-
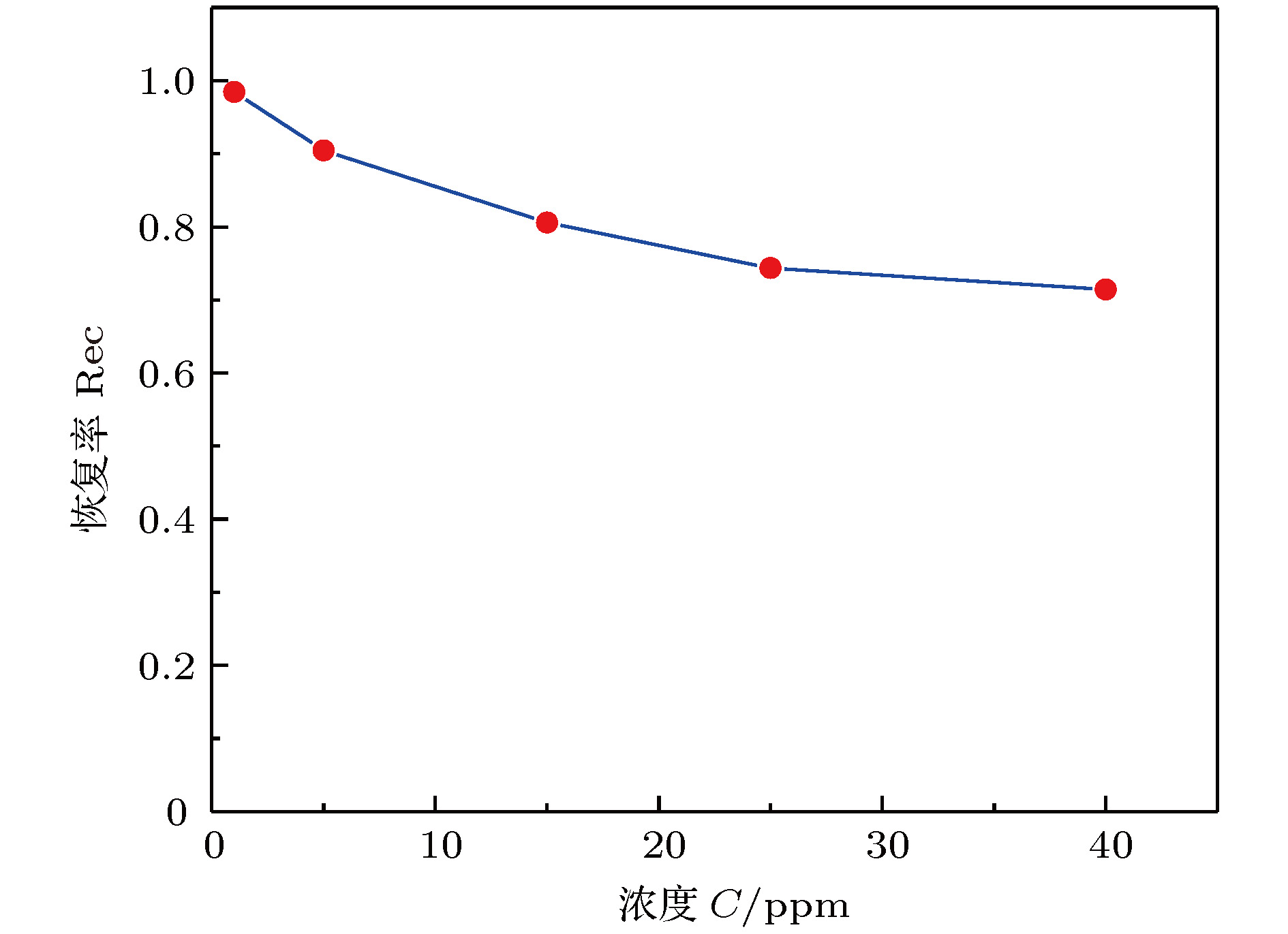
还原氧化石墨烯由于独特的原子结构, 作为气体检测领域有潜力的候选者引起了研究者们的广泛兴趣. 本文采用水合肼作为还原剂来制备还原氧化石墨烯, 并以此作为叉指电极的气体敏感层, 研究了其对NO2气体的响应特性. 结果表明, 水合肼还原的氧化石墨烯可以实现在室温下对浓度为1—40 ppm (1 ppm = 10–6)的NO2气体的检测, 具有较好的响应性和重复性, 恢复率可以达到71%以上, 但是灵敏度只有0.00201 ppm–1, 还有较大的提升空间. 此外, 对浓度5 ppm的NO2的响应和恢复时间分别是319 s和776 s. 水合肼还原的氧化石墨烯气体传感器的传感机制可归因于NO2分子和传感材料之间的电荷转移. 还原氧化石墨烯的突出电学特性促进了电子转移过程, 这使得传感器在室温下表现出优异的气体传感性能. 本实验研究可为石墨烯基传感器件的应用奠定一定的基础.Reduced graphene oxide, as a candidate for gas detection due to its unique atomic structure, is arousing the wide interest of researchers. In this paper, hydrazine hydrate is used to reduce graphene oxide prepared by the modified Hummers method. A chemical resistance gas sensor is fabricated. The prepared reduced graphene oxide is used as a gas sensitive layer of Au planar interdigital electrode. The gas sensing characteristics such as responsivity, recovery and repeatability of NO2 gas are studied. The results show that the graphene oxide reduced by hydrazine hydrate can detect the NO2 gas at a concentration of 1−40 ppm under room temperature. It has good responsivity and repeatability. The recovery rate can reach more than 71%. However, the sensitivity is only 0.00201 ppm–1, and there is much room for improvement. In addition, the response time and recovery time for NO2 at 5 ppm concentration are 319 s and 776 s, respectively. The sensing mechanism of the hydrazine hydrate-reduced graphene oxide gas sensor can be attributed to charge transfer between the NO2 molecule and the sensing material. The outstanding electrical properties of the reduced graphene oxide promote the electron transfer process. This allows the sensor to exhibit excellent gas sensing performance at room temperature. The reduced graphene oxide appears as a typical p-type semiconductor and the oxidizing gas NO2 acts as an electron acceptor. Therefore, the adsorption of NO2 gas leads to the enhancement of the hole density and conductivity of the reduced graphene oxide. Another reason is the presence of defects and oxygen-containing functional groups on graphene sheets. Some oxygen-containing groups remain on the graphene surface after an incomplete reduction reaction. Compared with pure graphene, the reduced graphene oxide has hydroxyl groups and epoxy groups remaining on the surface. These functional groups will functionalize the material and promote the adsorption of gases. At the same time, the reduction reaction will further produce vacancies and structural defects. This will provide more reaction sites and thus conduce to the material further adsorbing the gas. In summary, the experimental research in this paper is of significance for studying the mechanism and characteristics of the reduced graphene oxide by using hydrazine hydrate as a reducing agent, and it can provide reference and lay a foundation for the applications of future graphene sensors.
-
Keywords:
- reduced graphene oxide /
- hydrazine hydrate /
- nitrogen dioxide /
- gas sensing
[1] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[2] 孙建平, 缪应蒙, 曹相春 2013 62 036301
 Google Scholar
Google Scholar
Sun J P, Liao Y M, Cao X C 2013 Acta Phys. Sin. 62 036301
 Google Scholar
Google Scholar
[3] 魏晓林, 陈元平, 王如志, 钟建新 2013 62 057101
 Google Scholar
Google Scholar
Wei X L, Chen Y P, Wang R Z, Zhong J X 2013 Acta Phys. Sin. 62 057101
 Google Scholar
Google Scholar
[4] Choudhuri I, Patra N, Mahata A, Ahuja R, Pathak B 2015 J. Phys. Chem. C 119 24827
 Google Scholar
Google Scholar
[5] Gavgani J N, Hasani A, Nouri M, Mahyari M, Salehi A 2016 Sens. Actuator B: Chem. 229 239
 Google Scholar
Google Scholar
[6] Ye Z, Tai H, Xie T, Yuan Z, Liu C, Jiang Y 2016 Sens. Actuator B: Chem. 223 149
 Google Scholar
Google Scholar
[7] Matsuba K, Wang C, Saruwatari K, Uesusuki Y, Akatsuka K, Osada M, Ebina Y, Ma R, Sasaki T 2017 Sci. Adv. 3 e1700414
 Google Scholar
Google Scholar
[8] Pour M M, Lashkov A, Radocea A, Liu X, Sun T, Lipatov A, Korlacki R A, Shekhirev M, Aluru N R, Lyding J W 2017 Nat. Commun. 8 820
 Google Scholar
Google Scholar
[9] Singh E, Meyyappan M, Nalwa H S 2017 ACS Appl. Mater. Interfaces 9 34544
 Google Scholar
Google Scholar
[10] Liu J H, Yang X K, Zhang M L, Wei B, Li C, Dong D N, Li C 2019 IEEE Electron Device Lett. 40 220
 Google Scholar
Google Scholar
[11] Liu J H, Yang X K, Cui H Q, Wang S, Wei B, Li C, Li C, Dong D N 2019 J. Magn. Magn. Mater. 474 161
 Google Scholar
Google Scholar
[12] Zhang T, Ma Y D, Huang B B, Dai Y 2019 ACS Appl. Mater.Interfaces 11 6104
[13] Schedin F, Geim A, Morozov S, Hill E, Blake P, Katsnelson M, Novoselov K 2007 Nat. Mater. 6 652
 Google Scholar
Google Scholar
[14] Venugopal G, Krishnamoorthy K, Mohan R, Kim S J 2012 Mater. Chem. Phys. 132 29
 Google Scholar
Google Scholar
[15] Yasaei P, Kumar B, Hantehzadeh R, Kayyalha M, Baskin A, Repnin N, Wang C, Klie R F, Chen Y P, Král P 2014 Nat. Commun. 5 4911
 Google Scholar
Google Scholar
[16] Chung M G, Kim D H, Lee H M, Kim T, Choi J H, Kyun Seo D, Yoo J B, Hong S H, Kang T J, Kim Y H 2012 Sens. Actuator B: Chem. 166 172
[17] Yavari F, Koratkar N 2012 J. Phys. Chem. Lett. 3 1746
 Google Scholar
Google Scholar
[18] Novoselov K, Jiang D, Schedin F, Booth T, Khotkevich V, Morozov S, Geim A 2005 Proc. Natl. Acad. Sci. U. S. A. 102 10451
 Google Scholar
Google Scholar
[19] Park S, Ruoff R S 2009 Nat. Nanotechnol. 4 217
 Google Scholar
Google Scholar
[20] Berger C, Song Z, Li X, Wu X, Brown N, Naud C, Mayou D, Li T, Hass J, Marchenkov A N 2006 Science 312 1191
 Google Scholar
Google Scholar
[21] 韩林芷, 赵占霞, 马忠权 2014 63 248103
 Google Scholar
Google Scholar
Han L Z, Zhao Z X, Ma Z Q 2014 Acta Phys. Sin. 63 248103
 Google Scholar
Google Scholar
[22] Dua V, Surwade S P, Ammu S, Agnihotra S R, Jain S, Roberts K E, Park S, Ruoff R S, Manohar S K 2010 Angew. Chem. 122 2200
 Google Scholar
Google Scholar
[23] Li W, Li X, Cai L, Sun Y, Sun M, Xie D 2018 J. Nanosci. Nanotechnol. 18 7927
 Google Scholar
Google Scholar
[24] Li W, Teng C, Sun Y, Cai L, Xu J L, Sun M, Li X, Yang X, Xiang L, Xie D 2018 ACS Appl. Mater. Interfaces 10 34485
 Google Scholar
Google Scholar
[25] Hu N, Wang Y, Chai J, Gao R, Yang Z, Kong E S W, Zhang Y 2012 Sens. Actuator B: Chem. 163 107
 Google Scholar
Google Scholar
[26] Brodie B 1860 Ann. Chim. Phys. 59 e472
[27] Staudenmaier L 1898 Ber. Dtsch. Chem. Ges. 31 1481
 Google Scholar
Google Scholar
[28] Hummers Jr W S, Offeman R E 1958 J. Am. Chem. Soc. 80 1339
 Google Scholar
Google Scholar
[29] 洪菲, 周立群, 黄莹, 宋荷娟, 王婷, 罗辛茹, 伍珍 2012 化学与生物工程 29 31
 Google Scholar
Google Scholar
Hong F, Zhou L Q, Huang Y, Song H J, Wang T, Luo X R, Wu Z 2012 Chem. Bioeng. 29 31
 Google Scholar
Google Scholar
[30] Compton O C, Nguyen S T 2010 Small 6 711
 Google Scholar
Google Scholar
[31] Lipatov A, Varezhnikov A, Wilson P, Sysoev V, Kolmakov A, Sinitskii A 2013 Nanoscale 5 5426
 Google Scholar
Google Scholar
[32] Ren P G, Yan D X, Ji X, Chen T, Li Z M 2010 Nanotechnology 22 055705
[33] Wan W B, Zhao Z B, Hu H, Zhou Q, Fan Y R, Qiu J S 2011 Carbon 8 2878
[34] Alizadeh T, Soltani L H 2016 Sens. Actuator B: Chem. 234 361
 Google Scholar
Google Scholar
[35] Li D, Müller M B, Gilje S, Kaner R B, Wallace G G 2008 Nat. Nanotechnol. 3 101
 Google Scholar
Google Scholar
[36] Cho B, Hahm M G, Choi M, Yoon J, Kim A R, Lee Y J, Park S G, Kwon J D, Kim C S, Song M 2015 Sci. Rep. 5 8052
 Google Scholar
Google Scholar
[37] Jin C, Tang X, Tan X, Smith S C, Dai Y, Kou L 2019 J. Mater. Chem. A 7 1099
 Google Scholar
Google Scholar
[38] Yang L, Cai Z, Hao L, Ran L, Xu X, Dai Y, Pan S, Jing B, Zou J 2018 Electrochim. Acta 283 448
 Google Scholar
Google Scholar
[39] Ko K Y, Song J G, Kim Y, Choi T, Shin S, Lee C W, Lee K, Koo J, Lee H, Kim J 2016 ACS Nano 10 9287
 Google Scholar
Google Scholar
[40] Lu G, Ocola L E, Chen J 2009 Appl. Phys. Lett. 94 083111
 Google Scholar
Google Scholar
[41] Pearce R, Iakimov T, Andersson M, Hultman L, Spetz A L, Yakimova R 2011 Sens. Actuator B: Chem. 155 451
 Google Scholar
Google Scholar
[42] Yavari F, Castillo E, Gullapalli H, Ajayan P M, Koratkar N 2012 Appl. Phys. Lett. 100 203120
 Google Scholar
Google Scholar
[43] Qazi M, Nomani M W, Chandrashekhar M, Shields V B, Spencer M G, Koley G 2010 Appl. Phys. Express 3 075101
 Google Scholar
Google Scholar
-
表 1 不同石墨烯基气体传感器检测NO2性能对比
Table 1. Graphene based gas sensors for NO2 detection.
敏感材料 浓度 响应度 响应时间 恢复时间 文献 rGO 5 ppm $1.03\left( {{R_{\rm{a}}}/{R_{\rm{g}}}} \right)$ ~5.3 min ~13 min 本文 低温热还原rGO 2 ppm $1.56\left( {{G_{\rm{g}}} - {G_{\rm{a}}}} \right)/{G_{\rm{a}}}$ ~30 min > 30 min [40] 机械剥离石墨烯 2.5 ppm $0.005\left( {{R_{\rm{g}}}/{R_{\rm{a}}}} \right)$ ~1 h ~2.5 h [41] CVD石墨烯 10 ppm 15% $\left( {\Delta R/{R_{\rm{a}}}} \right)$ ~30 min ~45 min [42] 外延生长的石墨烯 15 ppm 7.50%$ \left[ {\left( {{G_{\rm{g}}} - {G_{\rm{a}}}} \right)/{G_{\rm{a}}}} \right]$ ~7.5 min ~17 min [43] 注: a) 1 ppb = 10-9; b) Ga, 在干燥空气中的电导; Gg, 在测试气体中的电导; c) Ra, 在干燥空气中的电阻; Rg, 在测试气体中的电阻; d) $\Delta R$, 由气体浓度变化导致的电阻的相对改变, $\Delta R={R_{\rm{a}}} - {R_{\rm{g}}}.$ -
[1] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[2] 孙建平, 缪应蒙, 曹相春 2013 62 036301
 Google Scholar
Google Scholar
Sun J P, Liao Y M, Cao X C 2013 Acta Phys. Sin. 62 036301
 Google Scholar
Google Scholar
[3] 魏晓林, 陈元平, 王如志, 钟建新 2013 62 057101
 Google Scholar
Google Scholar
Wei X L, Chen Y P, Wang R Z, Zhong J X 2013 Acta Phys. Sin. 62 057101
 Google Scholar
Google Scholar
[4] Choudhuri I, Patra N, Mahata A, Ahuja R, Pathak B 2015 J. Phys. Chem. C 119 24827
 Google Scholar
Google Scholar
[5] Gavgani J N, Hasani A, Nouri M, Mahyari M, Salehi A 2016 Sens. Actuator B: Chem. 229 239
 Google Scholar
Google Scholar
[6] Ye Z, Tai H, Xie T, Yuan Z, Liu C, Jiang Y 2016 Sens. Actuator B: Chem. 223 149
 Google Scholar
Google Scholar
[7] Matsuba K, Wang C, Saruwatari K, Uesusuki Y, Akatsuka K, Osada M, Ebina Y, Ma R, Sasaki T 2017 Sci. Adv. 3 e1700414
 Google Scholar
Google Scholar
[8] Pour M M, Lashkov A, Radocea A, Liu X, Sun T, Lipatov A, Korlacki R A, Shekhirev M, Aluru N R, Lyding J W 2017 Nat. Commun. 8 820
 Google Scholar
Google Scholar
[9] Singh E, Meyyappan M, Nalwa H S 2017 ACS Appl. Mater. Interfaces 9 34544
 Google Scholar
Google Scholar
[10] Liu J H, Yang X K, Zhang M L, Wei B, Li C, Dong D N, Li C 2019 IEEE Electron Device Lett. 40 220
 Google Scholar
Google Scholar
[11] Liu J H, Yang X K, Cui H Q, Wang S, Wei B, Li C, Li C, Dong D N 2019 J. Magn. Magn. Mater. 474 161
 Google Scholar
Google Scholar
[12] Zhang T, Ma Y D, Huang B B, Dai Y 2019 ACS Appl. Mater.Interfaces 11 6104
[13] Schedin F, Geim A, Morozov S, Hill E, Blake P, Katsnelson M, Novoselov K 2007 Nat. Mater. 6 652
 Google Scholar
Google Scholar
[14] Venugopal G, Krishnamoorthy K, Mohan R, Kim S J 2012 Mater. Chem. Phys. 132 29
 Google Scholar
Google Scholar
[15] Yasaei P, Kumar B, Hantehzadeh R, Kayyalha M, Baskin A, Repnin N, Wang C, Klie R F, Chen Y P, Král P 2014 Nat. Commun. 5 4911
 Google Scholar
Google Scholar
[16] Chung M G, Kim D H, Lee H M, Kim T, Choi J H, Kyun Seo D, Yoo J B, Hong S H, Kang T J, Kim Y H 2012 Sens. Actuator B: Chem. 166 172
[17] Yavari F, Koratkar N 2012 J. Phys. Chem. Lett. 3 1746
 Google Scholar
Google Scholar
[18] Novoselov K, Jiang D, Schedin F, Booth T, Khotkevich V, Morozov S, Geim A 2005 Proc. Natl. Acad. Sci. U. S. A. 102 10451
 Google Scholar
Google Scholar
[19] Park S, Ruoff R S 2009 Nat. Nanotechnol. 4 217
 Google Scholar
Google Scholar
[20] Berger C, Song Z, Li X, Wu X, Brown N, Naud C, Mayou D, Li T, Hass J, Marchenkov A N 2006 Science 312 1191
 Google Scholar
Google Scholar
[21] 韩林芷, 赵占霞, 马忠权 2014 63 248103
 Google Scholar
Google Scholar
Han L Z, Zhao Z X, Ma Z Q 2014 Acta Phys. Sin. 63 248103
 Google Scholar
Google Scholar
[22] Dua V, Surwade S P, Ammu S, Agnihotra S R, Jain S, Roberts K E, Park S, Ruoff R S, Manohar S K 2010 Angew. Chem. 122 2200
 Google Scholar
Google Scholar
[23] Li W, Li X, Cai L, Sun Y, Sun M, Xie D 2018 J. Nanosci. Nanotechnol. 18 7927
 Google Scholar
Google Scholar
[24] Li W, Teng C, Sun Y, Cai L, Xu J L, Sun M, Li X, Yang X, Xiang L, Xie D 2018 ACS Appl. Mater. Interfaces 10 34485
 Google Scholar
Google Scholar
[25] Hu N, Wang Y, Chai J, Gao R, Yang Z, Kong E S W, Zhang Y 2012 Sens. Actuator B: Chem. 163 107
 Google Scholar
Google Scholar
[26] Brodie B 1860 Ann. Chim. Phys. 59 e472
[27] Staudenmaier L 1898 Ber. Dtsch. Chem. Ges. 31 1481
 Google Scholar
Google Scholar
[28] Hummers Jr W S, Offeman R E 1958 J. Am. Chem. Soc. 80 1339
 Google Scholar
Google Scholar
[29] 洪菲, 周立群, 黄莹, 宋荷娟, 王婷, 罗辛茹, 伍珍 2012 化学与生物工程 29 31
 Google Scholar
Google Scholar
Hong F, Zhou L Q, Huang Y, Song H J, Wang T, Luo X R, Wu Z 2012 Chem. Bioeng. 29 31
 Google Scholar
Google Scholar
[30] Compton O C, Nguyen S T 2010 Small 6 711
 Google Scholar
Google Scholar
[31] Lipatov A, Varezhnikov A, Wilson P, Sysoev V, Kolmakov A, Sinitskii A 2013 Nanoscale 5 5426
 Google Scholar
Google Scholar
[32] Ren P G, Yan D X, Ji X, Chen T, Li Z M 2010 Nanotechnology 22 055705
[33] Wan W B, Zhao Z B, Hu H, Zhou Q, Fan Y R, Qiu J S 2011 Carbon 8 2878
[34] Alizadeh T, Soltani L H 2016 Sens. Actuator B: Chem. 234 361
 Google Scholar
Google Scholar
[35] Li D, Müller M B, Gilje S, Kaner R B, Wallace G G 2008 Nat. Nanotechnol. 3 101
 Google Scholar
Google Scholar
[36] Cho B, Hahm M G, Choi M, Yoon J, Kim A R, Lee Y J, Park S G, Kwon J D, Kim C S, Song M 2015 Sci. Rep. 5 8052
 Google Scholar
Google Scholar
[37] Jin C, Tang X, Tan X, Smith S C, Dai Y, Kou L 2019 J. Mater. Chem. A 7 1099
 Google Scholar
Google Scholar
[38] Yang L, Cai Z, Hao L, Ran L, Xu X, Dai Y, Pan S, Jing B, Zou J 2018 Electrochim. Acta 283 448
 Google Scholar
Google Scholar
[39] Ko K Y, Song J G, Kim Y, Choi T, Shin S, Lee C W, Lee K, Koo J, Lee H, Kim J 2016 ACS Nano 10 9287
 Google Scholar
Google Scholar
[40] Lu G, Ocola L E, Chen J 2009 Appl. Phys. Lett. 94 083111
 Google Scholar
Google Scholar
[41] Pearce R, Iakimov T, Andersson M, Hultman L, Spetz A L, Yakimova R 2011 Sens. Actuator B: Chem. 155 451
 Google Scholar
Google Scholar
[42] Yavari F, Castillo E, Gullapalli H, Ajayan P M, Koratkar N 2012 Appl. Phys. Lett. 100 203120
 Google Scholar
Google Scholar
[43] Qazi M, Nomani M W, Chandrashekhar M, Shields V B, Spencer M G, Koley G 2010 Appl. Phys. Express 3 075101
 Google Scholar
Google Scholar
计量
- 文章访问数: 11680
- PDF下载量: 115
- 被引次数: 0














 下载:
下载: