-
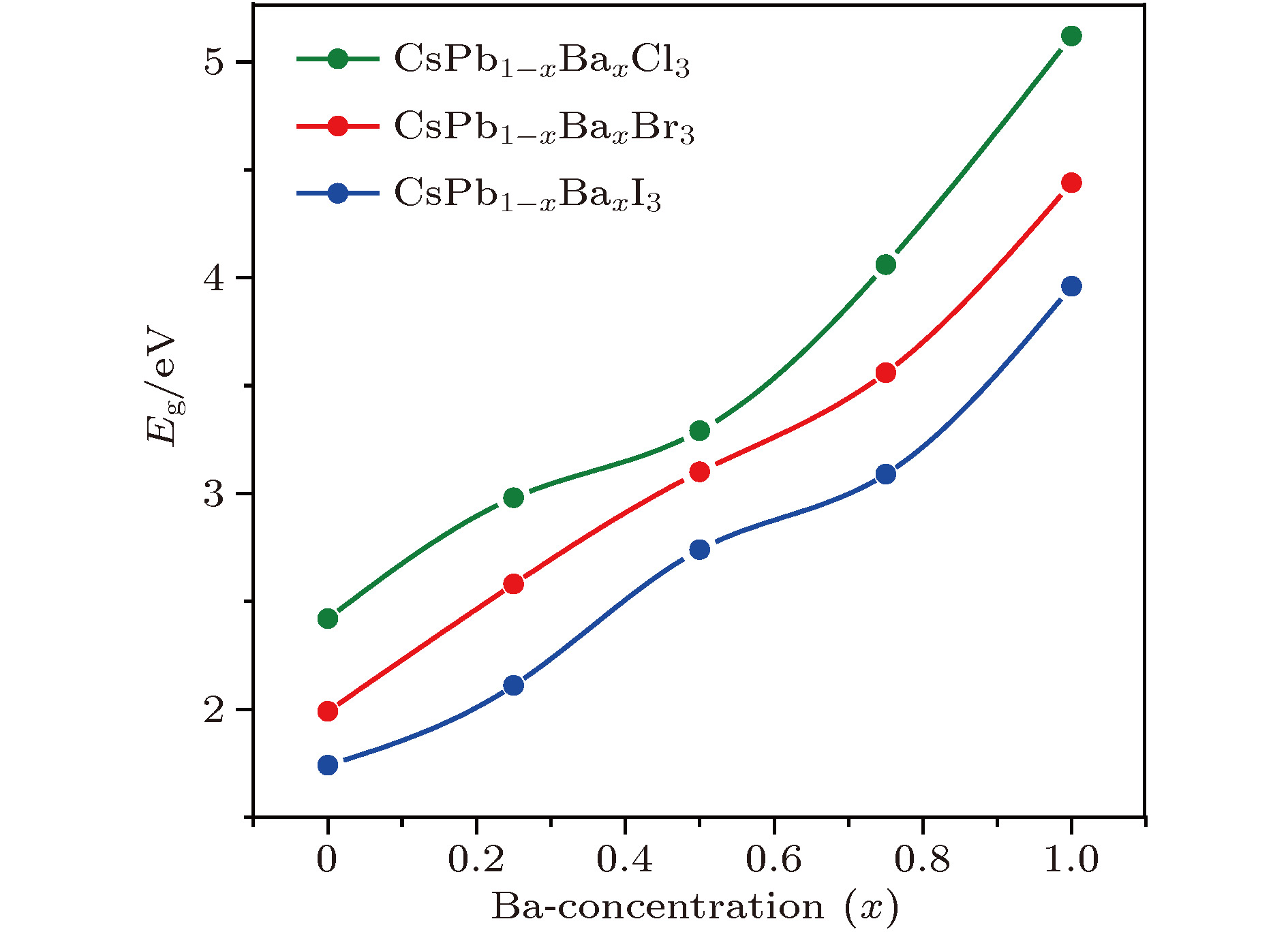
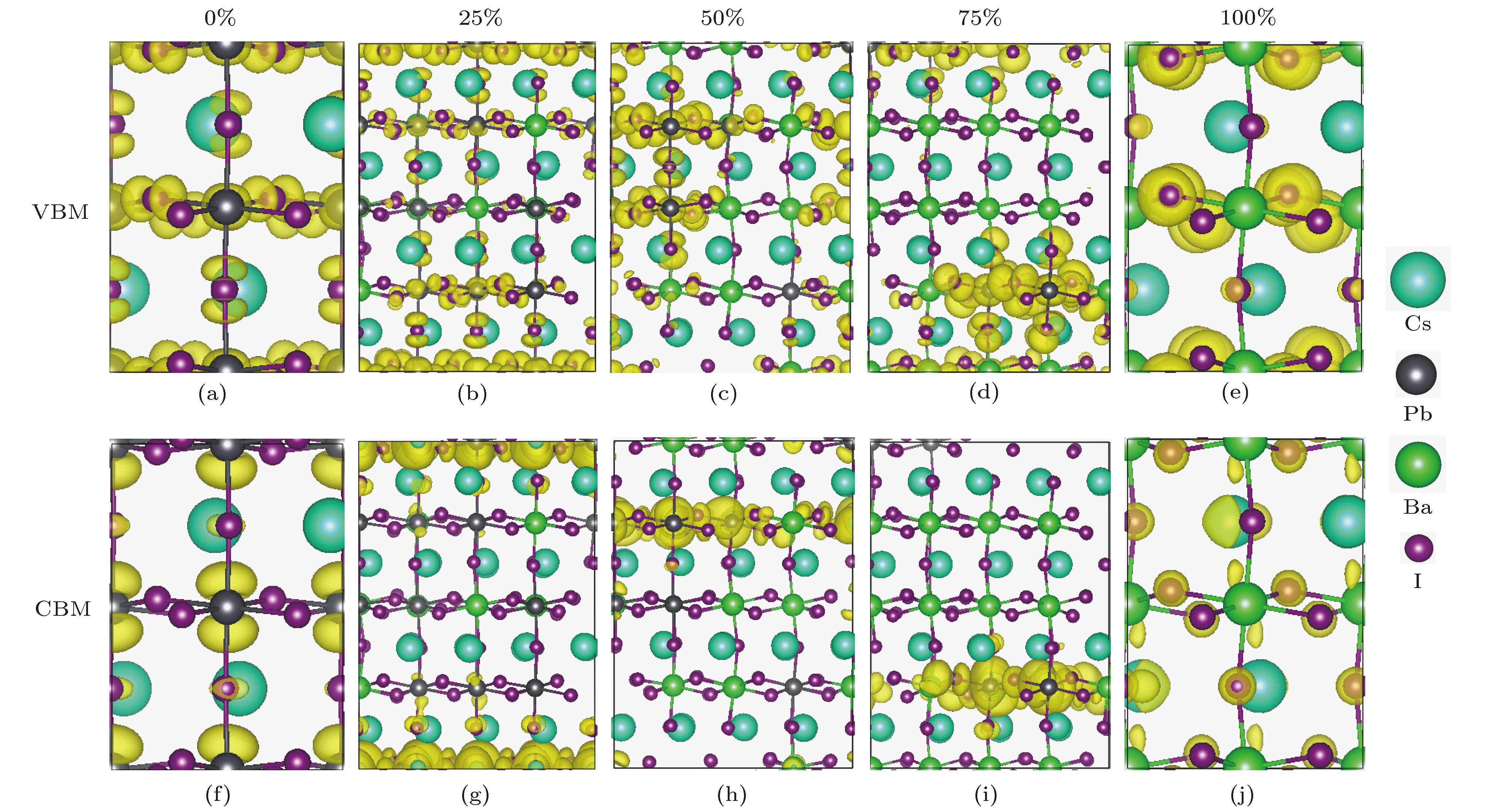
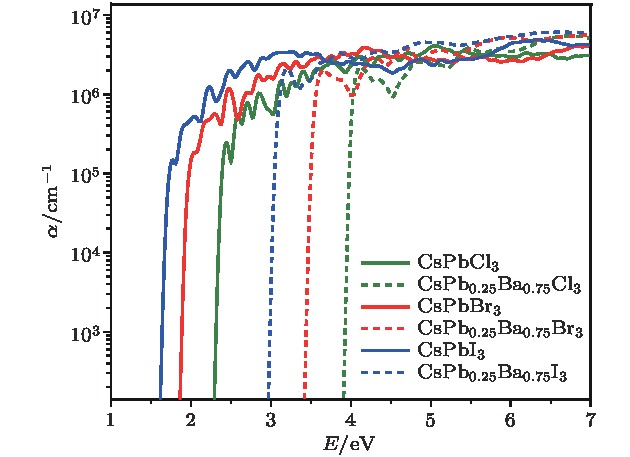
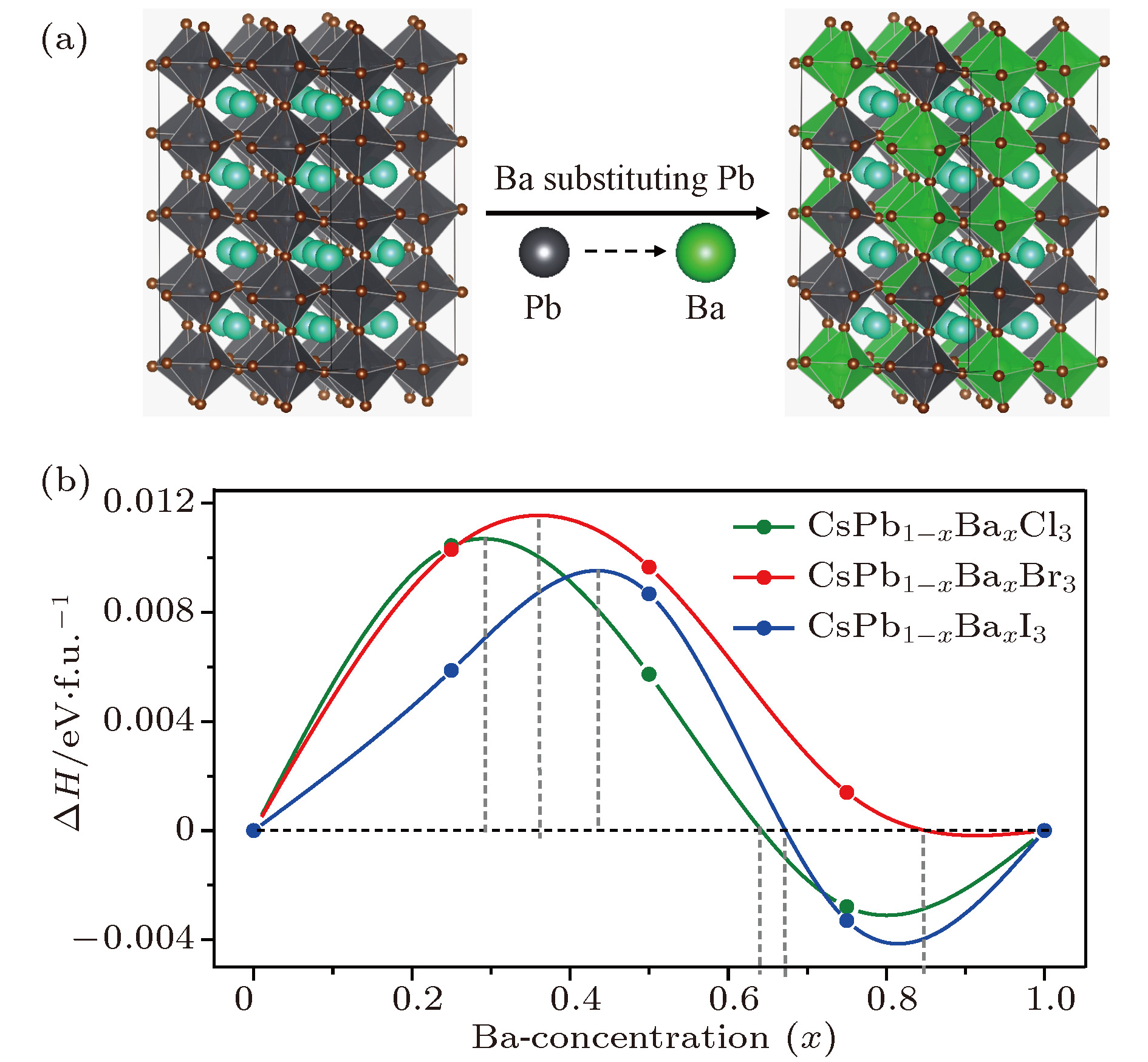
近年来, 有机-无机杂化卤化物钙钛矿材料(ABX3)由于具有优异的光电性质, 受到了材料、能源等领域的广泛关注. 但是, 卤化物钙钛矿存在两个明显阻碍其商业化应用的问题: 热稳定性差和含有有毒的铅(Pb)元素. 相比于有机-无机杂化卤化物钙钛矿, 全无机卤化物钙钛矿通常拥有更好的热稳定性. 同时, 采用一些无毒的元素部分替代B位的Pb, 可以在保持优异光电特性的同时减小材料的毒性. 本文结合无序合金结构搜索方法和第一性原理计算, 系统研究了Ba掺杂CsPbX3 (X = Cl, Br, I)钙钛矿体系的热稳定性和光电特性. 计算结果显示, 只有在高Ba浓度时, 可以在室温下形成无序固溶体. 由于Ba和Pb的离子半径和电负性等物理化学性质存在显著差异, 随着Ba掺杂浓度的增加, 带隙和载流子有效质量都呈现上升趋势, 且带隙具有很宽的调节范围. 因此, 我们预测高Ba掺杂浓度的CsPbX3 (X = Cl, Br, I)钙钛矿体系在短波段(如紫外或近紫外光)发光二极管或辐射探测器等领域具有潜在的应用价值.Organic-inorganic halide perovskites ABX3 (A = CH3NH3, HC(NH2)2; B = Pb; X = Cl, Br, I) have recently attracted increasing attention due to their advanced optoelectronic properties. However, the poor stability and toxicity of organic lead halogen perovskites are still a major challenge for deploying the outdoor solar cells. Element substitution is a simple and effective strategy to solve these problems. For example, the substitution of the I ions with Cl and Br has been regarded as a reliable method to improve the device stability. A-site engineering, i.e., replacing organic ions with inorganic cations (such as Cs+, Rb+), has also been reported. The B-site alloying approach has been demonstrated with Zn, Sr, Sn, etc. Inorganic halide perovskites can be synthesized by the low-cost solution spin-coating method and have similar optoelectronic properties and improved stability to their organic counterparts. Here in this paper, we report a comprehensive study of the alloyed perovskite CsPb1–xBaxX3 (X = Cl, Br, I) by combining the disorder alloy structure search method with first-principles energy calculations. We find that it is not easy to dope barium into the perovskite lattice when Ba concentration is low and the stable disordered solid solution can exist in the high Ba concentration case. Carrier effective mass and bandgap increase with the increase of Ba concentration and the bandgap change range is wide, owing to the difference in both electronegativity and ionic radius between Pb and Ba. After inducing Ba into CsPb1–xBaxX3 (X = Cl, Br, I), the higher electron concentration on the I sites also enhances the Coulomb interaction of the Pb—I bonds. Moreover, the electrons and holes tend to be located on Pb sites, and this may give rise to the formation of local potential wells, which would further induce the large lattice deformation to accommodate the self-trapped excitons. Especially, CsPbI3-Pnma perovskite is metastable in the ambient environment with a suitable photon absorption threshold. The CsPb1–xBaxI3 can be used as a capping layer on CsPbI3 in solar cells, thereby significantly improving the power conversion efficiency and long-term stability. Overall, the alloyed perovskite CsPb1–xBaxX3 (X = Cl, Br, I) with high Ba concentration can be stable and less-toxic, and they can be used in short wave light-emitting diodes, radiation detectors or other fields because of their large bandgaps (> 2.8 eV).
-
Keywords:
- lead-based halide perovskite /
- elemental substitution /
- optoelectronic properties /
- first-principles calculations
[1] Bell L E 2008 Science 321 1457
 Google Scholar
Google Scholar
[2] Zou C, Zhao Q, Zhang G, Xiong B 2016 Natural Gas Industry B 3 1
 Google Scholar
Google Scholar
[3] Lenzen M 2008 Energy Conversion and Management 49 2178
 Google Scholar
Google Scholar
[4] Polman A, Knight M, Garnett E C, Ehrler B, Sinke W C 2016 Science 352 aad4424
 Google Scholar
Google Scholar
[5] Milan P, Wächter M, Peinke J 2013 Phys. Rev. Lett. 110 138701
 Google Scholar
Google Scholar
[6] Lewis N S 2007 Science 315 798
 Google Scholar
Google Scholar
[7] Dong Q, Fang Y, Shao Y, Mulligan P, Qiu J, Cao L, Huang J 2015 Science 347 967
 Google Scholar
Google Scholar
[8] Xing G, Mathews N, Sun S, Lim S S, Lam Y M, Gratzel M, Mhaisalkar S, Sum T C 2013 Science 342 344
 Google Scholar
Google Scholar
[9] Han Q, Bae S H, Sun P, Hsieh Y T, Yang Y M, Rim Y S, Zhao H, Chen Q, Shi W, Li G, Yang Y 2016 Adv. Mater. 28 2253
 Google Scholar
Google Scholar
[10] Chen H, Xiang S, Li W, Liu H, Zhu L, Yang S 2018 Solar RRL 2 1700188
 Google Scholar
Google Scholar
[11] Shang M H, Zhang J, Zhang P, Yang Z, Zheng J, Haque M A, Yang W, Wei S H, Wu T 2019 J. Phys. Chem. Lett. 10 59
 Google Scholar
Google Scholar
[12] Huang, Y, Sun Q D, Xu W, He Y, Yin W J 2017 Acta Phys.-Chim. Sin. 33 1730
[13] Qin X, Zhao Z, Wang Y, Wu J, Jiang Q, You J 2017 J. Semicond. 38 011002
 Google Scholar
Google Scholar
[14] Linaburg M R, McClure E T, Majher J D, Woodward P M 2017 Chem. Mater. 29 3507
 Google Scholar
Google Scholar
[15] Wu M C, Chen W C, Chan S H, Su W F 2018 Appl. Surf. Sci. 429 9
 Google Scholar
Google Scholar
[16] Lau C F J, Zhang M, Deng X, Zheng J, Bing J, Ma Q, Kim J, Hu L, Green M A, Huang S, Ho-Baillie A 2017 ACS Energy Lett. 2 2319
 Google Scholar
Google Scholar
[17] Navas J, Sánchez-Coronilla A, Gallardo J J, Cruz Hernández N, Piñero J C, Alcántara R, Fernández-Lorenzo C, De los Santos D M, Aguilar T, Martín-Calleja J 2015 Nanoscale 7 6216
 Google Scholar
Google Scholar
[18] Li F, Xia Z, Gong Y, Gu L, Liu Q 2017 J. Mater. Chem. C 5 9281
 Google Scholar
Google Scholar
[19] Bechtel J S, van der Ven A 2018 Phys. Rev. Mater. 2 045401
 Google Scholar
Google Scholar
[20] Fu Y, Rea M T, Chen J, Morrow D J, Hautzinger M P, Zhao Y, Pan D, Manger L H, Wright J C, Goldsmith R H, Jin S 2017 Chem. Mater. 29 8385
 Google Scholar
Google Scholar
[21] Wang P, Zhang X, Zhou Y, Jiang Q, Ye Q, Chu Z, Li X, Yang X, Yin Z, You J 2018 Nat. Commun. 9 2225
 Google Scholar
Google Scholar
[22] Ju M G, Dai J, Ma L, Zeng X C 2017 J. Am. Chem. Soc. 139 8038
 Google Scholar
Google Scholar
[23] Hao F, Stoumpos C C, Cao D H, Chang R P H, Kanatzidis M G 2014 Nature Photonics 8 489
 Google Scholar
Google Scholar
[24] Swarnkar A, Mir W J, Nag A 2018 ACS Energy Lett. 3 286
 Google Scholar
Google Scholar
[25] Xiang W, Wang Z, Kubicki D J, Tress W, Luo J, Prochowicz D, Akin S, Emsley L, Zhou J, Dietler G, Grätzel M, Hagfeldt A 2019 Joule 3 205
 Google Scholar
Google Scholar
[26] Pazoki M, Jacobsson T J, Hagfeldt A, Boschloo G, Edvinsson T 2016 Phys. Rev. B 93 144105
 Google Scholar
Google Scholar
[27] Huang Q, Zou Y, Bourelle S A, Zhai T, Wu T, Tan Y, Li Y, Li J, Duhm S, Song T, Wang L, Deschler F, Sun B 2019 Nanoscale Horizons DOI: 10.1039.C9NH00066F
[28] Kumar A, Balasubramaniam K R, Kangsabanik J, Vikram, Alam A 2016 Phys. Rev. B 94 180105
 Google Scholar
Google Scholar
[29] Song J, Li J, Li X, Xu L, Dong Y, Zeng H 2015 Adv. Mater. 27 7162
 Google Scholar
Google Scholar
[30] Li X, Wu Y, Zhang S, Cai B, Gu Y, Song J, Zeng H 2016 Adv. Funct. Mater. 26 2435
 Google Scholar
Google Scholar
[31] van de Walle A, Tiwary P, de Jong M, Olmsted D L, Asta M, Dick A, Shin D, Wang Y, Chen L Q, Liu Z K 2013 Calphad 42 13
 Google Scholar
Google Scholar
[32] Hass K C, Davis L C, Zunger A 1990 Phys. Rev. B 42 3757
 Google Scholar
Google Scholar
[33] Jiang C, Stanek C R, Sickafus K E, Uberuaga B P 2009 Phys. Rev. B 79 104203
 Google Scholar
Google Scholar
[34] Shin D, van de Walle A, Wang Y, Liu Z K 2007 Phys. Rev. B 76 144204
 Google Scholar
Google Scholar
[35] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[36] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[37] Grimme S 2006 J. Comput. Chem. 27 1787
 Google Scholar
Google Scholar
[38] Gajdoš M, Hummer K, Kresse G, Furthmüller J, Bechstedt F 2006 Phys. Rev. B 73 045112
 Google Scholar
Google Scholar
[39] Hu J, Alicea J, Wu R, Franz M 2012 Phys. Rev. Lett. 109 266801
 Google Scholar
Google Scholar
[40] Feng Y, Ding H C, Du Y, Wan X, Wang B, Savrasov S Y, Duan C G 2014 J. Appl. Phys. 115 233901
 Google Scholar
Google Scholar
[41] Yun S, Zhou X, Even J, Hagfeldt A 2017 Angew. Chem. Int. Ed. 56 15806
 Google Scholar
Google Scholar
[42] Krukau A V, Vydrov O A, Izmaylov A F, Scuseria G E 2006 J. Chem. Phys. 125 224106
 Google Scholar
Google Scholar
[43] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[44] Medeiros P V C, Stafström S, Björk J 2014 Phys. Rev. B 89 041407
 Google Scholar
Google Scholar
[45] Medeiros P V C, Tsirkin S S, Stafström S, Björk J 2015 Phys. Rev. B 91 041116
 Google Scholar
Google Scholar
[46] Pauling L 1932 J. Am. Chem. Soc. 54 3570
 Google Scholar
Google Scholar
[47] Yu J, Kong J, Hao W, Guo X, He H, Leow W R, Liu Z, Cai P, Qian G, Li S, Chen X, Chen X 2019 Adv. Mater. 31 1806385
[48] Tanaka K, Kondo T 2003 Sci. Technol. Adv. Mater. 4 599
 Google Scholar
Google Scholar
[49] Lee K J, Turedi B, Sinatra L, Zhumekenov A A, Maity P, Dursun I, Naphade R, Merdad N, Alsalloum A, Oh S, Wehbe N, Hedhili M N, Kang C H, Subedi R C, Cho N, Kim J S, Ooi B S, Mohammed O F, Bakr O M 2019 Nano Lett. 19 3535
[50] Jiang Y, Qin C, Cui M, He T, Liu K, Huang Y, Luo M, Zhang L, Xu H, Wei J, Liu Z, Wang H, Kim G H, Yuan M, Chen J 2019 Nat. Commun. 10 1868
 Google Scholar
Google Scholar
[51] Zhang S, Yi C, Wang N, Sun Y, Zou W, Wei Y, Cao Y, Miao Y, Li R, Yin Y, Zhao N, Wang J, Huang W 2017 Adv. Mater. 29 1606600
 Google Scholar
Google Scholar
[52] Blancon J C, Stier A V, Tsai H, Nie W, Stoumpos C C, Traoré B, Pedesseau L, Kepenekian M, Katsutani F, Noe G T, Kono J, Tretiak S, Crooker S A, Katan C, Kanatzidis M G, Crochet J J, Evan J, Mohite A D 2018 Nat. Commun. 9 2254
 Google Scholar
Google Scholar
[53] Kulbak M, Cahen D, Hodes G 2015 J. Phys. Chem. Lett. 6 2452
 Google Scholar
Google Scholar
-
图 2 计算得到的CsPb1–xBaxI3合金钙钛矿体系的能带结构 (a) x = 0%; (b) x = 25%; (c) x = 50%; (d) x = 75%; (e) x = 100%; 其中, 图(b)−(d)是通过能带展开技术得到的, 彩色刻度尺代表指定波矢下穿过能量区间的原胞能带数目
Fig. 2. Calculated band structures of the alloyed perovskite CsPb1–xBaxI3, x = (a) 0%, (b) 25%, (c) 50%, (d) 75%, (e) 100%; panels (b)−(d) are obtained by band unfolding technique. The color scale represents the number of the primitive cell bands crossing the energy interval at a given primitive wave vector.
-
[1] Bell L E 2008 Science 321 1457
 Google Scholar
Google Scholar
[2] Zou C, Zhao Q, Zhang G, Xiong B 2016 Natural Gas Industry B 3 1
 Google Scholar
Google Scholar
[3] Lenzen M 2008 Energy Conversion and Management 49 2178
 Google Scholar
Google Scholar
[4] Polman A, Knight M, Garnett E C, Ehrler B, Sinke W C 2016 Science 352 aad4424
 Google Scholar
Google Scholar
[5] Milan P, Wächter M, Peinke J 2013 Phys. Rev. Lett. 110 138701
 Google Scholar
Google Scholar
[6] Lewis N S 2007 Science 315 798
 Google Scholar
Google Scholar
[7] Dong Q, Fang Y, Shao Y, Mulligan P, Qiu J, Cao L, Huang J 2015 Science 347 967
 Google Scholar
Google Scholar
[8] Xing G, Mathews N, Sun S, Lim S S, Lam Y M, Gratzel M, Mhaisalkar S, Sum T C 2013 Science 342 344
 Google Scholar
Google Scholar
[9] Han Q, Bae S H, Sun P, Hsieh Y T, Yang Y M, Rim Y S, Zhao H, Chen Q, Shi W, Li G, Yang Y 2016 Adv. Mater. 28 2253
 Google Scholar
Google Scholar
[10] Chen H, Xiang S, Li W, Liu H, Zhu L, Yang S 2018 Solar RRL 2 1700188
 Google Scholar
Google Scholar
[11] Shang M H, Zhang J, Zhang P, Yang Z, Zheng J, Haque M A, Yang W, Wei S H, Wu T 2019 J. Phys. Chem. Lett. 10 59
 Google Scholar
Google Scholar
[12] Huang, Y, Sun Q D, Xu W, He Y, Yin W J 2017 Acta Phys.-Chim. Sin. 33 1730
[13] Qin X, Zhao Z, Wang Y, Wu J, Jiang Q, You J 2017 J. Semicond. 38 011002
 Google Scholar
Google Scholar
[14] Linaburg M R, McClure E T, Majher J D, Woodward P M 2017 Chem. Mater. 29 3507
 Google Scholar
Google Scholar
[15] Wu M C, Chen W C, Chan S H, Su W F 2018 Appl. Surf. Sci. 429 9
 Google Scholar
Google Scholar
[16] Lau C F J, Zhang M, Deng X, Zheng J, Bing J, Ma Q, Kim J, Hu L, Green M A, Huang S, Ho-Baillie A 2017 ACS Energy Lett. 2 2319
 Google Scholar
Google Scholar
[17] Navas J, Sánchez-Coronilla A, Gallardo J J, Cruz Hernández N, Piñero J C, Alcántara R, Fernández-Lorenzo C, De los Santos D M, Aguilar T, Martín-Calleja J 2015 Nanoscale 7 6216
 Google Scholar
Google Scholar
[18] Li F, Xia Z, Gong Y, Gu L, Liu Q 2017 J. Mater. Chem. C 5 9281
 Google Scholar
Google Scholar
[19] Bechtel J S, van der Ven A 2018 Phys. Rev. Mater. 2 045401
 Google Scholar
Google Scholar
[20] Fu Y, Rea M T, Chen J, Morrow D J, Hautzinger M P, Zhao Y, Pan D, Manger L H, Wright J C, Goldsmith R H, Jin S 2017 Chem. Mater. 29 8385
 Google Scholar
Google Scholar
[21] Wang P, Zhang X, Zhou Y, Jiang Q, Ye Q, Chu Z, Li X, Yang X, Yin Z, You J 2018 Nat. Commun. 9 2225
 Google Scholar
Google Scholar
[22] Ju M G, Dai J, Ma L, Zeng X C 2017 J. Am. Chem. Soc. 139 8038
 Google Scholar
Google Scholar
[23] Hao F, Stoumpos C C, Cao D H, Chang R P H, Kanatzidis M G 2014 Nature Photonics 8 489
 Google Scholar
Google Scholar
[24] Swarnkar A, Mir W J, Nag A 2018 ACS Energy Lett. 3 286
 Google Scholar
Google Scholar
[25] Xiang W, Wang Z, Kubicki D J, Tress W, Luo J, Prochowicz D, Akin S, Emsley L, Zhou J, Dietler G, Grätzel M, Hagfeldt A 2019 Joule 3 205
 Google Scholar
Google Scholar
[26] Pazoki M, Jacobsson T J, Hagfeldt A, Boschloo G, Edvinsson T 2016 Phys. Rev. B 93 144105
 Google Scholar
Google Scholar
[27] Huang Q, Zou Y, Bourelle S A, Zhai T, Wu T, Tan Y, Li Y, Li J, Duhm S, Song T, Wang L, Deschler F, Sun B 2019 Nanoscale Horizons DOI: 10.1039.C9NH00066F
[28] Kumar A, Balasubramaniam K R, Kangsabanik J, Vikram, Alam A 2016 Phys. Rev. B 94 180105
 Google Scholar
Google Scholar
[29] Song J, Li J, Li X, Xu L, Dong Y, Zeng H 2015 Adv. Mater. 27 7162
 Google Scholar
Google Scholar
[30] Li X, Wu Y, Zhang S, Cai B, Gu Y, Song J, Zeng H 2016 Adv. Funct. Mater. 26 2435
 Google Scholar
Google Scholar
[31] van de Walle A, Tiwary P, de Jong M, Olmsted D L, Asta M, Dick A, Shin D, Wang Y, Chen L Q, Liu Z K 2013 Calphad 42 13
 Google Scholar
Google Scholar
[32] Hass K C, Davis L C, Zunger A 1990 Phys. Rev. B 42 3757
 Google Scholar
Google Scholar
[33] Jiang C, Stanek C R, Sickafus K E, Uberuaga B P 2009 Phys. Rev. B 79 104203
 Google Scholar
Google Scholar
[34] Shin D, van de Walle A, Wang Y, Liu Z K 2007 Phys. Rev. B 76 144204
 Google Scholar
Google Scholar
[35] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[36] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[37] Grimme S 2006 J. Comput. Chem. 27 1787
 Google Scholar
Google Scholar
[38] Gajdoš M, Hummer K, Kresse G, Furthmüller J, Bechstedt F 2006 Phys. Rev. B 73 045112
 Google Scholar
Google Scholar
[39] Hu J, Alicea J, Wu R, Franz M 2012 Phys. Rev. Lett. 109 266801
 Google Scholar
Google Scholar
[40] Feng Y, Ding H C, Du Y, Wan X, Wang B, Savrasov S Y, Duan C G 2014 J. Appl. Phys. 115 233901
 Google Scholar
Google Scholar
[41] Yun S, Zhou X, Even J, Hagfeldt A 2017 Angew. Chem. Int. Ed. 56 15806
 Google Scholar
Google Scholar
[42] Krukau A V, Vydrov O A, Izmaylov A F, Scuseria G E 2006 J. Chem. Phys. 125 224106
 Google Scholar
Google Scholar
[43] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[44] Medeiros P V C, Stafström S, Björk J 2014 Phys. Rev. B 89 041407
 Google Scholar
Google Scholar
[45] Medeiros P V C, Tsirkin S S, Stafström S, Björk J 2015 Phys. Rev. B 91 041116
 Google Scholar
Google Scholar
[46] Pauling L 1932 J. Am. Chem. Soc. 54 3570
 Google Scholar
Google Scholar
[47] Yu J, Kong J, Hao W, Guo X, He H, Leow W R, Liu Z, Cai P, Qian G, Li S, Chen X, Chen X 2019 Adv. Mater. 31 1806385
[48] Tanaka K, Kondo T 2003 Sci. Technol. Adv. Mater. 4 599
 Google Scholar
Google Scholar
[49] Lee K J, Turedi B, Sinatra L, Zhumekenov A A, Maity P, Dursun I, Naphade R, Merdad N, Alsalloum A, Oh S, Wehbe N, Hedhili M N, Kang C H, Subedi R C, Cho N, Kim J S, Ooi B S, Mohammed O F, Bakr O M 2019 Nano Lett. 19 3535
[50] Jiang Y, Qin C, Cui M, He T, Liu K, Huang Y, Luo M, Zhang L, Xu H, Wei J, Liu Z, Wang H, Kim G H, Yuan M, Chen J 2019 Nat. Commun. 10 1868
 Google Scholar
Google Scholar
[51] Zhang S, Yi C, Wang N, Sun Y, Zou W, Wei Y, Cao Y, Miao Y, Li R, Yin Y, Zhao N, Wang J, Huang W 2017 Adv. Mater. 29 1606600
 Google Scholar
Google Scholar
[52] Blancon J C, Stier A V, Tsai H, Nie W, Stoumpos C C, Traoré B, Pedesseau L, Kepenekian M, Katsutani F, Noe G T, Kono J, Tretiak S, Crooker S A, Katan C, Kanatzidis M G, Crochet J J, Evan J, Mohite A D 2018 Nat. Commun. 9 2254
 Google Scholar
Google Scholar
[53] Kulbak M, Cahen D, Hodes G 2015 J. Phys. Chem. Lett. 6 2452
 Google Scholar
Google Scholar
计量
- 文章访问数: 21085
- PDF下载量: 349
- 被引次数: 0















 下载:
下载: