mater. 32 4065
 Google Scholar
Google Scholar
[22] Zheng F, Kotobuki M, Song S, Lai M O, Lu L 2018 J. Power Sources 389 198
 Google Scholar
Google Scholar
[23] Jia H, Peng L, Zhang Z, An T, Xie J 2020 J. Energy Chem. 48 102
 Google Scholar
Google Scholar
[24] Narayanan S, Reid S, Butler S, Thangadurai V 2019 Solid State Ionics 331 22
 Google Scholar
Google Scholar
[25] Zhao Y S, Daemen L L 2012 J. Am. Chem. Soc. 134 15042
 Google Scholar
Google Scholar
[26] Wang Y, Wang Q, Liu Z, Zhou Z, Li S, Zhu J, Zou R, Wang Y, Lin J, Zhao Y 2015 J. Power Sources 293 735
 Google Scholar
Google Scholar
[27] Fan S S, Lei M, Wu H, Hu J, Yin C L, Liang T X, Li C L 2020 Energy Storage Mater. 31 87
 Google Scholar
Google Scholar
[28] Yang Q F, Li C L 2018 Energy Storage Mater. 14 100
 Google Scholar
Google Scholar
[29] Nguyen H, Hy S, Wu E, Deng Z, Samiee M, Yersak T, Luo J, Ong S P, Meng Y S 2016 J. Electrochem. Soc. 163 A2165
 Google Scholar
Google Scholar
[30] Braga M H, Ferreira J A, Murchison A J, Goodenough J B 2016 J. Electrochem. Soc. 164 A207
 Google Scholar
Google Scholar
[31] Braga M H, Murchison A J, Ferreira J A, Singh P, Goodenough J B 2016 Energy Environ. Sci. 9 948
 Google Scholar
Google Scholar
[32] Sun Y, Wang Y, Liang X, Xia Y, Peng L, Jia H, Li H, Bai L, Feng J, Jiang H, Xie J 2019 J. Am. Chem. Soc. 141 5640
 Google Scholar
Google Scholar
[33] Wang Y, Wen T, Park C, Kenney B C, Pravica M, Yang W, Zhao Y 2016 J. Appl. Phys. 119 025901
 Google Scholar
Google Scholar
[34] Zhu J, Wang Y, Li S, Howard J W, Neuefeind J, Ren Y, Wang H, Liang C, Yang W, Zou R, Jin C, Zhao Y 2016 Inorg. Chem. 55 5993
 Google Scholar
Google Scholar
[35] Lv Z L, Cui H L, Wang H, Li X H, Ji G F 2017 Phys. Status Solidi B) 254 1700089
 Google Scholar
Google Scholar
[36] Dawson J A, Chen H, Islam M S 2018 J. Phys. Chem. C 122 23978
 Google Scholar
Google Scholar
[37] Pham T L, Samad A, Kim H J, Shin Y H 2018 J. Appl. Phys. 124 164106
 Google Scholar
Google Scholar
[38] Wan T H, Lu Z, Ciucci F 2018 J. Power Sources 390 61
 Google Scholar
Google Scholar
[39] Fang H, Jena P 2019 ACS Appl. Mater. Interfaces 11 963
 Google Scholar
Google Scholar
[40] Yu Y, Wang Z, Shao G 2019 J. Mater. Chem. A 7 21985
 Google Scholar
Google Scholar
[41] Hippler K, Sitta S, Vogt P, Sabrowsky H 1990 Acta Cryst. C 46 736
[42] Hu J L, Yao Z G, Chen K Y, Li C L 2020 Energy Storage Mater. 28 37
 Google Scholar
Google Scholar
-
图 2 通过(a)冷压和(b)热压方法制备得到的Na3OBr0.5I0.5电解质片的SEM图; (c)不同温度下测得的热压Na3OBr0.5I0.5片的Nyquist曲线; (d) Na3OBrxI1–x (x = 0.3, 0.5, 0.7)的logσ与1000/T对应曲线
Figure 2. SEM images of (a) cold-pressed and (b) hot-pressed Na3OBr0.5I0.5 solid electrolyte pellets; (c) Nyquist plots of hot-pressed Na3OBr0.5I0.5 measured at different temperatures; (d) logσ versus 1000/T plots for Na3OBrxI1–x (x = 0.3, 0.5, 0.7).
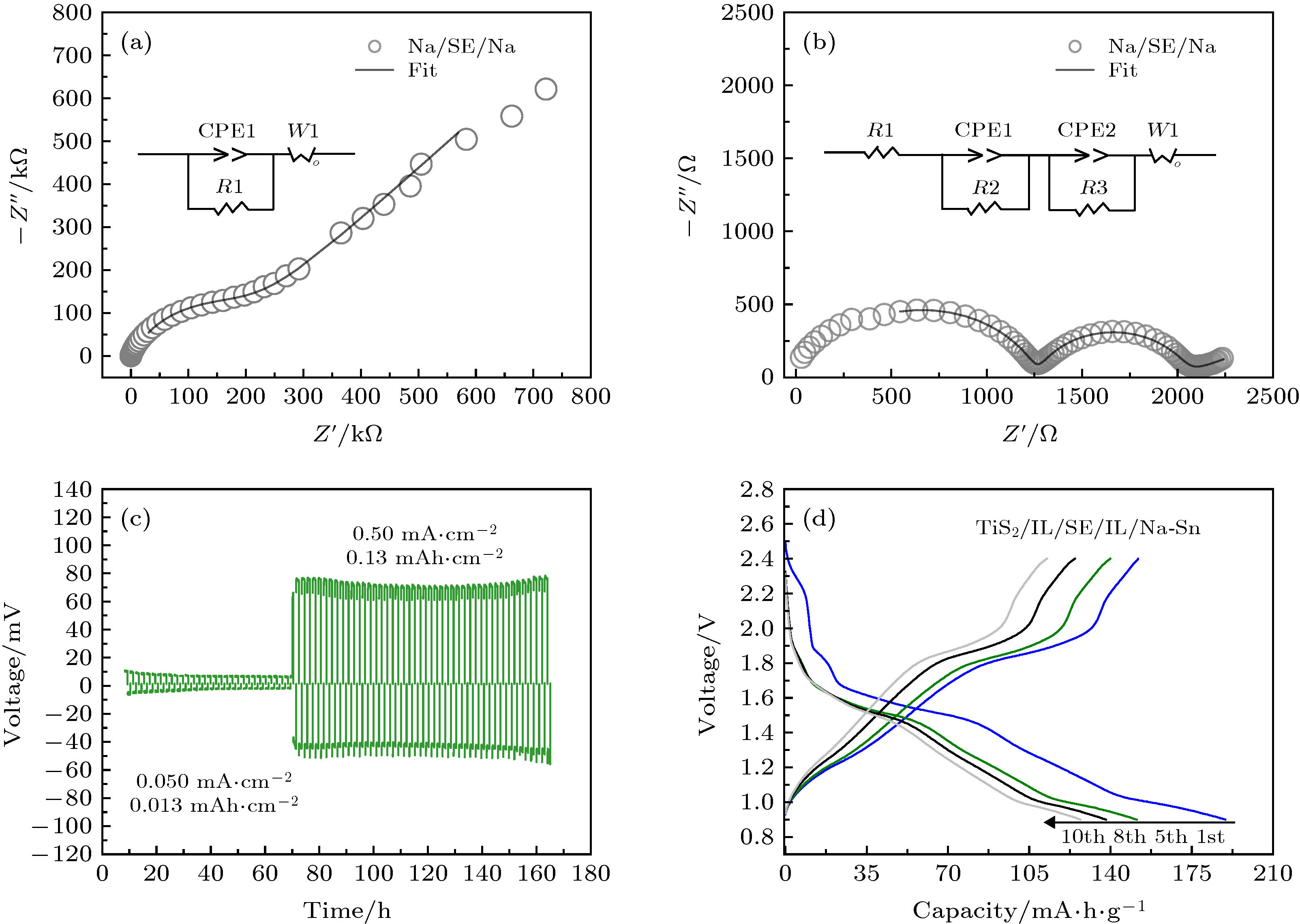
图 4 (a) Na/Na3OBr0.5I0.5/Na对称电池的电化学阻抗谱; (b) 添加了离子液体的Na/IL/Na3OBr0.5I0.5/IL/Na对称电池的电化学阻抗谱; (c) Na/IL/Na3OBr0.5I0.5/IL/Na对称电池在不同电流密度下的充放电曲线; (d) TiS2/IL/Na3OBr0.5I0.5/IL/Na-Sn在50 ℃, 0.1 C条件下充放电曲线
Figure 4. (a) Electrochemical impedance plot of Na/Na3OBr0.5I0.5/Na symmetrical cell; (b) electrochemical impedance plot of Na/IL/Na3OBr0.5I0.5/IL/Na symmetrical cell with ionic liquid; (c) charge-discharge curves of Na/IL/Na3OBr0.5I0.5/IL/Na symmetrical cell at different current density; (d) charge-discharge curves of TiS2/IL/Na3OBr0.5I0.5/IL/Na-Sn operated at 50°C, 0.1 C.
表 1 Na3OBr0.5I0.5在冷压和热压下的密度
Table 1. Density of hot- and cold-pressed Na3OBr0.5I0.5
密度/ g·cm–3 致密度 冷压 2.11 69% 热压 2.55 83% 真实密度* 3.06 — *真实密度基于XRD谱得到的晶格参数计算, 晶格参数计算基于简单的立方相[26], 忽略结构对称性破坏引起的细微变化. 表 2 Na3OBrxI1–x (x = 0.3, 0.5, 0.7)离子电导率
Table 2. Ionic conductivity of Na3OBrxI1–x (x = 0.3, 0.5, 0.7).
温度/℃ 离子电导率/ S·cm–1 Na3OBr0.7I0.3 Na3OBr0.5I0.5 Na3OBr0.3I0.7 130 1.67 × 10–3 — — 110 8.96 × 10–4 1.32 × 10–3 5.55 × 10–3 100 4.50 × 10–4 6.56 × 10–4 1.47 × 10–3 80 9.73 × 10–5 2.01 × 10–4 3.93 × 10–4 60 1.22 × 10–5 6.78 × 10–5 2.05 × 10–4 40 — 1.06 × 10–5 — -
[1] Li M, Lu J, Chen Z, Amine K 2018 Adv. Mater. 30 e1800561
 Google Scholar
Google Scholar
[2] Service R F 2019 Science 366 292
 Google Scholar
Google Scholar
[3] Yabuuchi N, Kubota K, Dahbi M, Komaba S 2014 Chem. Rev. 114 11636
 Google Scholar
Google Scholar
[4] Lee J M, Singh G, Cha W, Kim S, Yi J, Hwang S J, Vinu A 2020 ACS Energy Lett. 5 1939
 Google Scholar
Google Scholar
[5] Yang C, Xin S, Mai L, You Y 2020 Adv. Energy Mater. 10.1002/aenm.202000974
 Google Scholar
Google Scholar
[6] Rajagopalan R, Tang Y, Jia C, Ji X, Wang H 2020 Energy Environ. Sci. 13 1568
 Google Scholar
Google Scholar
[7] Xiao Y H, Wang Y, Bo S H, Kim J C, Miara L J, Ceder G 2020 Nat. Rev. Mater. 5 105
 Google Scholar
Google Scholar
[8] Feng X, Ren D, He X, Ouyang M 2020 Joule 4 743
 Google Scholar
Google Scholar
[9] Chen R, Li Q, Yu X, Chen L, Li H 2019 Chem. Rev. 120 6820
 Google Scholar
Google Scholar
[10] Xu L, Li J, Deng W, Shuai H, Li S, Xu Z, Li J, Hou H, Peng H, Zou G, Ji X 2020 Adv. Energy Mater. 10.1002/aenm. 202000648
 Google Scholar
Google Scholar
[11] Lu Y, Li L, Zhang Q, Niu Z, Chen J 2018 Joule 2 1747
 Google Scholar
Google Scholar
[12] Kamaya N, Homma K, Yamakawa Y, Hirayama M, Kanno R, Yonemura M, Kamiyama T, Kato Y, Hama S, Kawamoto K, Mitsui A 2011 Nat. Mater. 10 682
 Google Scholar
Google Scholar
[13] Kato Y, Hori S, Saito T, Suzuki K, Hirayama M, Mitsui A, Yonemura M, Iba H, Kanno R 2016 Nat. Energy 1 16030
 Google Scholar
Google Scholar
[14] Zhang Z, Sun Y, Duan X, Peng L, Jia H, Zhang Y, Shan B, Xie J 2019 J. Mater. Chem. A 7 2717
 Google Scholar
Google Scholar
[15] Zhou L, Assoud A, Zhang Q, Wu X, Nazar L F 2019 J. Am. Chem. Soc. 141 19002
 Google Scholar
Google Scholar
[16] Wang C, Fu K, Kammampata S P, McOwen D W, Samson A J, Zhang L, Hitz G T, Nolan A M, Wachsman E D, Mo Y, Thangadurai V, Hu L 2020 Chem. Rev. 120 4257
 Google Scholar
Google Scholar
[17] Zhang Z, Zhang J, Jia H, Peng L, An T, Xie J 2020 J. Power Sources 450
 Google Scholar
Google Scholar
[18] Fuchs T, Culver S P, Till P, Zeier W G 2019 ACS Energy Lett. 5 146
 Google Scholar
Google Scholar
[19] Jia H, Sun Y, Zhang Z, Peng L, An T, Xie J 2019 Energy Storage Mater. 23 508
 Google Scholar
Google Scholar
[20] Hayashi A, Masuzawa N, Yubuchi S, Tsuji F, Hotehama C, Sakuda A, Tatsumisago M 2019 Nat. Commun. 10 5266
 Google Scholar
Google Scholar
[21] Jia H, Liang X, An T, Peng L, Feng J, Xie J 2020 Chem. Mater. 32 4065
 Google Scholar
Google Scholar
[22] Zheng F, Kotobuki M, Song S, Lai M O, Lu L 2018 J. Power Sources 389 198
 Google Scholar
Google Scholar
[23] Jia H, Peng L, Zhang Z, An T, Xie J 2020 J. Energy Chem. 48 102
 Google Scholar
Google Scholar
[24] Narayanan S, Reid S, Butler S, Thangadurai V 2019 Solid State Ionics 331 22
 Google Scholar
Google Scholar
[25] Zhao Y S, Daemen L L 2012 J. Am. Chem. Soc. 134 15042
 Google Scholar
Google Scholar
[26] Wang Y, Wang Q, Liu Z, Zhou Z, Li S, Zhu J, Zou R, Wang Y, Lin J, Zhao Y 2015 J. Power Sources 293 735
 Google Scholar
Google Scholar
[27] Fan S S, Lei M, Wu H, Hu J, Yin C L, Liang T X, Li C L 2020 Energy Storage Mater. 31 87
 Google Scholar
Google Scholar
[28] Yang Q F, Li C L 2018 Energy Storage Mater. 14 100
 Google Scholar
Google Scholar
[29] Nguyen H, Hy S, Wu E, Deng Z, Samiee M, Yersak T, Luo J, Ong S P, Meng Y S 2016 J. Electrochem. Soc. 163 A2165
 Google Scholar
Google Scholar
[30] Braga M H, Ferreira J A, Murchison A J, Goodenough J B 2016 J. Electrochem. Soc. 164 A207
 Google Scholar
Google Scholar
[31] Braga M H, Murchison A J, Ferreira J A, Singh P, Goodenough J B 2016 Energy Environ. Sci. 9 948
 Google Scholar
Google Scholar
[32] Sun Y, Wang Y, Liang X, Xia Y, Peng L, Jia H, Li H, Bai L, Feng J, Jiang H, Xie J 2019 J. Am. Chem. Soc. 141 5640
 Google Scholar
Google Scholar
[33] Wang Y, Wen T, Park C, Kenney B C, Pravica M, Yang W, Zhao Y 2016 J. Appl. Phys. 119 025901
 Google Scholar
Google Scholar
[34] Zhu J, Wang Y, Li S, Howard J W, Neuefeind J, Ren Y, Wang H, Liang C, Yang W, Zou R, Jin C, Zhao Y 2016 Inorg. Chem. 55 5993
 Google Scholar
Google Scholar
[35] Lv Z L, Cui H L, Wang H, Li X H, Ji G F 2017 Phys. Status Solidi B) 254 1700089
 Google Scholar
Google Scholar
[36] Dawson J A, Chen H, Islam M S 2018 J. Phys. Chem. C 122 23978
 Google Scholar
Google Scholar
[37] Pham T L, Samad A, Kim H J, Shin Y H 2018 J. Appl. Phys. 124 164106
 Google Scholar
Google Scholar
[38] Wan T H, Lu Z, Ciucci F 2018 J. Power Sources 390 61
 Google Scholar
Google Scholar
[39] Fang H, Jena P 2019 ACS Appl. Mater. Interfaces 11 963
 Google Scholar
Google Scholar
[40] Yu Y, Wang Z, Shao G 2019 J. Mater. Chem. A 7 21985
 Google Scholar
Google Scholar
[41] Hippler K, Sitta S, Vogt P, Sabrowsky H 1990 Acta Cryst. C 46 736
[42] Hu J L, Yao Z G, Chen K Y, Li C L 2020 Energy Storage Mater. 28 37
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 11048
- PDF Downloads: 394
- Cited By: 0















 DownLoad:
DownLoad: