-
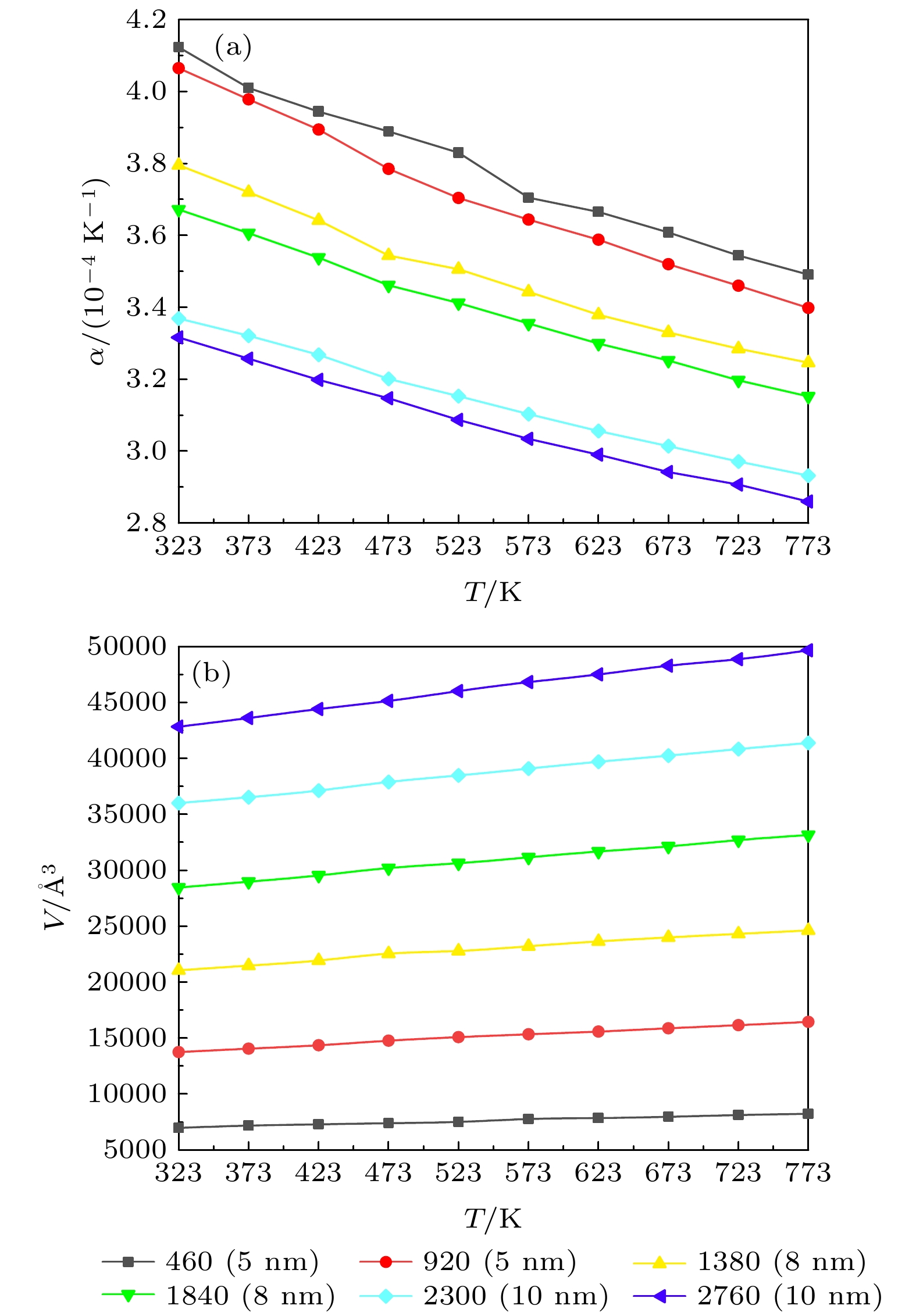
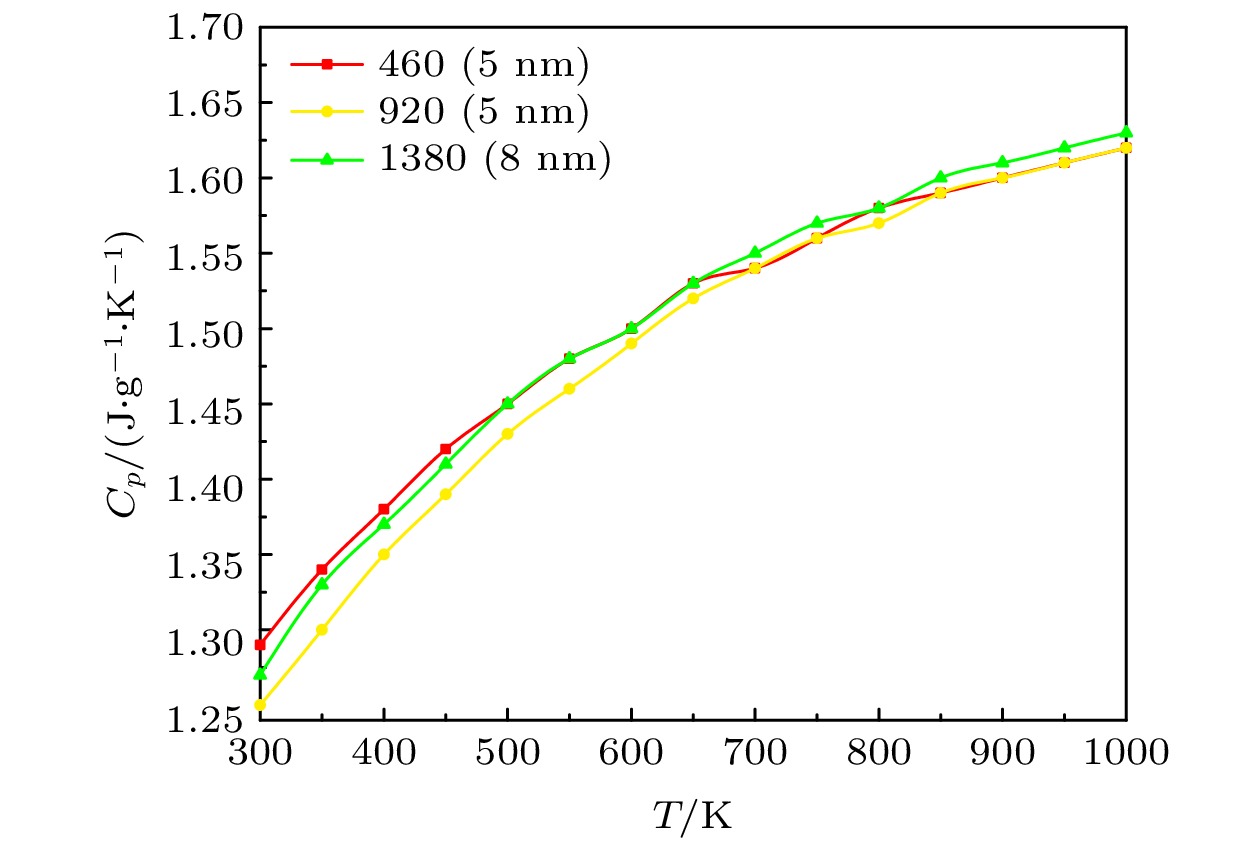
Molecular dynamics method is used to simulate the influence of the mesopore size and structure on the heat transport characteristics of the mixed nitrate. The Material Studio software is used to establish the mixed nitrate models of different scales and two structures, and the NaNO3-KNO3 models of different proportions that reach the eutectic state. By calculating the models and sorting out the calculation results, the phase transition of mixed nitrates on a nanometer scale is calculated and the micro-mechanism of heat transport characteristics is analyzed. The results show that the phase transition temperature of the solar salt first increases and then decreases with the increase of the nanopore size, and finally is consistent with the melting point on a macro scale. The proportion of cations has a great influence on the phase transition temperature of mixed nitrate, and the nanowire structures also change the phase transition temperature of nitrate. The bulk thermal expansion coefficient of nitrate decreases with the increase of mesoporous size, increases with the increase of NaNO3 content, and changes with the mesoporous structure. The enhancement of the interaction between ions will increase the thermal conductivity, but it will not have much effect on the specific heat capacity at a constant pressure.
-
Keywords:
- mixed nitrate /
- scale /
- structure /
- heat transport characteristics
[1] Kannan N, Vakeesan D 2016 Renew. Sust. Energy Rev. 62 1092
 Google Scholar
Google Scholar
[2] Awad A, Navarro H, Ding Y L 2018 Renew. Energy 120 275
 Google Scholar
Google Scholar
[3] Chieruzzia M, Gian F. C, Miliozzi A 2017 Sol. Energy Mater. Sol. Cells 167 60
 Google Scholar
Google Scholar
[4] Zhang Y, Li J L, Gao L 2020 Sol. Energy Mater. Sol. Cells 216 110727
 Google Scholar
Google Scholar
[5] Han C J, Gu H Z, Zhang M J 2020 Sol. Energy Mater. Sol. Cells 217 110697
 Google Scholar
Google Scholar
[6] Deng Y, Qian T T, Guan W M 2017 J. Mater. Sci. Technol. 33 198
 Google Scholar
Google Scholar
[7] Rena Y, Lia P 2019 Sol. Energy Mater. Sol. Cells 200 110005
 Google Scholar
Google Scholar
[8] 尹辉斌, 王文豪, 陈昌杰 2017 广东化工 22 33
 Google Scholar
Google Scholar
Yin H B, Wang W H, Chen C J 2017 Guangdong Chemical 22 33
 Google Scholar
Google Scholar
[9] 李进, 王峰, 张世广, 吴玉庭 2020 华电技术 42 17
 Google Scholar
Google Scholar
Li J, Wang F, Chang S G, Wu Y T 2020 Huadian Technologies 42 17
 Google Scholar
Google Scholar
[10] 李彦, 李鹏, 朱群志, 余杨敏 2018 硅酸盐学报 46 625
 Google Scholar
Google Scholar
Li Y, Li P, Zhu Q Z, Yu Y Y 2018 Journal of Silicate 46 625
 Google Scholar
Google Scholar
[11] 冯妍卉, 冯黛丽, 张欣欣 2019 介孔复合材料的相变及热输运特性 (北京: 科学出版社) 第5−138页
Feng Y H, Feng D L, Zhang X X 2019 Phase Transition and Thermal Transport Properties of Mesoporous Composite (Beijing: Science Press) pp5−138 (in Chinese)
[12] 袁思伟, 冯妍卉, 王鑫, 张欣欣 2014 63 014402
 Google Scholar
Google Scholar
Yuan S Y, Feng Y H, Wang X, Zhang X X 2014 Acta Phys. Sin. 63 014402
 Google Scholar
Google Scholar
[13] Bore M T, Pham H N, Switzer E E 2005 J. Phys. Chem. B 109 2873
 Google Scholar
Google Scholar
[14] Zhang J R, Feng Y H, Yuan H B 2015 Comput. Mater. Sci. 109 300
 Google Scholar
Google Scholar
[15] Wang L P, Sui J, Zhai M 2015 J. Phys. Chem. C 119 18697
 Google Scholar
Google Scholar
[16] 赵亚溥 2012 表面与界面物理力学 (北京: 科学出版社) 第212−220页
Zhao Y P 2012 Physical Mechanics of Surfaces and Interfaces (Beijing: Science Press) pp212−220 (in Chinese)
[17] 李亚琼, 梁凯彦, 王静静, 黄秀兵 2020 工程科学学报 42 1229
 Google Scholar
Google Scholar
Li Y Q, Liang K Y, Wang J J, Huang X B 2020 J. Eng. Sci. 42 1229
 Google Scholar
Google Scholar
[18] Min X, Fang M H, Huang Z H 2015 Sci. Rep. 5 12964
 Google Scholar
Google Scholar
[19] Gao J K, Tao W W, Chen D 2018 Nanomaterials 8 385
 Google Scholar
Google Scholar
[20] Sirota E B 2007 Macromolecules 40 1043
 Google Scholar
Google Scholar
[21] 冯黛丽, 冯妍卉, 张欣欣 2013 62 083602
 Google Scholar
Google Scholar
Feng D L, Feng Y H, Zhang X X 2013 Acta Phys. Sin. 62 083602
 Google Scholar
Google Scholar
[22] Asegun H, Chen G 2008 Phys. Rev. Lett. 101 235502
 Google Scholar
Google Scholar
[23] 黄丛亮, 冯黛丽, 张欣欣, 李静, 王戈, 侴爱辉 2013 62 026501
 Google Scholar
Google Scholar
Huang C L, Feng Y H, Zhang X X, Li J, Wang G, Chou A H 2013 Acta Phys. Sin. 62 026501
 Google Scholar
Google Scholar
[24] Ni H O, Wu J, Sun Z 2019 Chem. Eng. J. 377 120029
 Google Scholar
Google Scholar
[25] Elena N, Anabel P, Tomos H 2017 Energy Stor. Sci. Tech. 6 688
[26] 吴玉庭, 王涛, 马重芳 2012 太阳能学报 33 148
 Google Scholar
Google Scholar
Wu Y T, Wang T, Ma C F 2012 J. Sol. Energy 33 148
 Google Scholar
Google Scholar
[27] 官云许, 杨启容, 何卓亚, 王力伟 2021 功能材料 52 2153
 Google Scholar
Google Scholar
Guan Y X, Yang Q R, He Z Y, Wang L W 2021 Funct. Mater. 52 2153
 Google Scholar
Google Scholar
[28] 李成祥, 孟庆元, 杨立军 2008 哈尔滨工业大学学报 40 705
 Google Scholar
Google Scholar
Li C X, Meng Q Y, Yang L J 2008 Journal of Harbin Institute of Technology 40 705
 Google Scholar
Google Scholar
[29] Pan G, Ding J, Wang W L 2016 Int. J. Heat Mass Transf. 103 417
 Google Scholar
Google Scholar
[30] 宫薛菲, 杨启容, 姚尔人, 刘亭 2020 功能材料 51 1214
 Google Scholar
Google Scholar
Gong X F, Yang Q R, Yao E R, L T 2020 Funct. Mater. 51 1214
 Google Scholar
Google Scholar
[31] Jayaraman S, Thompson A P, von Lilienfeld O Anatole 2010 Ind. Eng. Chem. Res. 49 559
 Google Scholar
Google Scholar
[32] Anagnostopoulos A, Alexiadis A, Ding Y 2019 Sol. Energy Mater. Sol. Cells 200 109897
 Google Scholar
Google Scholar
[33] 倪海鸥, 孙泽, 路贵民 2017 储能科学与技术 6 669
 Google Scholar
Google Scholar
Ni H O, Sun Z, Lu G M 2017 Energy Stor. Mater. 6 669
 Google Scholar
Google Scholar
[34] 车德勇, 沈辉, 蒋文强 2015 无机盐工业 47 30
Che D Y, Shen H, Jiang W Q 2015 Inorganic salt industry 47 30
[35] Couchman P R, Jesser W A 1977 Nature 269 481
 Google Scholar
Google Scholar
[36] David T B, Lereah Y, Deutscher G 1995 Philos. Mag. A 71 1135
 Google Scholar
Google Scholar
[37] Reiss H, Wilson I B 1948 J. Colloid Sci. 3 551
 Google Scholar
Google Scholar
[38] 王海龙, 王秀喜, 梁海弋 2005 金属学报 41 568
 Google Scholar
Google Scholar
Wang H L, Wang X X, Liang H Y 2005 J. Metal Sci. 41 568
 Google Scholar
Google Scholar
[39] 姜小宝 2013 博士学位论文 (长春: 吉林大学)
Jiang X B 2013 Ph. D. Dissertation (Changchun: Jilin University) (in Chinese)
[40] Sememchenko V K 1961 Surface Phenomena in Metals and Alloys (Oxford Pergamon) pp2−81
[41] 温元凯, 李振民 1978 科学通报 23 225
 Google Scholar
Google Scholar
Wen Y K 1978 Chin. Sci. Bull. 23 225
 Google Scholar
Google Scholar
[42] Lonappan M 1955 Proceedings of the Indian Academy of Sciences-Section A 41 239
 Google Scholar
Google Scholar
[43] 张景胤 2017 硕士学位论文 (北京: 华北电力大学)
Zhang J Y 2017 M. S. Thesis (Beijing: North China Electric Power University) (in Chinese)
[44] Kenisarin M 2010 Renew. Sust. Energ. Rev. 14 955
 Google Scholar
Google Scholar
[45] 李杨 2018 硕士学位论文 (郑州: 郑州大学)
Li Y 2018 M. S. Thesis (Zhengzhou: Zhengzhou University) (in Chinese)
[46] 王长宝 2013 硕士学位论文 (北京: 北京工业大学)
Wang C B 2013 M. S. Thesis (Beijing: Beijing University of Technology) (in Chinese)
-
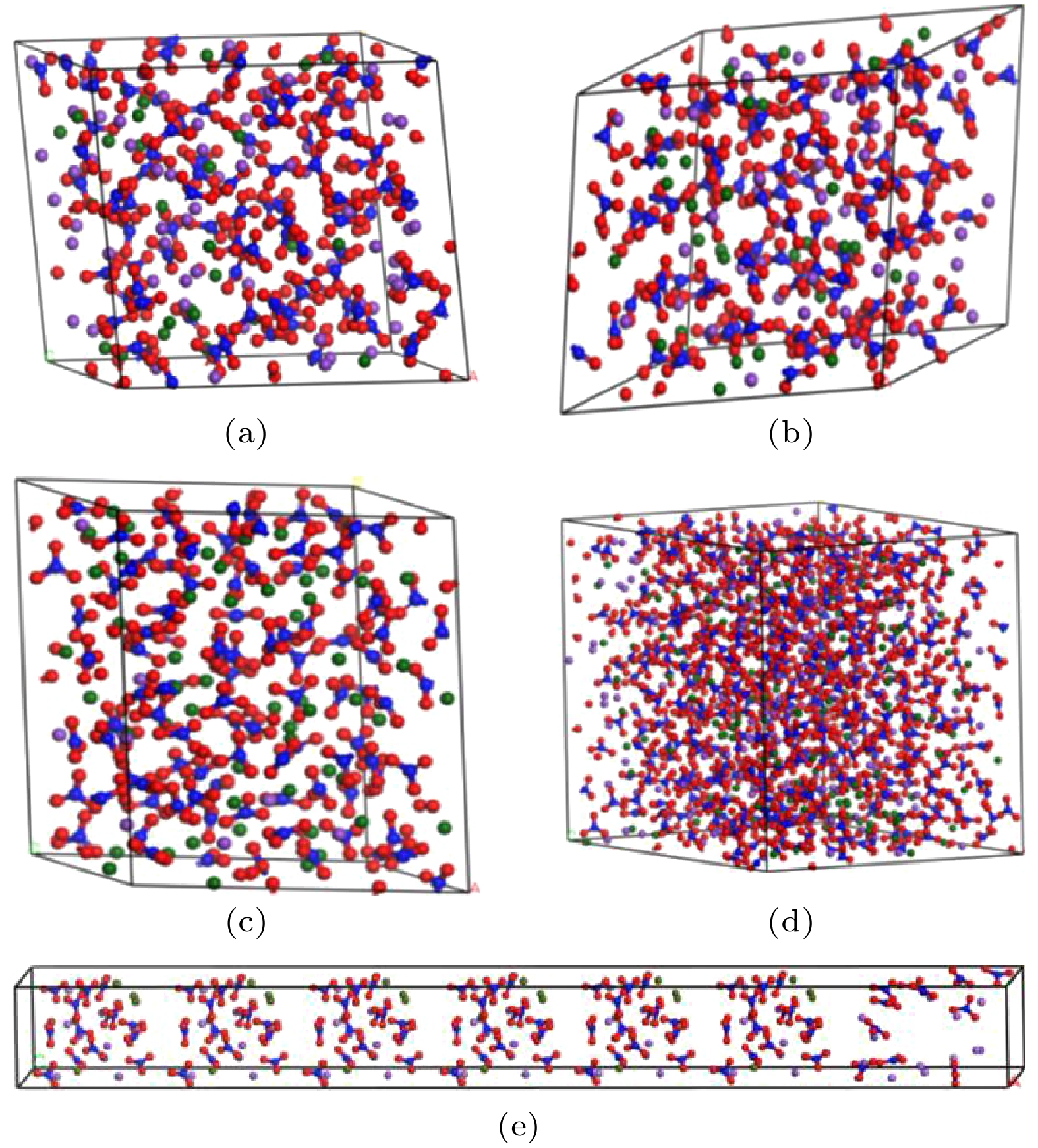
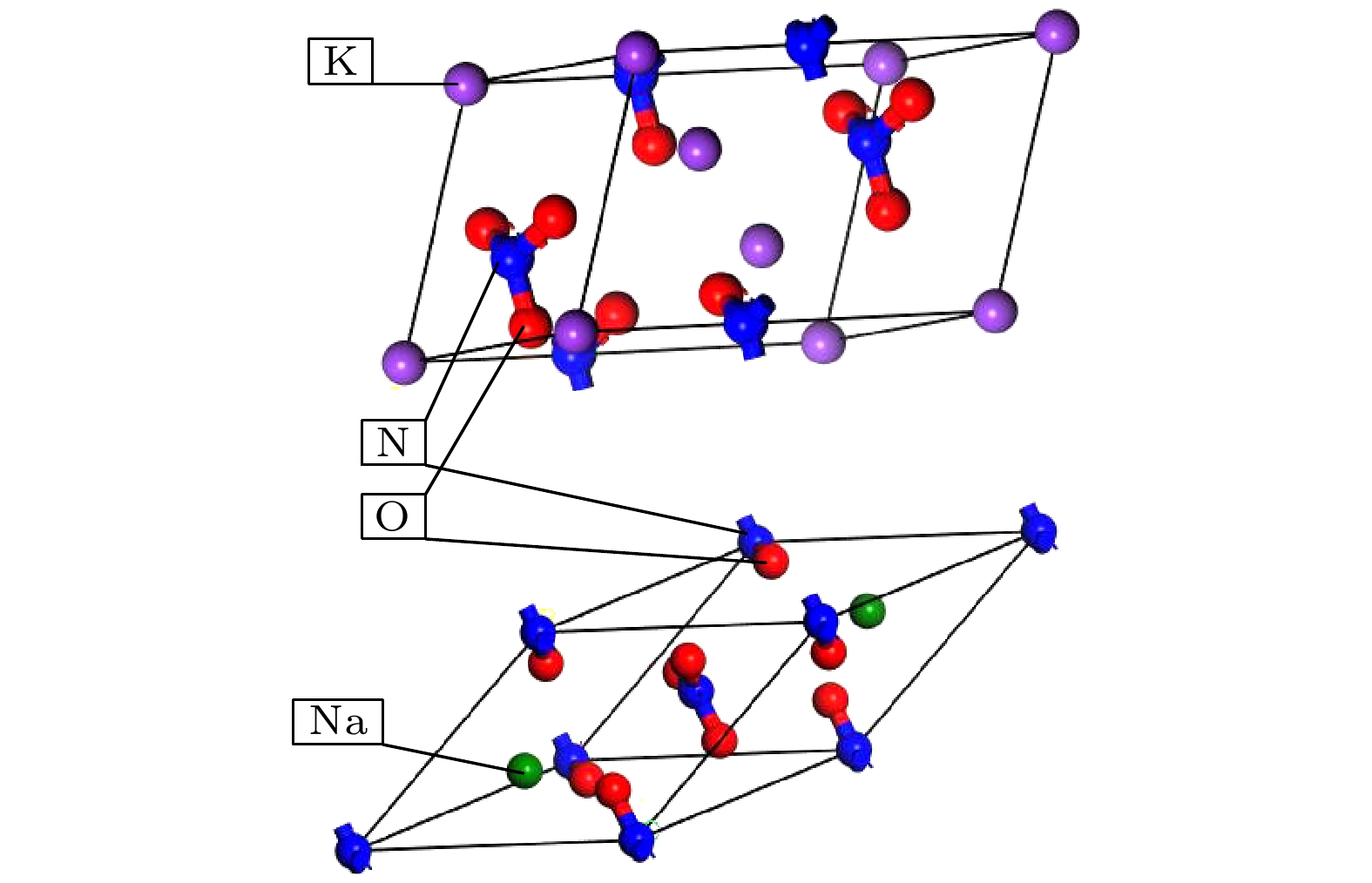
图 2 混合硝酸盐模型 (a) w (NaNO3)∶w (KNO3) = 4∶6混合硝酸盐; (b) w (NaNO3) ∶w (KNO3) = 5∶5混合硝酸盐; (c) w (NaNO3) ∶w (KNO3) = 9∶1混合硝酸盐; (d) w (NaNO3)∶w (KNO3) = 6∶4太阳盐; (e) w(NaNO3)∶w (KNO3) = 6∶4异结构太阳盐
Figure 2. Mixed nitrate model: (a) w (NaNO3)∶w (KNO3) = 4∶6 mixed nitrate; (b) w (NaNO3)∶w (KNO3) = 5∶5 mixed nitrate; (c) w (NaNO3)∶w (KNO3) = 9∶1 mixed nitrate; (d) w (NaNO3)∶w (KNO3) = 6∶4 solar salt; (e) w (NaNO3)∶w (KNO3) = 6∶4 heterogeneous solar salts.
表 1 太阳盐(
$w({\rm NaNO_3}): w({\rm KNO_3}) = 6:4$ )中NaNO3和KNO3的离子数Table 1. Ion numbers of NaNO3 and KNO3 in solar salts (
$w({\rm NaNO_3}): w({\rm KNO_3}) = 6: 4$ )离子数 种类 Model size/nm Na+ K+ N– O2– 460 60 32 92 276 5 920 120 64 184 552 5 1380 180 96 276 828 8 1840 240 128 368 1104 8 2300 300 160 460 1380 10 2760 360 192 552 1656 10 表 2 混合硝酸盐中NaNO3和KNO3的离子数(总离子数为460)
Table 2. Ion numbers of NaNO3 and KNO3 in mixed nitrate (total number of ions is 460).
$w({\rm NaNO_3}): w({\rm KNO_3})$ 种类 Model
size/nmNa+ K+ N– O2– 4∶6 41 51 92 276 5 5∶5 49 43 92 276 5 6∶4 60 32 92 276 5 9∶1 84 8 92 276 5 表 3 不同尺度下太阳盐的相变温度
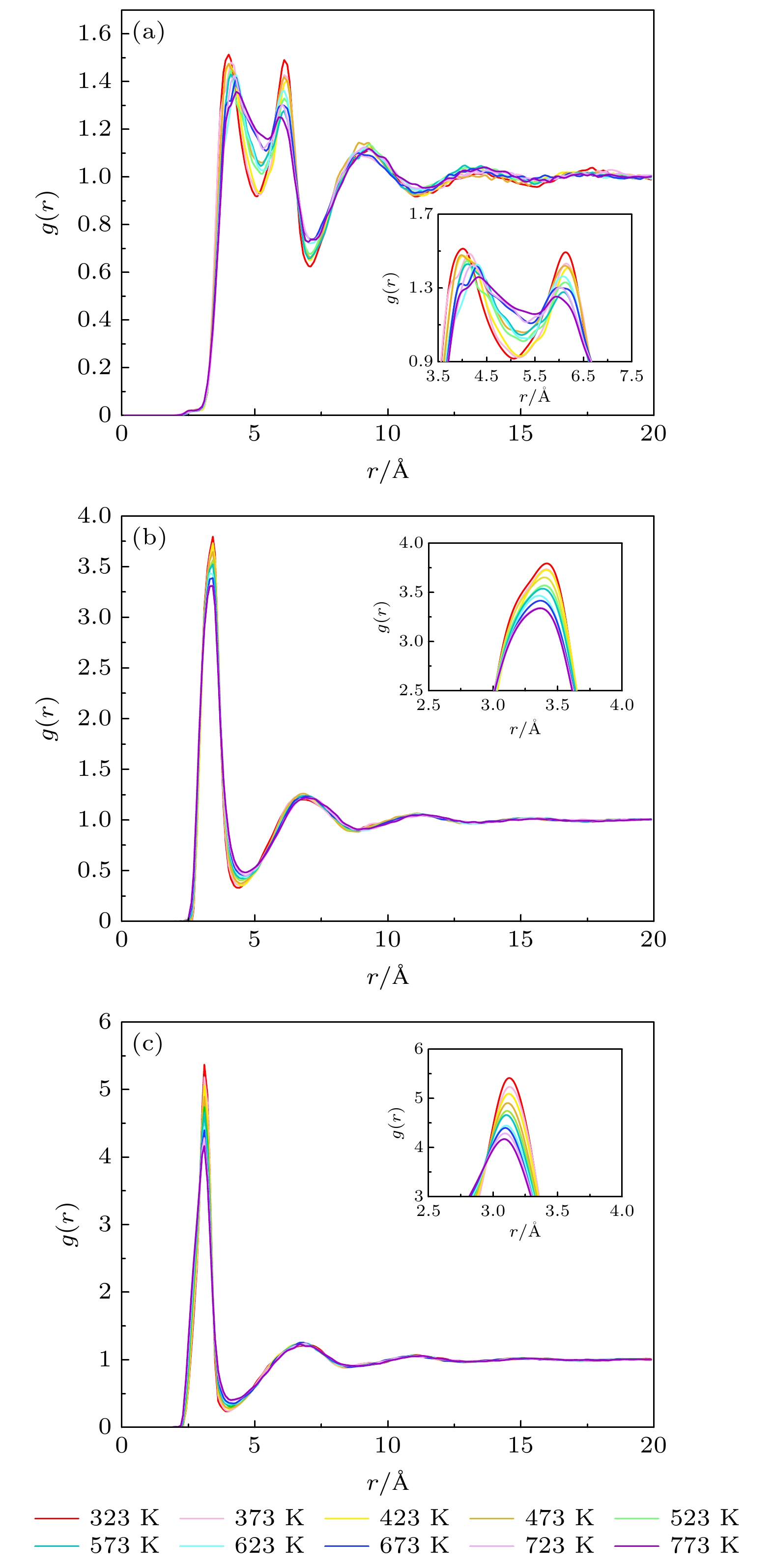
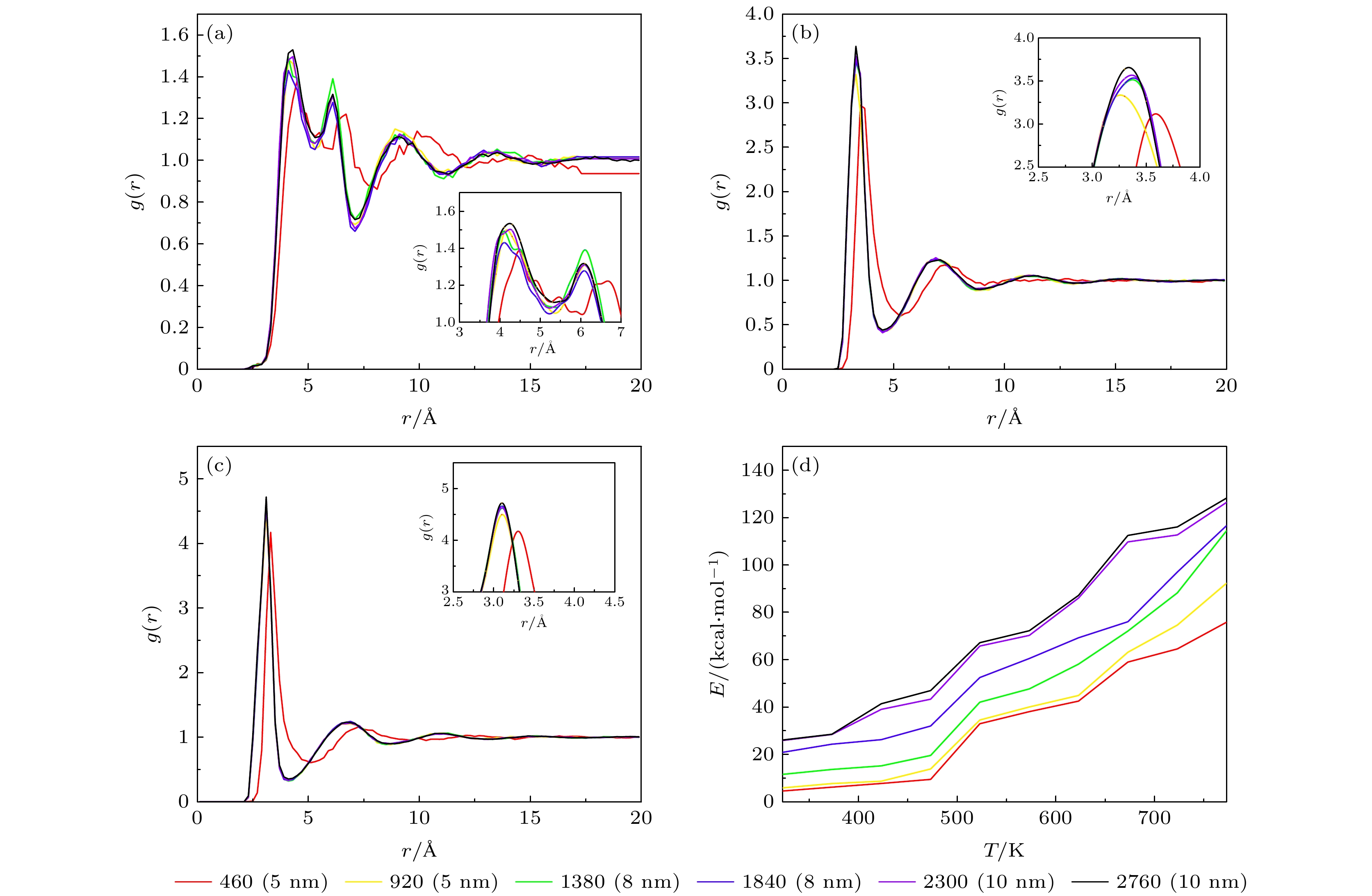
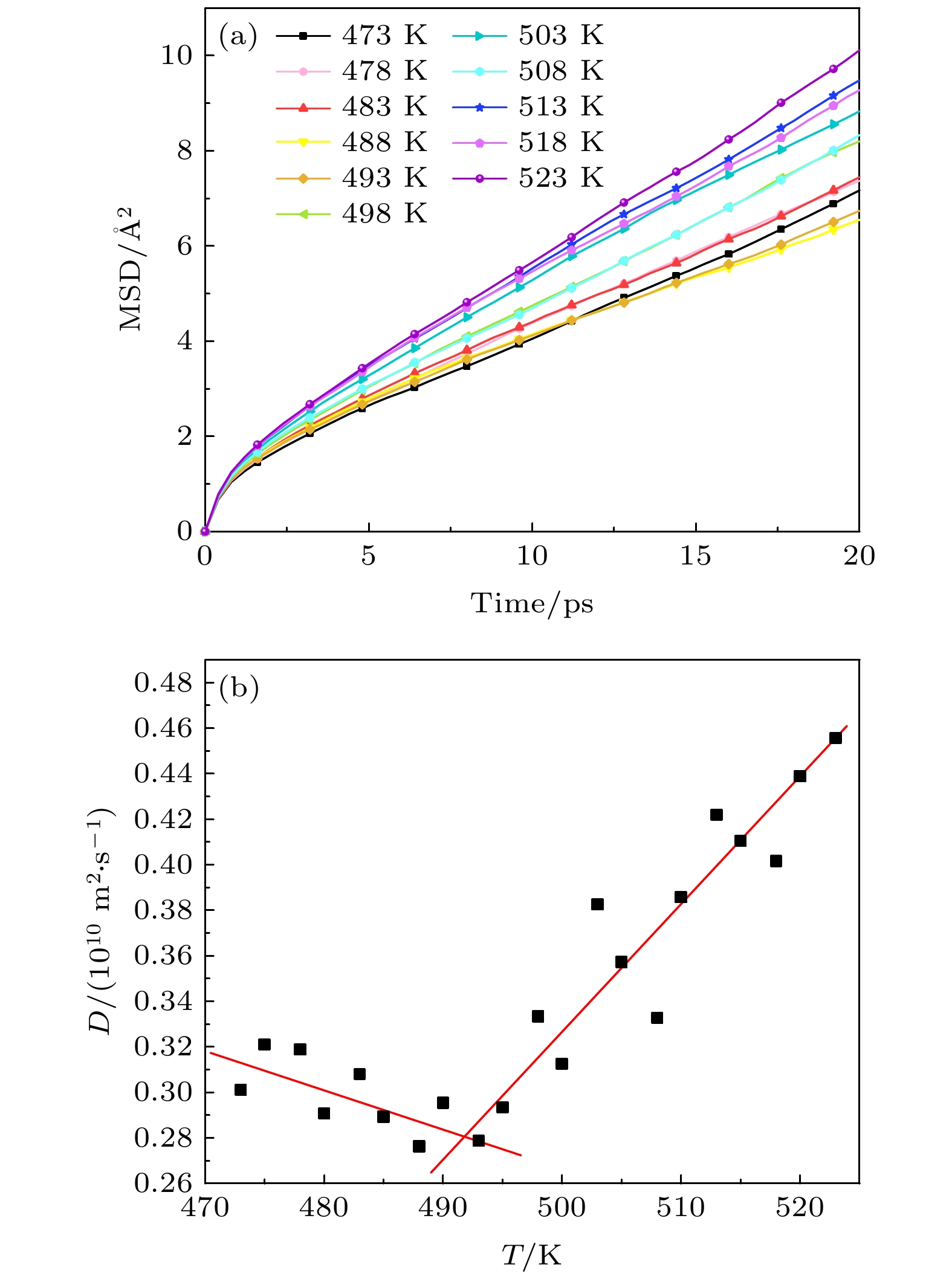
Table 3. Phase transition temperature of solar salts at different scales.
离子数 460 920 1380 1840 2300 2760 5520 温度/K 493 493 503 518 508 492 493 表 4 不同比例的混合硝酸盐的相变温度
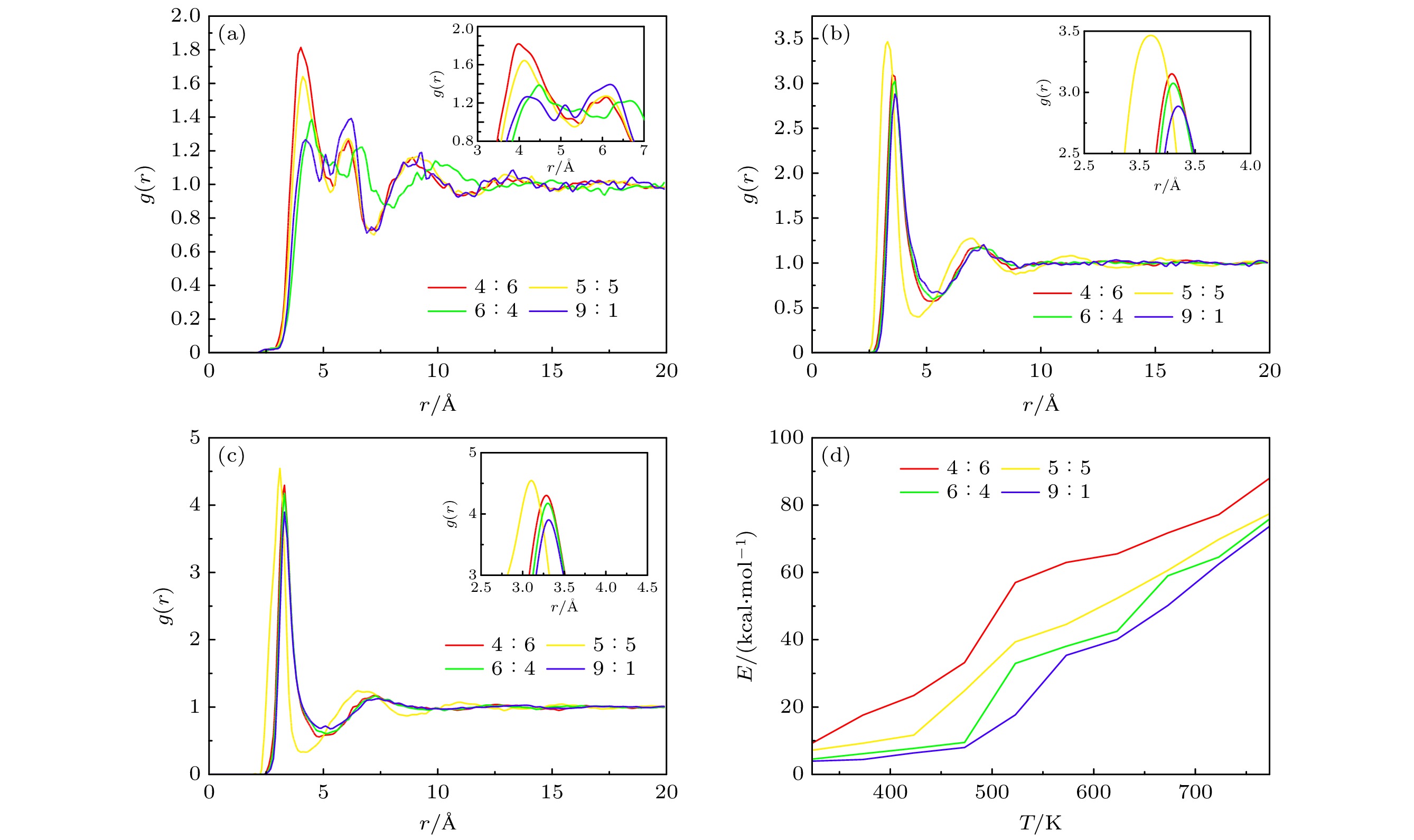
Table 4. Phase transition temperature of mixed nitrates at different proportions
w(NaNO3)∶w(KNO3) 4∶6 5∶5 6∶4 9∶1 温度/K 493 488 493 548 表 5 纳米线结构太阳盐的相变温度
Table 5. Phase transition temperature of nanostructured solar salts.
离子数 460 920 温度/K 528 548 表 6 两种结构下太阳盐导热率
Table 6. Thermal conductivity of solar salts at two structures.
离子数 460-
纳米孔920-
纳米孔460-
纳米线920-
纳米线导热率/(W·m–1·K–1) 0.46 0.63 0.99 1.98 -
[1] Kannan N, Vakeesan D 2016 Renew. Sust. Energy Rev. 62 1092
 Google Scholar
Google Scholar
[2] Awad A, Navarro H, Ding Y L 2018 Renew. Energy 120 275
 Google Scholar
Google Scholar
[3] Chieruzzia M, Gian F. C, Miliozzi A 2017 Sol. Energy Mater. Sol. Cells 167 60
 Google Scholar
Google Scholar
[4] Zhang Y, Li J L, Gao L 2020 Sol. Energy Mater. Sol. Cells 216 110727
 Google Scholar
Google Scholar
[5] Han C J, Gu H Z, Zhang M J 2020 Sol. Energy Mater. Sol. Cells 217 110697
 Google Scholar
Google Scholar
[6] Deng Y, Qian T T, Guan W M 2017 J. Mater. Sci. Technol. 33 198
 Google Scholar
Google Scholar
[7] Rena Y, Lia P 2019 Sol. Energy Mater. Sol. Cells 200 110005
 Google Scholar
Google Scholar
[8] 尹辉斌, 王文豪, 陈昌杰 2017 广东化工 22 33
 Google Scholar
Google Scholar
Yin H B, Wang W H, Chen C J 2017 Guangdong Chemical 22 33
 Google Scholar
Google Scholar
[9] 李进, 王峰, 张世广, 吴玉庭 2020 华电技术 42 17
 Google Scholar
Google Scholar
Li J, Wang F, Chang S G, Wu Y T 2020 Huadian Technologies 42 17
 Google Scholar
Google Scholar
[10] 李彦, 李鹏, 朱群志, 余杨敏 2018 硅酸盐学报 46 625
 Google Scholar
Google Scholar
Li Y, Li P, Zhu Q Z, Yu Y Y 2018 Journal of Silicate 46 625
 Google Scholar
Google Scholar
[11] 冯妍卉, 冯黛丽, 张欣欣 2019 介孔复合材料的相变及热输运特性 (北京: 科学出版社) 第5−138页
Feng Y H, Feng D L, Zhang X X 2019 Phase Transition and Thermal Transport Properties of Mesoporous Composite (Beijing: Science Press) pp5−138 (in Chinese)
[12] 袁思伟, 冯妍卉, 王鑫, 张欣欣 2014 63 014402
 Google Scholar
Google Scholar
Yuan S Y, Feng Y H, Wang X, Zhang X X 2014 Acta Phys. Sin. 63 014402
 Google Scholar
Google Scholar
[13] Bore M T, Pham H N, Switzer E E 2005 J. Phys. Chem. B 109 2873
 Google Scholar
Google Scholar
[14] Zhang J R, Feng Y H, Yuan H B 2015 Comput. Mater. Sci. 109 300
 Google Scholar
Google Scholar
[15] Wang L P, Sui J, Zhai M 2015 J. Phys. Chem. C 119 18697
 Google Scholar
Google Scholar
[16] 赵亚溥 2012 表面与界面物理力学 (北京: 科学出版社) 第212−220页
Zhao Y P 2012 Physical Mechanics of Surfaces and Interfaces (Beijing: Science Press) pp212−220 (in Chinese)
[17] 李亚琼, 梁凯彦, 王静静, 黄秀兵 2020 工程科学学报 42 1229
 Google Scholar
Google Scholar
Li Y Q, Liang K Y, Wang J J, Huang X B 2020 J. Eng. Sci. 42 1229
 Google Scholar
Google Scholar
[18] Min X, Fang M H, Huang Z H 2015 Sci. Rep. 5 12964
 Google Scholar
Google Scholar
[19] Gao J K, Tao W W, Chen D 2018 Nanomaterials 8 385
 Google Scholar
Google Scholar
[20] Sirota E B 2007 Macromolecules 40 1043
 Google Scholar
Google Scholar
[21] 冯黛丽, 冯妍卉, 张欣欣 2013 62 083602
 Google Scholar
Google Scholar
Feng D L, Feng Y H, Zhang X X 2013 Acta Phys. Sin. 62 083602
 Google Scholar
Google Scholar
[22] Asegun H, Chen G 2008 Phys. Rev. Lett. 101 235502
 Google Scholar
Google Scholar
[23] 黄丛亮, 冯黛丽, 张欣欣, 李静, 王戈, 侴爱辉 2013 62 026501
 Google Scholar
Google Scholar
Huang C L, Feng Y H, Zhang X X, Li J, Wang G, Chou A H 2013 Acta Phys. Sin. 62 026501
 Google Scholar
Google Scholar
[24] Ni H O, Wu J, Sun Z 2019 Chem. Eng. J. 377 120029
 Google Scholar
Google Scholar
[25] Elena N, Anabel P, Tomos H 2017 Energy Stor. Sci. Tech. 6 688
[26] 吴玉庭, 王涛, 马重芳 2012 太阳能学报 33 148
 Google Scholar
Google Scholar
Wu Y T, Wang T, Ma C F 2012 J. Sol. Energy 33 148
 Google Scholar
Google Scholar
[27] 官云许, 杨启容, 何卓亚, 王力伟 2021 功能材料 52 2153
 Google Scholar
Google Scholar
Guan Y X, Yang Q R, He Z Y, Wang L W 2021 Funct. Mater. 52 2153
 Google Scholar
Google Scholar
[28] 李成祥, 孟庆元, 杨立军 2008 哈尔滨工业大学学报 40 705
 Google Scholar
Google Scholar
Li C X, Meng Q Y, Yang L J 2008 Journal of Harbin Institute of Technology 40 705
 Google Scholar
Google Scholar
[29] Pan G, Ding J, Wang W L 2016 Int. J. Heat Mass Transf. 103 417
 Google Scholar
Google Scholar
[30] 宫薛菲, 杨启容, 姚尔人, 刘亭 2020 功能材料 51 1214
 Google Scholar
Google Scholar
Gong X F, Yang Q R, Yao E R, L T 2020 Funct. Mater. 51 1214
 Google Scholar
Google Scholar
[31] Jayaraman S, Thompson A P, von Lilienfeld O Anatole 2010 Ind. Eng. Chem. Res. 49 559
 Google Scholar
Google Scholar
[32] Anagnostopoulos A, Alexiadis A, Ding Y 2019 Sol. Energy Mater. Sol. Cells 200 109897
 Google Scholar
Google Scholar
[33] 倪海鸥, 孙泽, 路贵民 2017 储能科学与技术 6 669
 Google Scholar
Google Scholar
Ni H O, Sun Z, Lu G M 2017 Energy Stor. Mater. 6 669
 Google Scholar
Google Scholar
[34] 车德勇, 沈辉, 蒋文强 2015 无机盐工业 47 30
Che D Y, Shen H, Jiang W Q 2015 Inorganic salt industry 47 30
[35] Couchman P R, Jesser W A 1977 Nature 269 481
 Google Scholar
Google Scholar
[36] David T B, Lereah Y, Deutscher G 1995 Philos. Mag. A 71 1135
 Google Scholar
Google Scholar
[37] Reiss H, Wilson I B 1948 J. Colloid Sci. 3 551
 Google Scholar
Google Scholar
[38] 王海龙, 王秀喜, 梁海弋 2005 金属学报 41 568
 Google Scholar
Google Scholar
Wang H L, Wang X X, Liang H Y 2005 J. Metal Sci. 41 568
 Google Scholar
Google Scholar
[39] 姜小宝 2013 博士学位论文 (长春: 吉林大学)
Jiang X B 2013 Ph. D. Dissertation (Changchun: Jilin University) (in Chinese)
[40] Sememchenko V K 1961 Surface Phenomena in Metals and Alloys (Oxford Pergamon) pp2−81
[41] 温元凯, 李振民 1978 科学通报 23 225
 Google Scholar
Google Scholar
Wen Y K 1978 Chin. Sci. Bull. 23 225
 Google Scholar
Google Scholar
[42] Lonappan M 1955 Proceedings of the Indian Academy of Sciences-Section A 41 239
 Google Scholar
Google Scholar
[43] 张景胤 2017 硕士学位论文 (北京: 华北电力大学)
Zhang J Y 2017 M. S. Thesis (Beijing: North China Electric Power University) (in Chinese)
[44] Kenisarin M 2010 Renew. Sust. Energ. Rev. 14 955
 Google Scholar
Google Scholar
[45] 李杨 2018 硕士学位论文 (郑州: 郑州大学)
Li Y 2018 M. S. Thesis (Zhengzhou: Zhengzhou University) (in Chinese)
[46] 王长宝 2013 硕士学位论文 (北京: 北京工业大学)
Wang C B 2013 M. S. Thesis (Beijing: Beijing University of Technology) (in Chinese)
Catalog
Metrics
- Abstract views: 6145
- PDF Downloads: 73
- Cited By: 0














 DownLoad:
DownLoad: