-
As a clean and efficient unconventional energy source, natural gas hydrate has been highly valued and vigorously developed by many countries in recent years. In order to solve the problem that the existing hydrate structure symmetry is not high, which leads the theoretical research to be restricted, it is imperative to explore a new type of methane hydrate structure with high symmetry. Using the first-principles method which is based on the density functional theory (DFT), the structure and electronic properties of N-methane hydrate are calculated in the generalized gradient approximation (GGA) for Grimme dispersion correction. The obtained results are shown below. 1) The water cage structure of N-methane hydrate is a truncated octahedron (4668), which is composed of 8 regular hexagons and 6 squares, and the average length of the hexagons and the average length of the squares are both 2.723 Å. The average bond length of water molecules is optimized to be 1.056 Å, and the average bond angle of water molecules is 107.738°. The average bond length of methane molecules is 1.0973 Å. The average distance from methane molecules to water molecules is 4.2831 Å that is longer than the distance in the I- methane hydrate. So N-methane hydrate can accommodate larger volumes of gas molecules. The symmetric group is
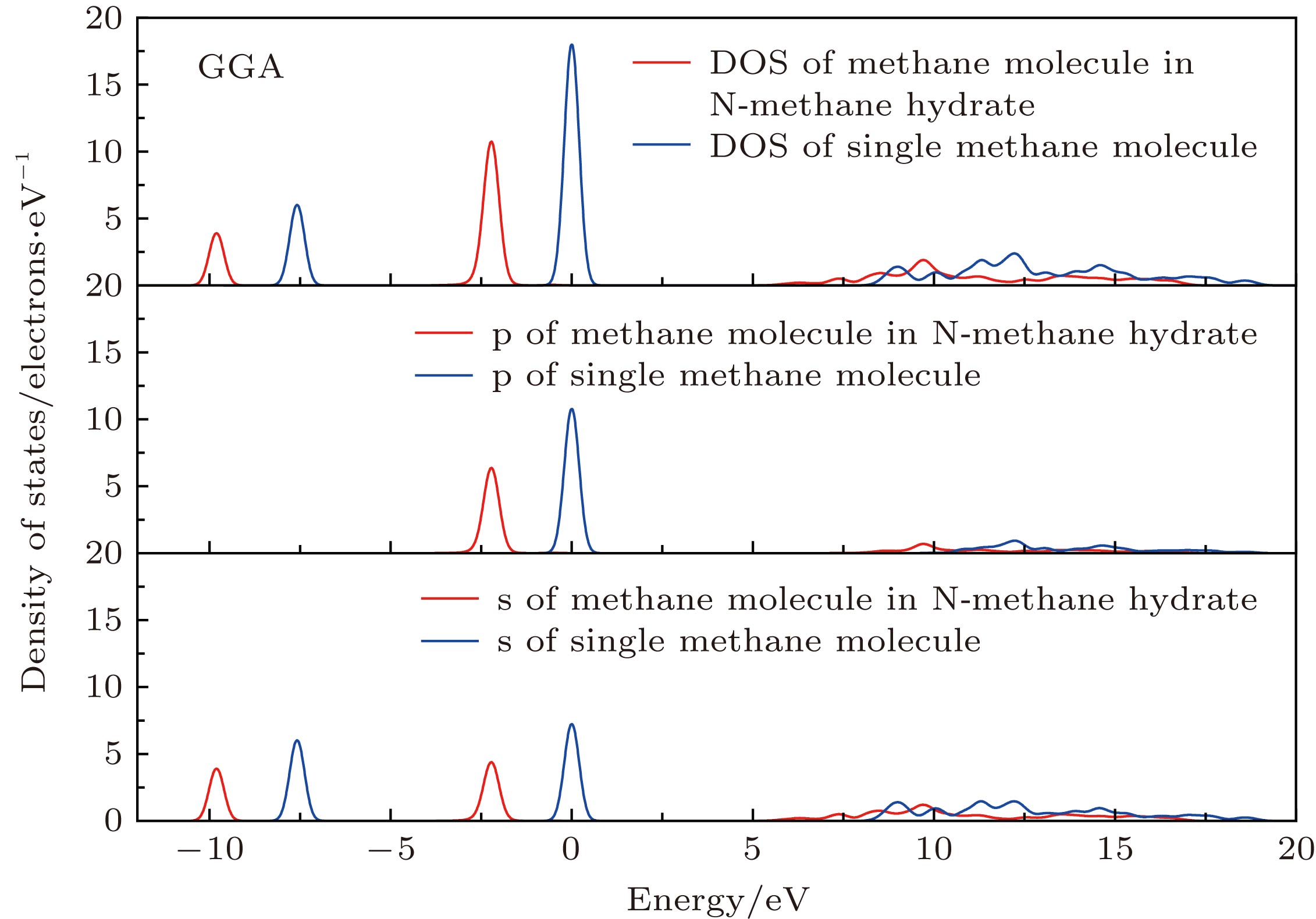
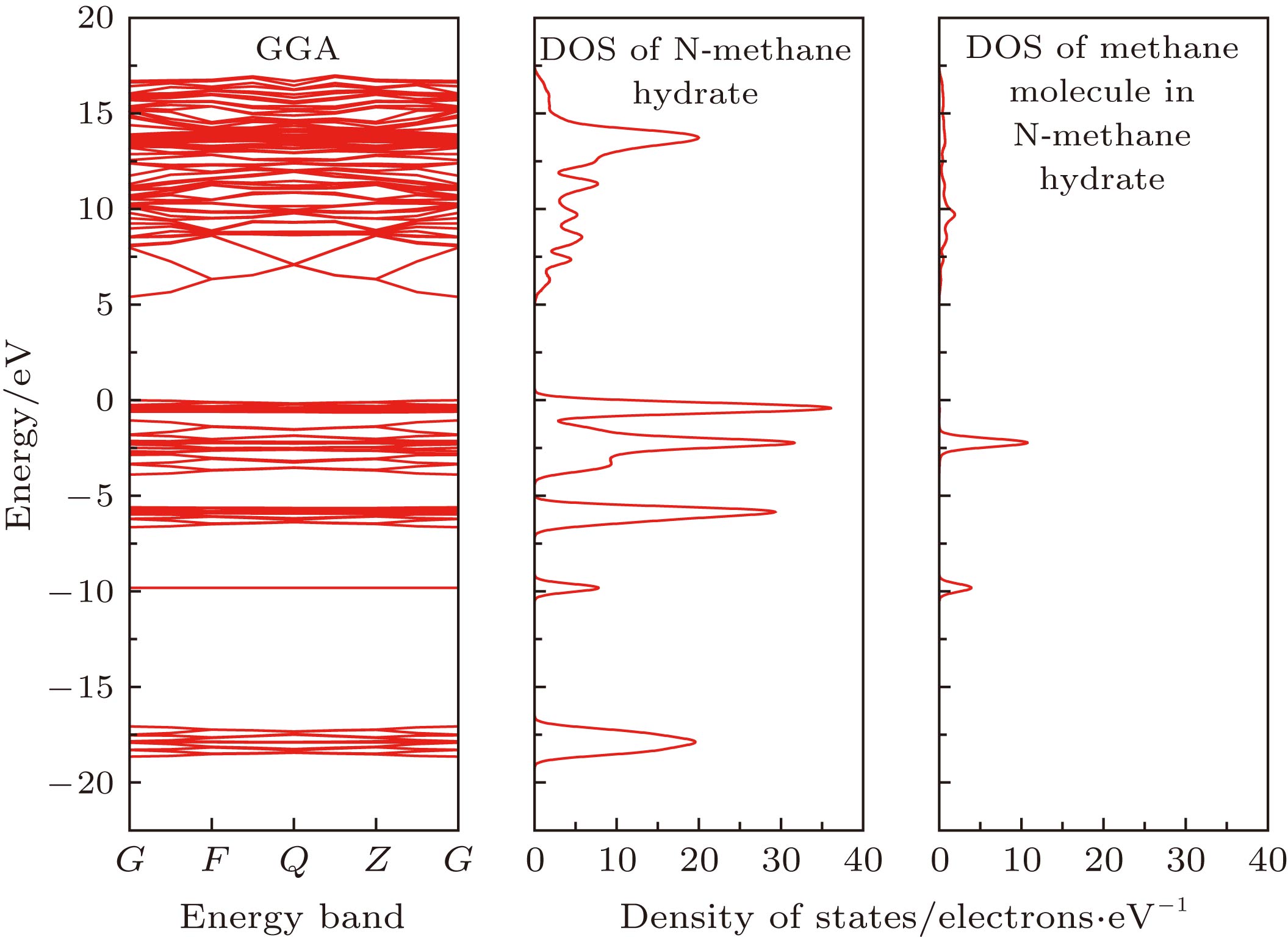
${\rm{IM}}\bar 3{\rm{M}}$ for N-methane hydrate, which has a simple and strict periodic stable structure. 2) The lattice parameter of N-methane hydrate is 7.70 Å, and the density is 0.903 g/cm3, which is greater than I-, II- and H-type hydrate density. 3) The x-ray diffraction(XRD) pattern of N-methane hydrateis calculated and is close to that of of I-methane hydrate, while the water cage of N-methane hydrate is larger. 4) The interaction between methane molecules and the water cage is van der Waals force, and the formation energy of N- methane hydrate is –0.247 eV, which indicates that the N-methane hydrate is easy to form. Both the density of states and partial density of states indicate that the interaction between methane and water cage is weak, and it relies on molecular force. 5) In addition, N-methane hydrate is an insulator material with the energy gap greater than 5 eV.-
Keywords:
- N-methane hydrate /
- structure /
- electronic properties /
- density functional theory
[1] Holder G, Kamath V, Godbole S 1984 Annu. Rev. Energy 9 427
[2] Max M, Lowrie A 1996 J. Petrol. Geol. 19 41
 Google Scholar
Google Scholar
[3] Collett T S 2002 AAPG Bull. 86 1971
[4] Kvamme B, Kuznetsova T, Sapate A, Qorbani K 2016 J. Nat. Gas Sci. Eng. 35 1594
 Google Scholar
Google Scholar
[5] Zhao J, Chen X, Song Y, Zhu Z, Yang L, Tian Y, Wang J, Yang M, Zhang Y 2014 Energy Procedia 61 75
 Google Scholar
Google Scholar
[6] Khlebnikov V, Antonov S, Mishin A, Bakulin D, Khamidullina I, 梁萌, Vinokurov V, Gushchin P A 2016 天然气工业 36 40
 Google Scholar
Google Scholar
Khlebnikov V, Antonov S, Mishin A, Bakulin D, Khamidullina I, Liang M, Vinokurov V, Gushchin P A 2016 Nat. Gas Ind. 36 40
 Google Scholar
Google Scholar
[7] Lim D, Ro H, Seo Y, Seo Y, Lee J, Kim S, Lee J , Lee H 2017 J. Chem. Thermodyn. 106 16
 Google Scholar
Google Scholar
[8] Partoon B, Nashed O, Kassim Z, Sabil K, Sangwai J, Lal B 2016 Process Eng. 148 1220
[9] Zhang L X, Yang L, Wang J Q, Zhao J F, Dong H S, Yang M J, Liu Y, Song Y C 2017 Chem. Eng. J. 308 40
 Google Scholar
Google Scholar
[10] Castellani B, Rossetti G, Tupsakhare S, Rossi F, Nicolini A, Castaldi M 2016 J. Petrol. Sci. Eng. 147 515
 Google Scholar
Google Scholar
[11] Michael K, Roland B, Edward B, Lewis R N 2004 J. Am. Chem. Soc. 126 9407
 Google Scholar
Google Scholar
[12] Xu C G, Cai J, Lin F H, Chen Z Y, Li X S 2015 Energy 79 111
 Google Scholar
Google Scholar
[13] Naeiji P, Varaminian F, Rahmati M 2017 J. Nat. Gas Sci. Eng. 44 122
 Google Scholar
Google Scholar
[14] 朱金龙, 赵予生, 靳常青 2019 68 018203
Zhu J L, Zhao Y S, Jin C Q 2019 Acta Phys. Sin. 68 018203
[15] Choudhary N, Kushwaha O S, Bhattacharjee G, Chakrabarty S, Kumara R 2017 Energt Procedia 105 5026
 Google Scholar
Google Scholar
[16] Alavi S, Ohmura R 2016 J. Chem. Phys. 145 154708
 Google Scholar
Google Scholar
[17] Huang Y, Yuan L, Su Y, Zhao J 2015 Mol. Simul. 41 1086
 Google Scholar
Google Scholar
[18] Nguyen A H, Molinero V 2014 J. Chem. Phys. 140 3440
[19] Yagasaki T, Matsumoto M, Andoh Y, Okazaki S, Tanaka H 2014 J. Phys. Chem. B 118 11797
[20] Druart M L, Michel L, Van D H 2014 Fluid Phase Equilib. 381 108
 Google Scholar
Google Scholar
[21] 耿春宇, 丁丽颖, 韩清珍, 温浩 2008 物理化学学报 24 595
 Google Scholar
Google Scholar
Geng C Y, Ding L Y, Han Q Z, Wen H 2008 Acta Phys. Chim. Sin. 24 595
 Google Scholar
Google Scholar
[22] Lasich M, Ramjugernath D 2016 Philos. Mag. 96 15
 Google Scholar
Google Scholar
[23] Zong X, Cheng G, Qiu N, Huang Q, He J, Du S, Li Y 2017 Chem. Lett. 46 1141
 Google Scholar
Google Scholar
[24] Liu Y, Ojamäe L 2014 J. Phys. Chem. A 118 51
[25] Zhang X, Qiu N, Huang Q, Zha X, He J, Li Y, Du S 2017 J. Mol. Struct. 1153 292
[26] Waldron C J, English N J 2017 J. Chem. Phys. 147 024506
 Google Scholar
Google Scholar
[27] Zquierdo-Ruiz F, Otero-de-la-Roza A, Contreras-García J, Menéndez B, Recio J M 2015 High Pressure Res. 35 49
 Google Scholar
Google Scholar
[28] Liu C, Zhang Z, Guo G J 2016 RSC Adv. 6 106443
 Google Scholar
Google Scholar
[29] Tang L, Shi R, Su Y, Zhao J 2015 J. Phys. Chem. A 119 10971
 Google Scholar
Google Scholar
[30] 郑朝阳, 赵纪军 2012 物理化学学报 28 1809
 Google Scholar
Google Scholar
Zheng C Y, Zhao J J 2012 Acta Phys. Chim. Sin. 28 1809
 Google Scholar
Google Scholar
[31] Huang Y, Zhu C, Wang L, Cao X, Su Y, Jiang X, Meng S, Zhao J, Zeng X C 2016 Sci. Adv. 2 e1501010
 Google Scholar
Google Scholar
[32] 曹潇潇 2016 博士学位论文(大连: 大连理工大学)
Cao X X 2016 Ph. D. Dissertation (Dalian: Dalian University of Technology University) (in Chinese)
[33] 曹潇潇, 苏艳, 赵纪军, 刘昌岭, 周潘旺 2014 物理化学学报 30 1437
 Google Scholar
Google Scholar
Cao X X, Su Y, Zhao J J, Liu C L, Zhou D W 2014 Acta Phys. Chim. Sin. 30 1437
 Google Scholar
Google Scholar
[34] Nityananda R, Hohenberg P, Kohn W 2017 Resonance 22 809
 Google Scholar
Google Scholar
[35] Kohn W, Sham L J 1965 Phys. Rev. 140 1133
 Google Scholar
Google Scholar
[36] Huang Y Y, Zhu C Q, Wang L 2017 Chem. Phys. Lett. 2017 186
 Google Scholar
Google Scholar
[37] Blöchl E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[38] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[39] 罗强, 唐斌, 张智, 冉曾令 2013 62 077101
 Google Scholar
Google Scholar
Luo Q, Tang B, Zhang Z, Ran Z L 2013 Acta Phys. Sin. 62 077101
 Google Scholar
Google Scholar
[40] Wang Z H, Li Y, Meng W J, Guo P, Luo Q, Ran Z L 2017 Appl. Ecol. Environ. Res. 15 861
 Google Scholar
Google Scholar
[41] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[42] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[43] Broyden C G 1970 J. Inst. Math. Applic. 6 76
 Google Scholar
Google Scholar
[44] Fletcher R 1970 Comput. J. 13 317
 Google Scholar
Google Scholar
[45] Goldfarb D 1970 Math. Comput. 24 23
 Google Scholar
Google Scholar
[46] Shanno D F 1970 Math. Comput. 24 647
 Google Scholar
Google Scholar
[47] Guo P, Qiu Y L, Li L L, Luo Q, Zhao J F, Pan Y K 2018 Chin. Phys. B 27 276
[48] 丁家祥, 史伶俐, 申小冬, 梁德青 2017 化工学报 68 4802
Ding J X, Shi L L, Sheng X D, Liang D Q 2017 J. Chem. Ind. Eng. 68 4802
-
表 1 平均晶格参数
Table 1. Average lattice parameters.
晶格参数 GGA 原始值 I型甲烷水合物[47] 六边形边长/Å 2.7231 2.6804 2.7152 四边形边长/Å 2.7233 2.7656 / 水分子键长/Å 1.0056 0.994 0.993 水分子键角/(°) 107.738 109.406 106.629 甲烷分子键长/Å 1.0973 1.1178 1.0917 甲烷分子键角/(°) 109.471 109.454 109.471 甲烷分子到水分子距离/Å 4.2831 4.278 3.78115 氢键键长/Å 1.7293 1.7025 1.7128 表 2 GGA近似下的形成能
Table 2. Formation energy of GGA approximation
能量 GGA I型甲烷水合物[47] ∆Eform/eV –0.247 –0.581 Etotal/eV –6074.782 –21186.729 Ecage/eV –5634.720 –20976.902 ECH4/eV –219.784 –209.246 -
[1] Holder G, Kamath V, Godbole S 1984 Annu. Rev. Energy 9 427
[2] Max M, Lowrie A 1996 J. Petrol. Geol. 19 41
 Google Scholar
Google Scholar
[3] Collett T S 2002 AAPG Bull. 86 1971
[4] Kvamme B, Kuznetsova T, Sapate A, Qorbani K 2016 J. Nat. Gas Sci. Eng. 35 1594
 Google Scholar
Google Scholar
[5] Zhao J, Chen X, Song Y, Zhu Z, Yang L, Tian Y, Wang J, Yang M, Zhang Y 2014 Energy Procedia 61 75
 Google Scholar
Google Scholar
[6] Khlebnikov V, Antonov S, Mishin A, Bakulin D, Khamidullina I, 梁萌, Vinokurov V, Gushchin P A 2016 天然气工业 36 40
 Google Scholar
Google Scholar
Khlebnikov V, Antonov S, Mishin A, Bakulin D, Khamidullina I, Liang M, Vinokurov V, Gushchin P A 2016 Nat. Gas Ind. 36 40
 Google Scholar
Google Scholar
[7] Lim D, Ro H, Seo Y, Seo Y, Lee J, Kim S, Lee J , Lee H 2017 J. Chem. Thermodyn. 106 16
 Google Scholar
Google Scholar
[8] Partoon B, Nashed O, Kassim Z, Sabil K, Sangwai J, Lal B 2016 Process Eng. 148 1220
[9] Zhang L X, Yang L, Wang J Q, Zhao J F, Dong H S, Yang M J, Liu Y, Song Y C 2017 Chem. Eng. J. 308 40
 Google Scholar
Google Scholar
[10] Castellani B, Rossetti G, Tupsakhare S, Rossi F, Nicolini A, Castaldi M 2016 J. Petrol. Sci. Eng. 147 515
 Google Scholar
Google Scholar
[11] Michael K, Roland B, Edward B, Lewis R N 2004 J. Am. Chem. Soc. 126 9407
 Google Scholar
Google Scholar
[12] Xu C G, Cai J, Lin F H, Chen Z Y, Li X S 2015 Energy 79 111
 Google Scholar
Google Scholar
[13] Naeiji P, Varaminian F, Rahmati M 2017 J. Nat. Gas Sci. Eng. 44 122
 Google Scholar
Google Scholar
[14] 朱金龙, 赵予生, 靳常青 2019 68 018203
Zhu J L, Zhao Y S, Jin C Q 2019 Acta Phys. Sin. 68 018203
[15] Choudhary N, Kushwaha O S, Bhattacharjee G, Chakrabarty S, Kumara R 2017 Energt Procedia 105 5026
 Google Scholar
Google Scholar
[16] Alavi S, Ohmura R 2016 J. Chem. Phys. 145 154708
 Google Scholar
Google Scholar
[17] Huang Y, Yuan L, Su Y, Zhao J 2015 Mol. Simul. 41 1086
 Google Scholar
Google Scholar
[18] Nguyen A H, Molinero V 2014 J. Chem. Phys. 140 3440
[19] Yagasaki T, Matsumoto M, Andoh Y, Okazaki S, Tanaka H 2014 J. Phys. Chem. B 118 11797
[20] Druart M L, Michel L, Van D H 2014 Fluid Phase Equilib. 381 108
 Google Scholar
Google Scholar
[21] 耿春宇, 丁丽颖, 韩清珍, 温浩 2008 物理化学学报 24 595
 Google Scholar
Google Scholar
Geng C Y, Ding L Y, Han Q Z, Wen H 2008 Acta Phys. Chim. Sin. 24 595
 Google Scholar
Google Scholar
[22] Lasich M, Ramjugernath D 2016 Philos. Mag. 96 15
 Google Scholar
Google Scholar
[23] Zong X, Cheng G, Qiu N, Huang Q, He J, Du S, Li Y 2017 Chem. Lett. 46 1141
 Google Scholar
Google Scholar
[24] Liu Y, Ojamäe L 2014 J. Phys. Chem. A 118 51
[25] Zhang X, Qiu N, Huang Q, Zha X, He J, Li Y, Du S 2017 J. Mol. Struct. 1153 292
[26] Waldron C J, English N J 2017 J. Chem. Phys. 147 024506
 Google Scholar
Google Scholar
[27] Zquierdo-Ruiz F, Otero-de-la-Roza A, Contreras-García J, Menéndez B, Recio J M 2015 High Pressure Res. 35 49
 Google Scholar
Google Scholar
[28] Liu C, Zhang Z, Guo G J 2016 RSC Adv. 6 106443
 Google Scholar
Google Scholar
[29] Tang L, Shi R, Su Y, Zhao J 2015 J. Phys. Chem. A 119 10971
 Google Scholar
Google Scholar
[30] 郑朝阳, 赵纪军 2012 物理化学学报 28 1809
 Google Scholar
Google Scholar
Zheng C Y, Zhao J J 2012 Acta Phys. Chim. Sin. 28 1809
 Google Scholar
Google Scholar
[31] Huang Y, Zhu C, Wang L, Cao X, Su Y, Jiang X, Meng S, Zhao J, Zeng X C 2016 Sci. Adv. 2 e1501010
 Google Scholar
Google Scholar
[32] 曹潇潇 2016 博士学位论文(大连: 大连理工大学)
Cao X X 2016 Ph. D. Dissertation (Dalian: Dalian University of Technology University) (in Chinese)
[33] 曹潇潇, 苏艳, 赵纪军, 刘昌岭, 周潘旺 2014 物理化学学报 30 1437
 Google Scholar
Google Scholar
Cao X X, Su Y, Zhao J J, Liu C L, Zhou D W 2014 Acta Phys. Chim. Sin. 30 1437
 Google Scholar
Google Scholar
[34] Nityananda R, Hohenberg P, Kohn W 2017 Resonance 22 809
 Google Scholar
Google Scholar
[35] Kohn W, Sham L J 1965 Phys. Rev. 140 1133
 Google Scholar
Google Scholar
[36] Huang Y Y, Zhu C Q, Wang L 2017 Chem. Phys. Lett. 2017 186
 Google Scholar
Google Scholar
[37] Blöchl E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[38] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[39] 罗强, 唐斌, 张智, 冉曾令 2013 62 077101
 Google Scholar
Google Scholar
Luo Q, Tang B, Zhang Z, Ran Z L 2013 Acta Phys. Sin. 62 077101
 Google Scholar
Google Scholar
[40] Wang Z H, Li Y, Meng W J, Guo P, Luo Q, Ran Z L 2017 Appl. Ecol. Environ. Res. 15 861
 Google Scholar
Google Scholar
[41] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[42] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[43] Broyden C G 1970 J. Inst. Math. Applic. 6 76
 Google Scholar
Google Scholar
[44] Fletcher R 1970 Comput. J. 13 317
 Google Scholar
Google Scholar
[45] Goldfarb D 1970 Math. Comput. 24 23
 Google Scholar
Google Scholar
[46] Shanno D F 1970 Math. Comput. 24 647
 Google Scholar
Google Scholar
[47] Guo P, Qiu Y L, Li L L, Luo Q, Zhao J F, Pan Y K 2018 Chin. Phys. B 27 276
[48] 丁家祥, 史伶俐, 申小冬, 梁德青 2017 化工学报 68 4802
Ding J X, Shi L L, Sheng X D, Liang D Q 2017 J. Chem. Ind. Eng. 68 4802
Catalog
Metrics
- Abstract views: 18063
- PDF Downloads: 128
- Cited By: 0
















 DownLoad:
DownLoad: