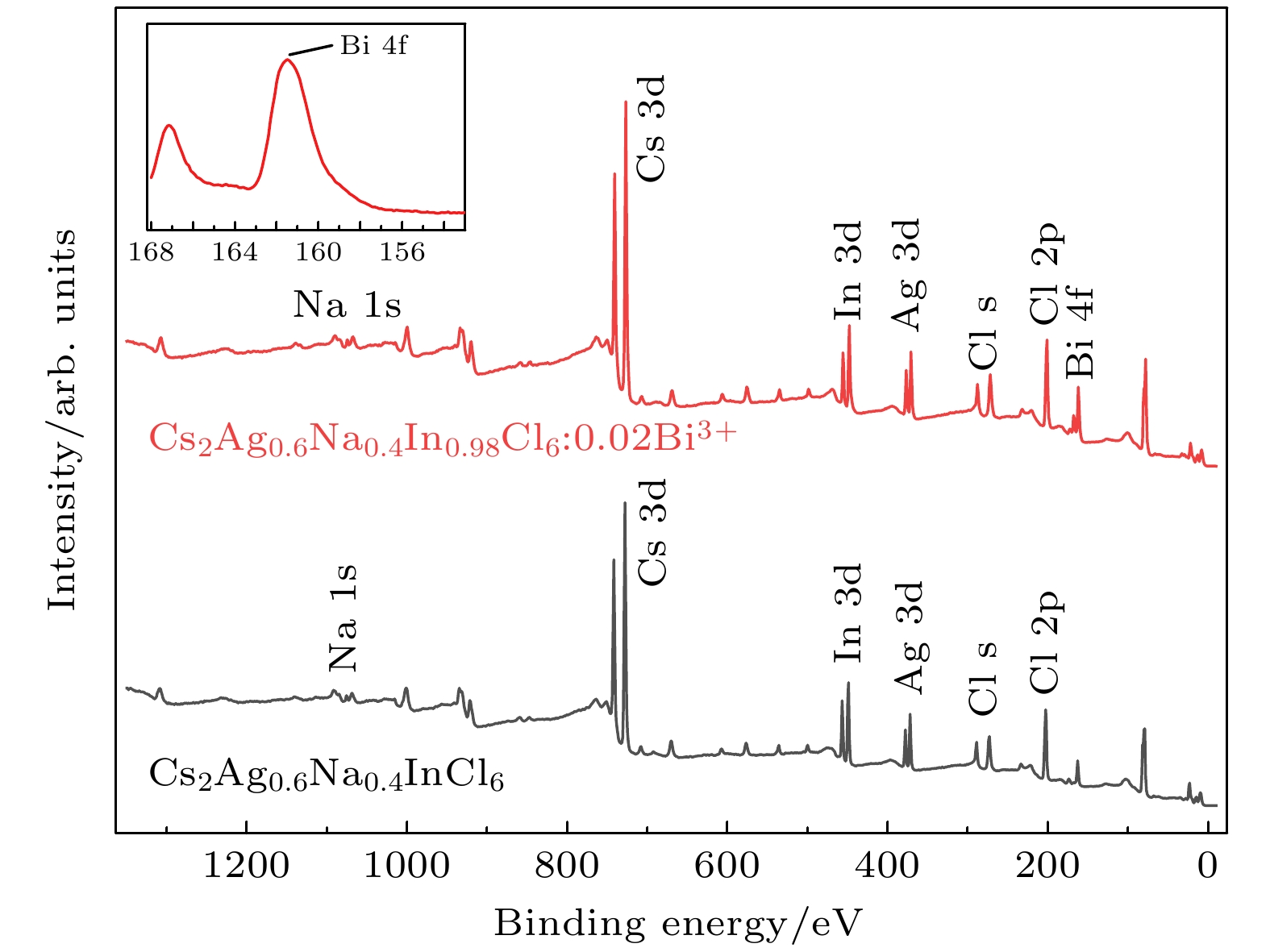
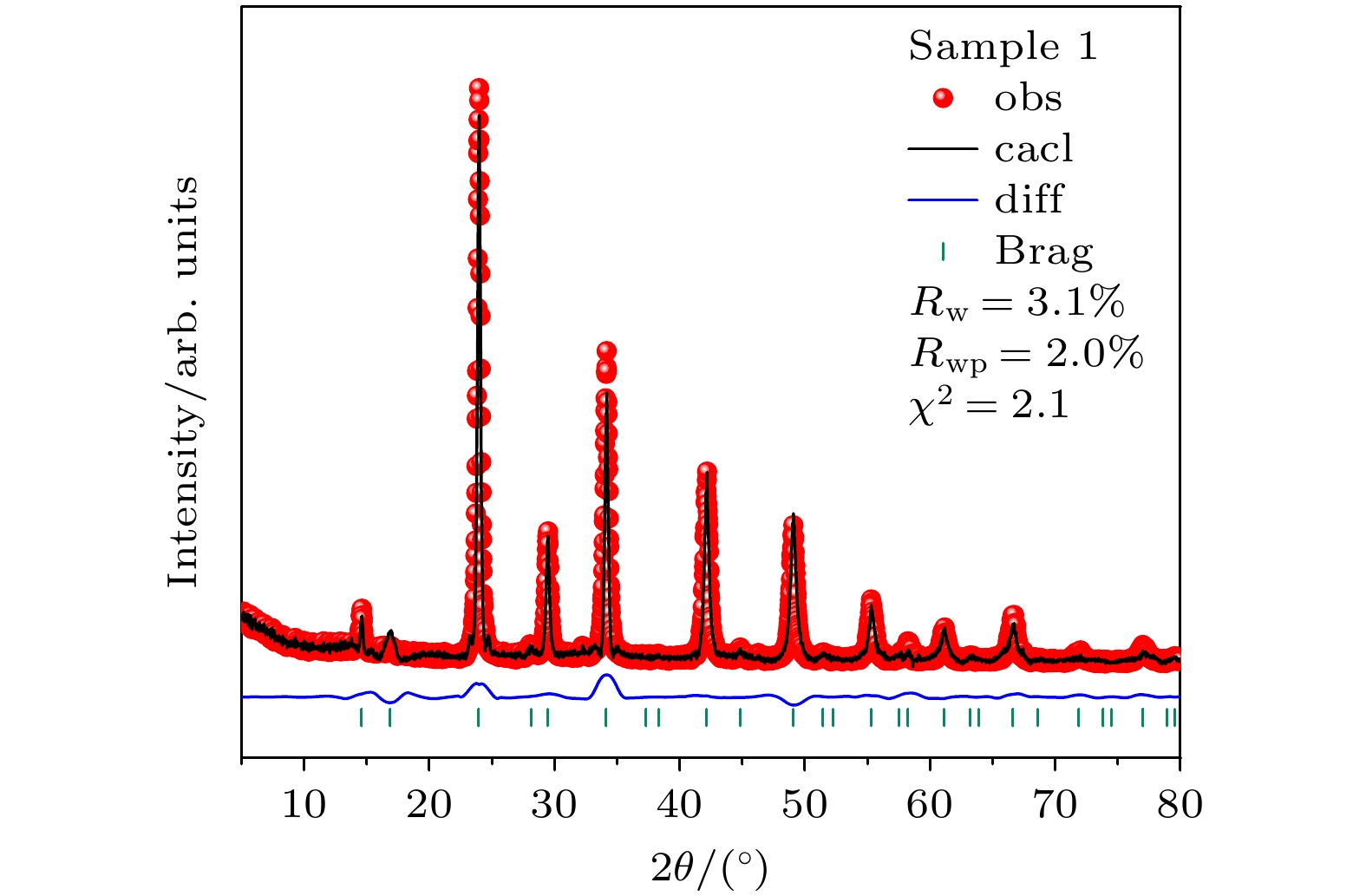
-
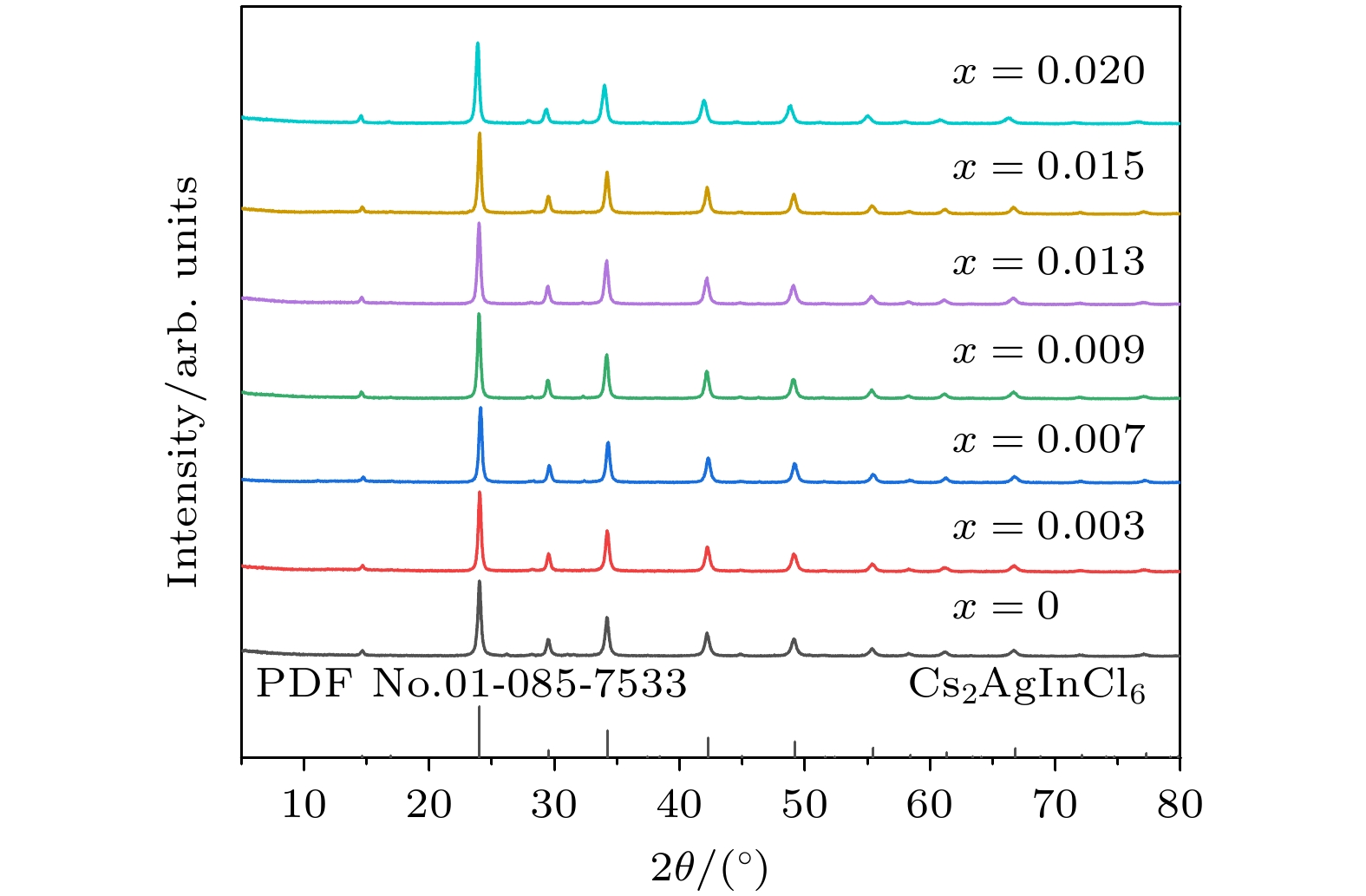
We need to develop a low energy consumption, green and environmentally friendly process for preparing double perovskite fluorescent powders, and incorporate other metal ions into the matrix to obtain a new type of luminescent material with high quantum efficiency. In this study a microwave solid-state method is used to prepare Bi3+ doped lead-free double perovskite Cs2Ag0.6Na0.4InCl6 fluorescent powders. This method does not require ligand assistance and is environmentally friendly. The crystal structure and morphology are characterized by X-ray diffraction and scanning electron microscopy, and the luminescence performance is studied by excitation spectroscopy, emission spectroscopy, time-resolved spectroscopy, and quantum efficiency. The results are shown below 1) The Cs2Ag0.6Na0.4InCl6 is a cubic crystal belonging to the
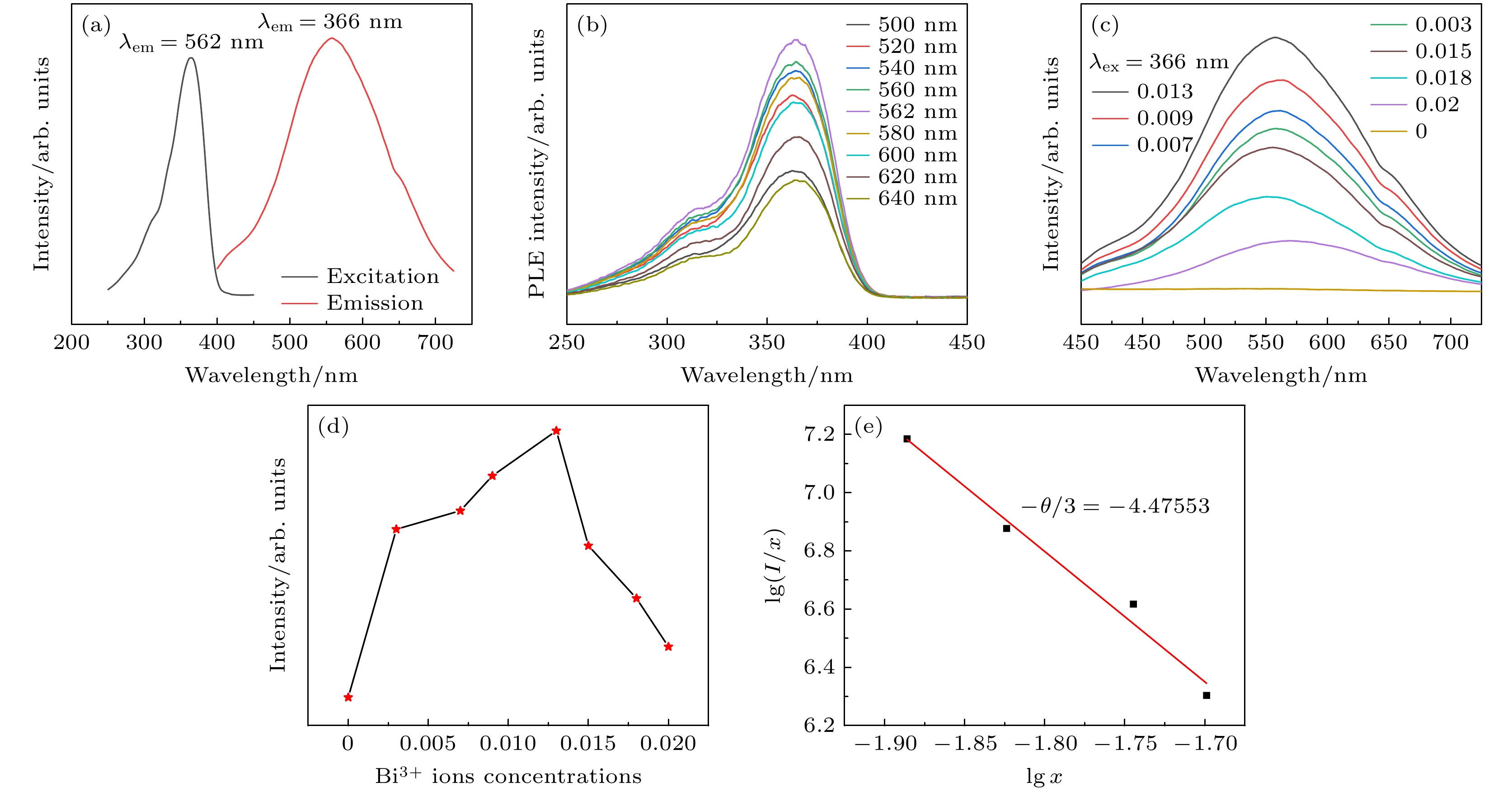
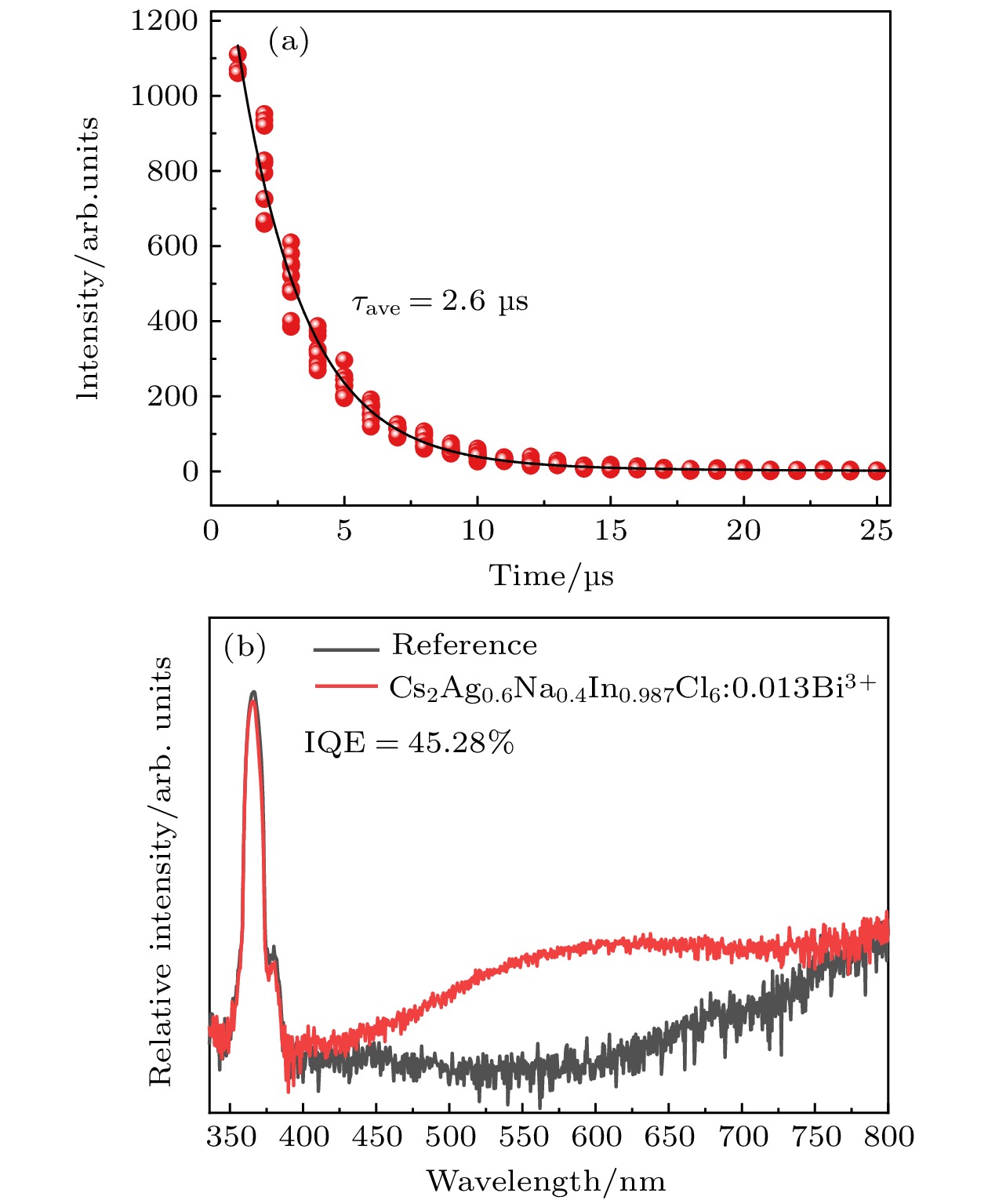
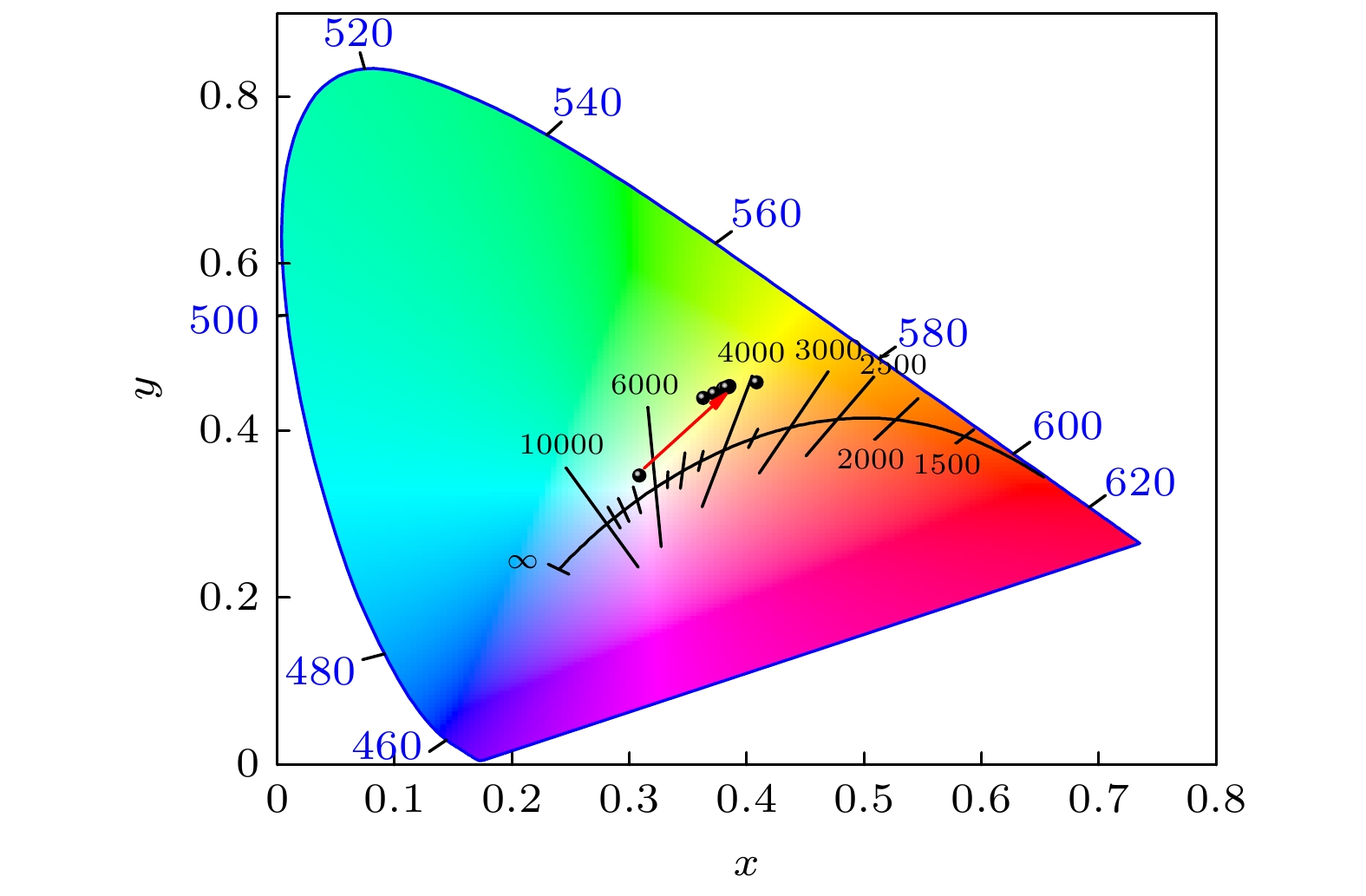
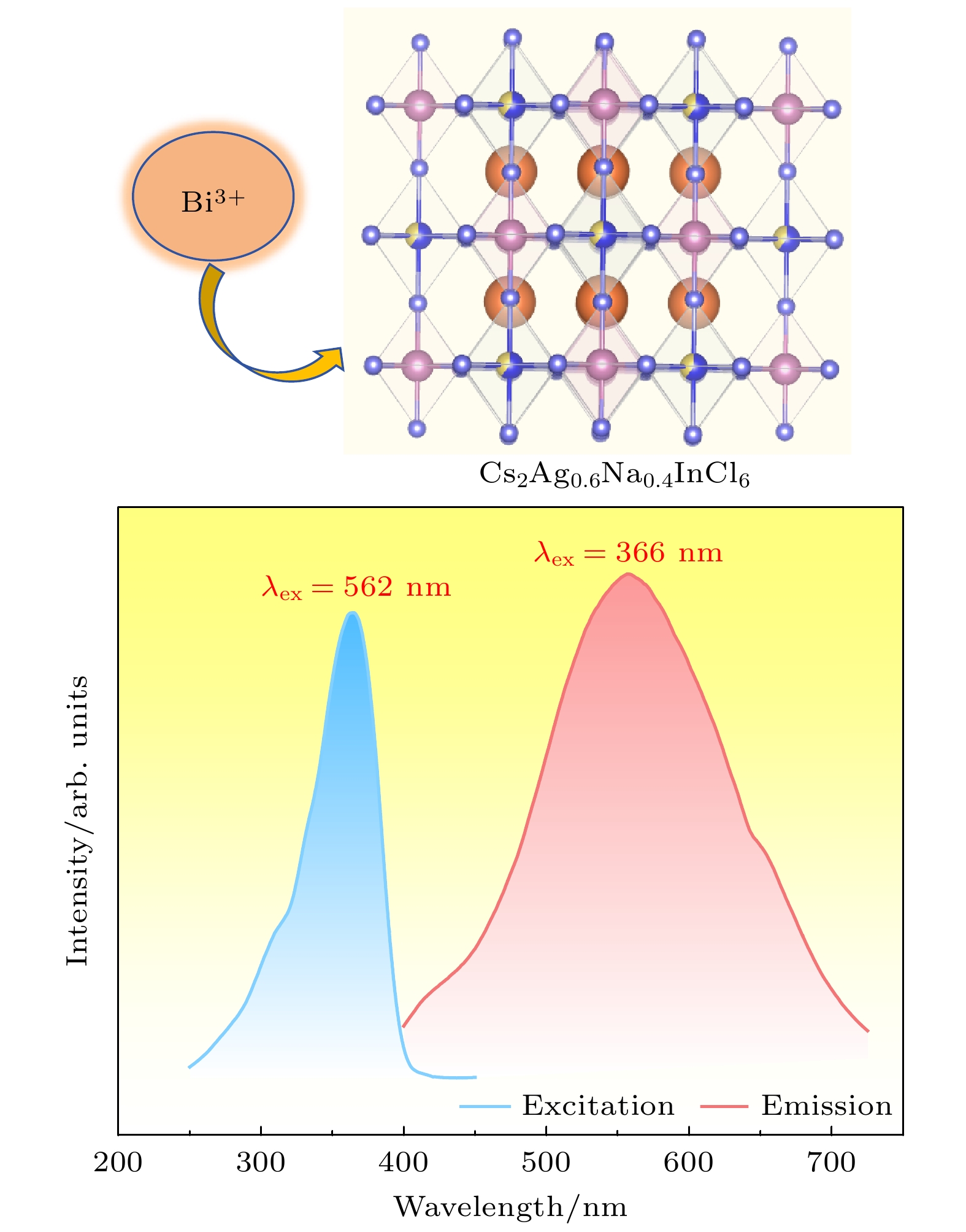
$ Fm\bar 3 m $ space group, and its grain morphology is irregular. 2) When the optimal doping concentration of Bi3+ is 0.0013 mmol, the emission center wavelength of Cs2Ag0.6Na0.4InCl6 material is 562 nm, the average fluorescence lifetime reaches 2.60 μs, and the quantum efficiency attains 45.28%. 3) When the concentration of Bi3+ ions exceeds 0.0013 mmol, a significant concentration quenching effect occurs, which is mainly due to the electric quadrupole electric quadrupole (q-q) interaction between Bi3+ ions. 4) The chromaticity coordinates of the Cs2Ag0.6Na0.4InCl6 doped Bi3+phosphor are located in the yellow-light region, making it a potential yellow phosphor for warm white light emitting diode (LED) applications.-
Keywords:
- lead free double perovskite /
- microwave solid-state method /
- Bi3+ doping /
- yellow fluorescent powder
[1] Yu Y X, Zhou Y F, Ang Y J, Zhang Y Q, Liu X D, Liang X J, Liu J P, Chen S Q, Xiang W D 2021 J. Lumin. 236 118129
 Google Scholar
Google Scholar
[2] Xu Q L, Yang D W, Lü J, Sun Y Y, Zhang L J 2018 Small Methods 2 1700316
 Google Scholar
Google Scholar
[3] Zhao X G, Yang D W, Ren J C, Sun Y H, Xiao Z W, Zhang L J 2018 Joule 2 1662
 Google Scholar
Google Scholar
[4] Filip M R, Hillman S, Haghighirad A A, Snaith H J, Giustino F 2016 J. Phys. Chem. Lett. 7 2579
 Google Scholar
Google Scholar
[5] Wei F X, Deng Z Y, Sun S J, Xie F, Kieslich G, Evans, Donald M 2016 Mater. Horiz. 3 328
 Google Scholar
Google Scholar
[6] Wei F X, Deng Z Y, Sun S J, Zhang F H, Evans-Donald M, Kieslich G, Tominaka S, Carpenter-Michael A, Zhang J, Bristowe-Paul D, Cheetham-Anthony K 2017 Chem. Mater. 29 1089
 Google Scholar
Google Scholar
[7] Tran T, Thao, Panella J R, Chamorro J R, Morey J R, McQueen T M 2017 Mater. Horiz. 4 688
 Google Scholar
Google Scholar
[8] Bekenstein Y, Dahl J C, Huang J M, Osowiecki W T, Swabeck J K, Chan E M, Yang P D, Alivisatos A P 2018 Nano Lett. 18 3502
 Google Scholar
Google Scholar
[9] Hao X G, Yang D G, Sun Y H, Li T S, Zhang L J, Yu L P, Zunger A 2017 J. Am. Chem. Soc. 139 6718
 Google Scholar
Google Scholar
[10] Jain A, Voznyy O, Sargent E H 2017 J. Phys. Chem. 121 7183
 Google Scholar
Google Scholar
[11] 皮慧慧, 李国辉, 周博林, 崔艳霞 2021 发光学报 42 650
 Google Scholar
Google Scholar
Pi H H, Li G H, Zhou B L, Cui Y X 2021 J. Lumin. 42 650
 Google Scholar
Google Scholar
[12] Li X, Gao X P, Zhang X T, Shen X Y, Lu M, Wu J L, Shi Z F, Colvin Vicki L, Hu J H, Bai X, Yu W W, Zhang Y 2021 Adv. Sci. Lett. 8 2003334
 Google Scholar
Google Scholar
[13] 李鑫 2023 博士学位论文 (吉林: 吉林大学)
Li X 2023 Ph. D. Dissertation (Jilin: Jilin University
[14] Akihiro K, Kenjiro T, Yasuo S, Tsutomu M 2009 J. Am. Chem. Soc. 131 6050
 Google Scholar
Google Scholar
[15] Aron W 2015 J. Phys. Chem. 119 5755
 Google Scholar
Google Scholar
[16] Hoefler S F, Trimmel G, Rath T 2017 Monatsh. Chem. 148 795
 Google Scholar
Google Scholar
[17] Hu H, Dong B H, Zhang W 2017 J. Mater. Chem. 5 11436
 Google Scholar
Google Scholar
[18] Cho H, Kim Y H, Wolf C, Lee H D, Lee T W 2018 Adv. Mater. 30 1704587
 Google Scholar
Google Scholar
[19] Quan L N, Rand B P, Friend R H, Mhaisalkar S G, Lee T W, Sargent E H 2019 Chem. Rev. 119 7444
 Google Scholar
Google Scholar
[20] Majher J D, Gray M B, Strom T A, Patrick M 2019 Chem. Mater. 31 1738
 Google Scholar
Google Scholar
[21] Zhang H N, Dun G H, Feng Q X, Zhao R, Liang R Q, Gao X Y, Thomas H, Chen M, Geng X S, Liu M Y, Huang Y, Zheng X R, Qin K, Tan X C, Wang X F, Xie D, Yang Y, Tian H, Zhou Y Y, Nitin P P, Wang X Y, Hong J W, Ren T L 2020 IEEE Trans. Electron Devices 67 3191
 Google Scholar
Google Scholar
[22] Bartel C J, Sutton C, Goldsmith B R, Ouyang R H, Charles B M, Luca M G, Matthias S 2019 Sci. Adv. 5 eaav0693
 Google Scholar
Google Scholar
[23] Blasse G 1968 Phys. Lett. A. 6 444
 Google Scholar
Google Scholar
[24] Xiao F, Yi R X, Yuan H L, Zang G J, Xie C N 2018 Spectrochim. Acta A 202 352
 Google Scholar
Google Scholar
[25] Luo J J, Wang X M, Li S R, Liu J, Guo Y M, Niu G D, Yao L, Fu Y H, Gao L, Dong Q S, Zhao C Y, Leng M Y, Ma F S, Liang W X, Wang L D, Jin S Y, Han J B, Zhang L J, Etheridge J, Wang J B, Yan Y F, Sargent E H, Tang J 2018 Nature 563 541
 Google Scholar
Google Scholar
[26] Tan Z F, Li J H, Zhang C, Li Z, Hu Q S, Xiao Z W, Toshio K, Hideo H, Guangda N, Efrat L, Cheng Y B, Tang J 2018 Adv. Funct. Mater. 28 1801131
 Google Scholar
Google Scholar
[27] Han P G, Mao X, Yang S Q, Zhang F, Yang B, Wei D H, Deng W Q, Han K L 2019 Angew. Chem. Int. Ed. 58 17231
 Google Scholar
Google Scholar
[28] Li J H, Tan Z F, Hu M C, Chen C, Luo J J, Li S R, Gao L, Xiao Z W, Niu G D, Tang J 2019 Front. Optoelectron. 12 352
 Google Scholar
Google Scholar
[29] Rainer W N 2013 J. Phys. Conf. Ser. 443 012080
 Google Scholar
Google Scholar
[30] Gong X K, Zhang X S, Li Q, Liu L, Zhang Y M, Li C, Kong L N, Xu J P, Li L 2023 J. Colloid Interface Sci. 648 865
 Google Scholar
Google Scholar
-
图 5 (a)室温下Cs2Ag0.6Na0.4In0.987Cl6:0.013Bi3+ 荧光粉的激发和发射光谱图; (b)依赖发射波长的Cs2Ag0.6Na0.4In0.987Cl6:0.013Bi3+激发光谱(250—450 nm); (c)室温下Cs2Ag0.6Na0.4In1–xCl6:xBi3+荧光粉的发射光谱图; (d) Bi3+掺杂浓度与发射荧光强度图; (e) Cs2Ag0.6Na0.4In1–xCl6:xBi3+的lg(I/x)与lg(x)关系曲线
Figure 5. (a) Excitation and emission spectra of Cs2Ag0.6Na0.4In0.987Cl6:0.013Bi3+ phosphor at room temperature; (b) emission wavelength dependent excitation spectrum of Cs2Ag0.6Na0.4In0.987Cl6:0.013Bi3+, from 250 nm to 450 nm; (c) emission spectra of Cs2Ag0.6Na0.4In1–xCl6:xBi3+ phosphor at room temperature; (d) emission fluorescence intensity versus Bi3+ doping concentration; (e) curve of lg(I/x) versus lg(x) for Cs2Ag0.6Na0.4In1–xCl6:xBi3+ .
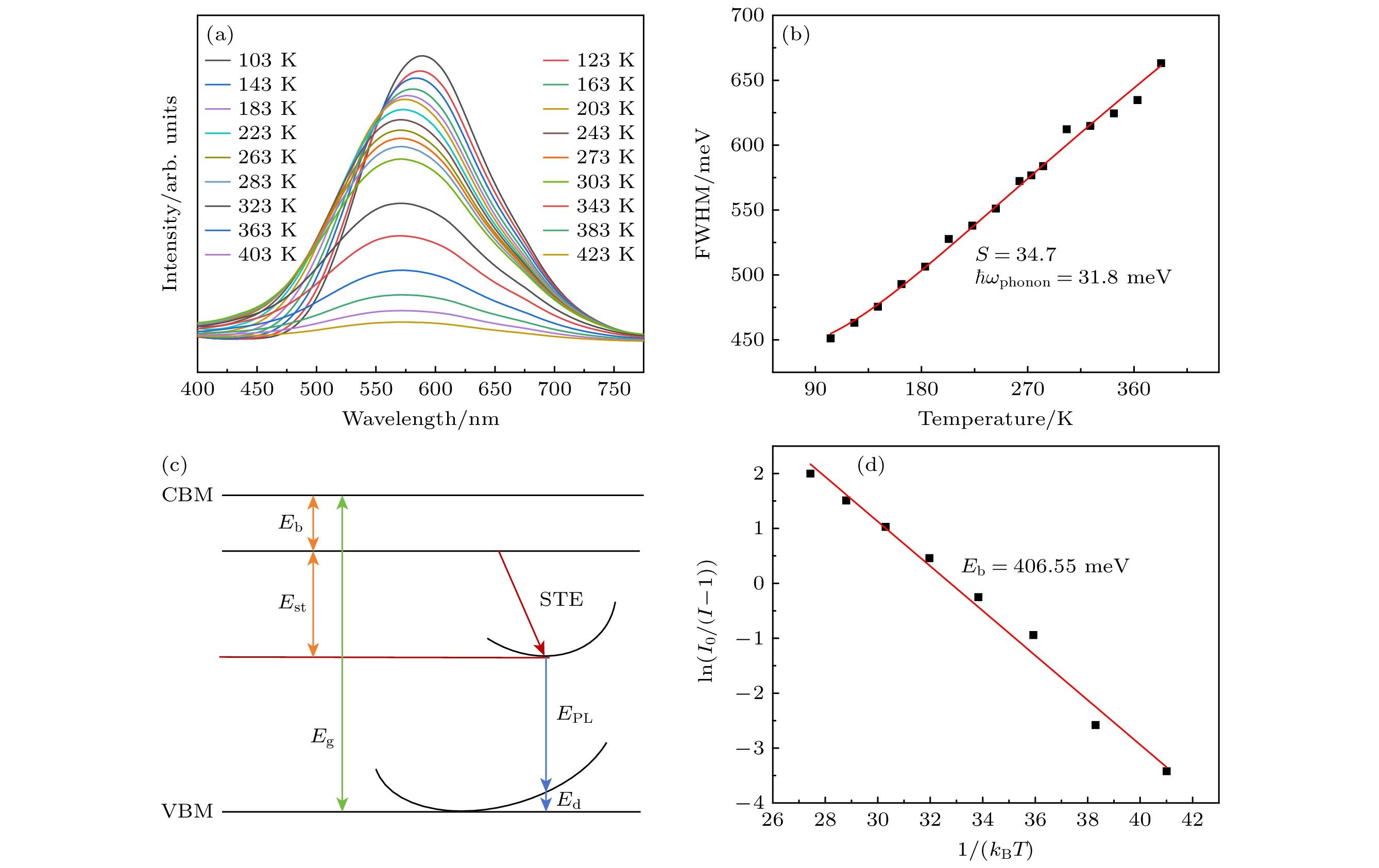
图 6 (a) Cs2Ag0.6Na0.4In0.987Cl6:0.013Bi3+的变温光谱图; (b) 荧光光谱半峰宽与温度的函数拟合结果; (c) STE发射的位形图; (d) Cs2Ag0.6Na0.4In0.987Cl6:0.013Bi3+的ln(I0/(I–1))与1/(kBT )关系曲线
Figure 6. (a) Temperature dependent spectrogram of Cs2Ag0.6Na0.4In0.987Cl6:0.013Bi3+; (b) the fitting results of the function between the half peak width of fluorescence spectrum and temperature; (c) stereogram of STE emission; (d) curve of ln(I0/(I–1)) versus 1/(kBT ) for Cs2Ag0.6Na0.4In0.987Cl6:xBi3+.
-
[1] Yu Y X, Zhou Y F, Ang Y J, Zhang Y Q, Liu X D, Liang X J, Liu J P, Chen S Q, Xiang W D 2021 J. Lumin. 236 118129
 Google Scholar
Google Scholar
[2] Xu Q L, Yang D W, Lü J, Sun Y Y, Zhang L J 2018 Small Methods 2 1700316
 Google Scholar
Google Scholar
[3] Zhao X G, Yang D W, Ren J C, Sun Y H, Xiao Z W, Zhang L J 2018 Joule 2 1662
 Google Scholar
Google Scholar
[4] Filip M R, Hillman S, Haghighirad A A, Snaith H J, Giustino F 2016 J. Phys. Chem. Lett. 7 2579
 Google Scholar
Google Scholar
[5] Wei F X, Deng Z Y, Sun S J, Xie F, Kieslich G, Evans, Donald M 2016 Mater. Horiz. 3 328
 Google Scholar
Google Scholar
[6] Wei F X, Deng Z Y, Sun S J, Zhang F H, Evans-Donald M, Kieslich G, Tominaka S, Carpenter-Michael A, Zhang J, Bristowe-Paul D, Cheetham-Anthony K 2017 Chem. Mater. 29 1089
 Google Scholar
Google Scholar
[7] Tran T, Thao, Panella J R, Chamorro J R, Morey J R, McQueen T M 2017 Mater. Horiz. 4 688
 Google Scholar
Google Scholar
[8] Bekenstein Y, Dahl J C, Huang J M, Osowiecki W T, Swabeck J K, Chan E M, Yang P D, Alivisatos A P 2018 Nano Lett. 18 3502
 Google Scholar
Google Scholar
[9] Hao X G, Yang D G, Sun Y H, Li T S, Zhang L J, Yu L P, Zunger A 2017 J. Am. Chem. Soc. 139 6718
 Google Scholar
Google Scholar
[10] Jain A, Voznyy O, Sargent E H 2017 J. Phys. Chem. 121 7183
 Google Scholar
Google Scholar
[11] 皮慧慧, 李国辉, 周博林, 崔艳霞 2021 发光学报 42 650
 Google Scholar
Google Scholar
Pi H H, Li G H, Zhou B L, Cui Y X 2021 J. Lumin. 42 650
 Google Scholar
Google Scholar
[12] Li X, Gao X P, Zhang X T, Shen X Y, Lu M, Wu J L, Shi Z F, Colvin Vicki L, Hu J H, Bai X, Yu W W, Zhang Y 2021 Adv. Sci. Lett. 8 2003334
 Google Scholar
Google Scholar
[13] 李鑫 2023 博士学位论文 (吉林: 吉林大学)
Li X 2023 Ph. D. Dissertation (Jilin: Jilin University
[14] Akihiro K, Kenjiro T, Yasuo S, Tsutomu M 2009 J. Am. Chem. Soc. 131 6050
 Google Scholar
Google Scholar
[15] Aron W 2015 J. Phys. Chem. 119 5755
 Google Scholar
Google Scholar
[16] Hoefler S F, Trimmel G, Rath T 2017 Monatsh. Chem. 148 795
 Google Scholar
Google Scholar
[17] Hu H, Dong B H, Zhang W 2017 J. Mater. Chem. 5 11436
 Google Scholar
Google Scholar
[18] Cho H, Kim Y H, Wolf C, Lee H D, Lee T W 2018 Adv. Mater. 30 1704587
 Google Scholar
Google Scholar
[19] Quan L N, Rand B P, Friend R H, Mhaisalkar S G, Lee T W, Sargent E H 2019 Chem. Rev. 119 7444
 Google Scholar
Google Scholar
[20] Majher J D, Gray M B, Strom T A, Patrick M 2019 Chem. Mater. 31 1738
 Google Scholar
Google Scholar
[21] Zhang H N, Dun G H, Feng Q X, Zhao R, Liang R Q, Gao X Y, Thomas H, Chen M, Geng X S, Liu M Y, Huang Y, Zheng X R, Qin K, Tan X C, Wang X F, Xie D, Yang Y, Tian H, Zhou Y Y, Nitin P P, Wang X Y, Hong J W, Ren T L 2020 IEEE Trans. Electron Devices 67 3191
 Google Scholar
Google Scholar
[22] Bartel C J, Sutton C, Goldsmith B R, Ouyang R H, Charles B M, Luca M G, Matthias S 2019 Sci. Adv. 5 eaav0693
 Google Scholar
Google Scholar
[23] Blasse G 1968 Phys. Lett. A. 6 444
 Google Scholar
Google Scholar
[24] Xiao F, Yi R X, Yuan H L, Zang G J, Xie C N 2018 Spectrochim. Acta A 202 352
 Google Scholar
Google Scholar
[25] Luo J J, Wang X M, Li S R, Liu J, Guo Y M, Niu G D, Yao L, Fu Y H, Gao L, Dong Q S, Zhao C Y, Leng M Y, Ma F S, Liang W X, Wang L D, Jin S Y, Han J B, Zhang L J, Etheridge J, Wang J B, Yan Y F, Sargent E H, Tang J 2018 Nature 563 541
 Google Scholar
Google Scholar
[26] Tan Z F, Li J H, Zhang C, Li Z, Hu Q S, Xiao Z W, Toshio K, Hideo H, Guangda N, Efrat L, Cheng Y B, Tang J 2018 Adv. Funct. Mater. 28 1801131
 Google Scholar
Google Scholar
[27] Han P G, Mao X, Yang S Q, Zhang F, Yang B, Wei D H, Deng W Q, Han K L 2019 Angew. Chem. Int. Ed. 58 17231
 Google Scholar
Google Scholar
[28] Li J H, Tan Z F, Hu M C, Chen C, Luo J J, Li S R, Gao L, Xiao Z W, Niu G D, Tang J 2019 Front. Optoelectron. 12 352
 Google Scholar
Google Scholar
[29] Rainer W N 2013 J. Phys. Conf. Ser. 443 012080
 Google Scholar
Google Scholar
[30] Gong X K, Zhang X S, Li Q, Liu L, Zhang Y M, Li C, Kong L N, Xu J P, Li L 2023 J. Colloid Interface Sci. 648 865
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 3699
- PDF Downloads: 63
- Cited By: 0















 DownLoad:
DownLoad: