-
采用分子动力学方法模拟介孔尺度和结构对太阳盐凝固特性的影响. 使用Material Studio软件分别建立不同尺度、两种结构的混合硝酸盐模型, 模型通过Lammps进行模拟计算, 总结凝固点、过冷度、相变潜热随尺度和结构的变化规律, 利用径向分布函数、势能-温度曲线、吉布斯自由能等表征参量对介孔内太阳盐凝固特性的微观机理进行分析. 结果表明, 太阳盐的凝固点随着纳米孔尺度的增大先增大后减小最终趋于稳定, 相同尺度下纳米线结构的凝固点高于纳米颗粒的凝固点. 太阳盐的过冷度整体呈现随介孔尺度增大而减小的规律, 但有反常增加现象. 两种不同结构下, 太阳盐凝固焓随着尺度增大均逐渐增大, 且纳米线结构较纳米粒子结构在相同尺度下提高了30%—37%.The effects of mesoporous size and structure on the solidification characteristics of solar salt are simulated by molecular dynamics (MD). The mixed nitrate model with different scales and two structures is established by using Material Studio software, and the model is applied to the Lammps software package for simulation calculation. The changes of freezing point, supercooling, and phase transformation latent heat are summarized. The micro mechanism of solidification characteristics of nano solar salt is analyzed by radial distribution function, potential energy temperature curve and Gibbs free energy theory. The results show that the freezing point of solar salt first increases and then decreases with the increase of nanopore scale. The nanowire structure will also increase the phase transition temperature on the same scale, and the phase transition points of the two eventually tend to be stable with the increase of scale. The supercooling of solar salt decreases with the increase of mesoporous scale, but there is an abnormal increase. Under the two different structures, the solidification enthalpy gradually decreases with the increase of scale, and the phase transition latent heat of nanowire solar salt is 30%–37% higher than that of nanoparticle structure on the same scale.
-
Keywords:
- solar salt /
- scale /
- supercooling /
- solidification characteristics
[1] Jiang Z, Leng G, Ye F, Ge Z, Liu C, Wang L 2015 Energy Convers. Manage. 106 165
 Google Scholar
Google Scholar
[2] Alehosseini E, Jafari S. M. 2019 Trends Food Sci. Tech. 91 116
 Google Scholar
Google Scholar
[3] Umair M M, Zhang Y, Zhang S, Tang B 2019 Applied Energy 235 846
 Google Scholar
Google Scholar
[4] Chen X, Gao H, Yang M, Xing L, Dong W, Li A 2019 Energy Storage Mater. 18 349
 Google Scholar
Google Scholar
[5] 冯妍卉, 冯黛丽, 张欣欣 2019 介孔复合材料的相变及热输运特性 (北京: 科学出版社) 第110—113页
Feng Y H, Feng D L, Zhang X X 2019 Phase Transition and Heat Transport Properties of Mesoporous Composites (Beijing: Science Press) pp110–113 (in Chinese)
[6] Chen X, Tang Z, Chang Y, Gao H, Lv J 2020 iScience 23 101606
 Google Scholar
Google Scholar
[7] Qian T T, Li J H, Xin M, Fan B 2018 ACS Sustain. Chem. Eng. 6 897
 Google Scholar
Google Scholar
[8] Xiao, C, Hg A, Lx A, Wd A, Al A, Pc B 2019 Energy Storage Mater. 18 280
 Google Scholar
Google Scholar
[9] Huang, X, Liu Z, Xia W, Zou R, Han R P S 2014 J. Mater. Chem. A 3 1935
 Google Scholar
Google Scholar
[10] 袁思伟, 冯妍卉, 王鑫, 张欣欣 2014 63 014402
 Google Scholar
Google Scholar
Yuan S W, Feng Y H, Wang X, Zhang X X 2014 Acta Phys. Sin. 63 014402
 Google Scholar
Google Scholar
[11] Feng D, Feng Y, Qiu L, Li P, Zang Y, Zou H 2019 Renew. Sust. Energ. Rev. 109 578
 Google Scholar
Google Scholar
[12] Lewis L J, Jensen P, Barrat J L 1997 Mrs Proceedings 56.4 2248-2257
 Google Scholar
Google Scholar
[13] 毋志民, 王新强 2006 原子与分子 23 167
 Google Scholar
Google Scholar
Wu Z M, Wang X Q 2006 Journal of Atomic and Molecular Physics 23 167
 Google Scholar
Google Scholar
[14] Alavi S, Thompson D L 2006 J. Phys. Chem. A 110 1518
 Google Scholar
Google Scholar
[15] Kang J W, Hwang H J 2003 Comp. Mater. Sci. 27 305
 Google Scholar
Google Scholar
[16] Wen Y H, Zhu Z Z, Zhu R, Shao G F 2005 Physica E: Low-Dimensional Systems and Nanostructures 25 47
[17] Goitandia A M, Beobide G, Aranzabe E, Aranzabe A 2015 Sol. Energ. Mat. Sol. C. 134 318
 Google Scholar
Google Scholar
[18] Nakano K, Masuda Y, Daiguji H 2015 J. Phys. Chem. C 119 4769
 Google Scholar
Google Scholar
[19] Zou T, X Liang, Wang S, Gao X, Fang, Y 2020 Micropor. Mesopor. Mater. 305 110403
 Google Scholar
Google Scholar
[20] Zhang P, Xiao X, Ma Z W 2016 Appl. Energy 165 472
 Google Scholar
Google Scholar
[21] 赵亚溥 2012 表面与界面物理力学 (北京: 科学出版社) 第212—220页
Zhao Y P 2012 Physical Mechanics of Surfaces and Interfaces (Beijing: Science Press) pp212–220 (in Chinese)
[22] 何卓亚, 杨启容, 李昭莹, 毛蕊, 王力伟, 闫晨宣 2022 71 030503
 Google Scholar
Google Scholar
He Z Y, Yang Q R, Li Z Y, Mao R, Wang L W, Yan C X 2022 Acta Phys. Sin. 71 030503
 Google Scholar
Google Scholar
[23] Anagnostopoulos A, Alexiadis A, Ding Y 2019 Sol. Energ. Mater. Sol. C 200 109897
[24] Hu G J, Cao B Y 2013 J. Appl. Phys. 114 96
 Google Scholar
Google Scholar
[25] 吴晨光, 李蓓 2022 复合材料学报 32 1
 Google Scholar
Google Scholar
[26] Karasawa N, Goddard WA 1992 Macromolecules 25 7268
 Google Scholar
Google Scholar
[27] Pan G, Ding J, Wang W L 2016 Int. J. Heat Mass Tran. 103 417
 Google Scholar
Google Scholar
[28] Sheppard D, Terrell R, Henkelman G 2008 J. Chem. Phys. 128 385
 Google Scholar
Google Scholar
[29] Zhang C, Chen Y, Yang L, Shi M 2011 Int. J. Heat Mass Transfer 54 4770
 Google Scholar
Google Scholar
[30] Li P T, Yang Y Q, Zhang W, Luo X, Jin N, Liu G 2016 RSC Advances 6 54763
 Google Scholar
Google Scholar
[31] 李昌, 侯兆阳, 牛媛, 高全华, 王真, 王晋国, 邹鹏飞 2022 71 016101
 Google Scholar
Google Scholar
Li C, Hou Z Y, Niu Y, Gao Q H, Wang Z, Wang J G, Zou P F 2022 Acta Phys. Sin 71 016101
 Google Scholar
Google Scholar
[32] Nicole P, Thomas B, Claudia M, Markus E, Antje W 2015 Beilstein J. Nanotech. 6 1487
 Google Scholar
Google Scholar
[33] Danneman D M, Johansen J B, Furbo S 2016 Sol. Energ. Mater. Sol. C. 145 287
 Google Scholar
Google Scholar
[34] Kibria M A, Anisur M R, Mahfuz M H, Saidur R, Metselaar I 2015 Energy Convers. Manage. 95 69
 Google Scholar
Google Scholar
[35] Al-Shannaq R, Kurdi J, Al-Muhtaseb S, Dickinson M, Farid M 2015 Energy 87 654
[36] Song Z, Deng Y, Li J, Nian H 2018 Mater. Res. Bull. 102 203
 Google Scholar
Google Scholar
[37] Cao F, Bao Y 2014 Appl. Energy 113 1512
 Google Scholar
Google Scholar
[38] Fang G, Hui L, Fan Y, Xu L, Wu S 2009 Chem. Eng. J. 153 217
 Google Scholar
Google Scholar
[39] Wei L L, Kenichi, Ohsasa 2010 ISIJ Int. 50 1265
 Google Scholar
Google Scholar
[40] Sun J, Simon S L 2007 Thermochim. Acta 463 32
 Google Scholar
Google Scholar
[41] Wang W, Zhong Y, Li D, Wang P, Cai Y, Duan Z 2015 J. Electr. Mater. 44 4920
 Google Scholar
Google Scholar
[42] Saranprabhu M K, Rajan K S 2019 Renew. Energy 141 451
 Google Scholar
Google Scholar
[43] Pan K, Li Y, Zhao Q, Zhang S 2018 JOM 71 737
 Google Scholar
Google Scholar
[44] Li Y 2018 Ph. D. Dissertation (Zhengzhou: Zhengzhou University) (in Chinese) [李扬 2018 博士学位论文 (郑州: 郑州大学)]
[45] Eryürek M, Güven 2007 Physica A:Stat. Mech. Appl. 377 514
 Google Scholar
Google Scholar
-
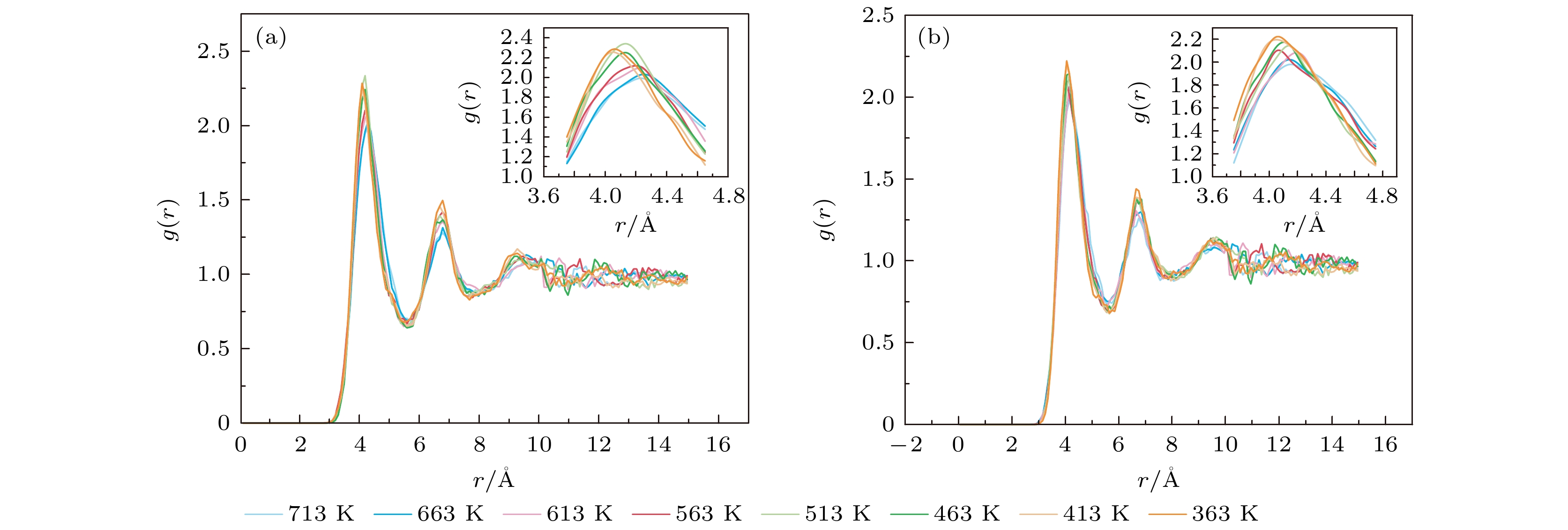
图 10 势能-温度曲线 (a) 460分子数升降温曲线; (b) 不同尺度纳米粒子降温曲线; (c) 不同尺度纳米线降温曲线; (d) 不同结构降温曲线
Fig. 10. Potential energy-temperature curve: (a) 460 molecular number rise and drop temperature curve; (b) cooling curves of nanoparticles with different scales; (c) cooling curves of nanowires with different scales; (d) cooling curves of different structures
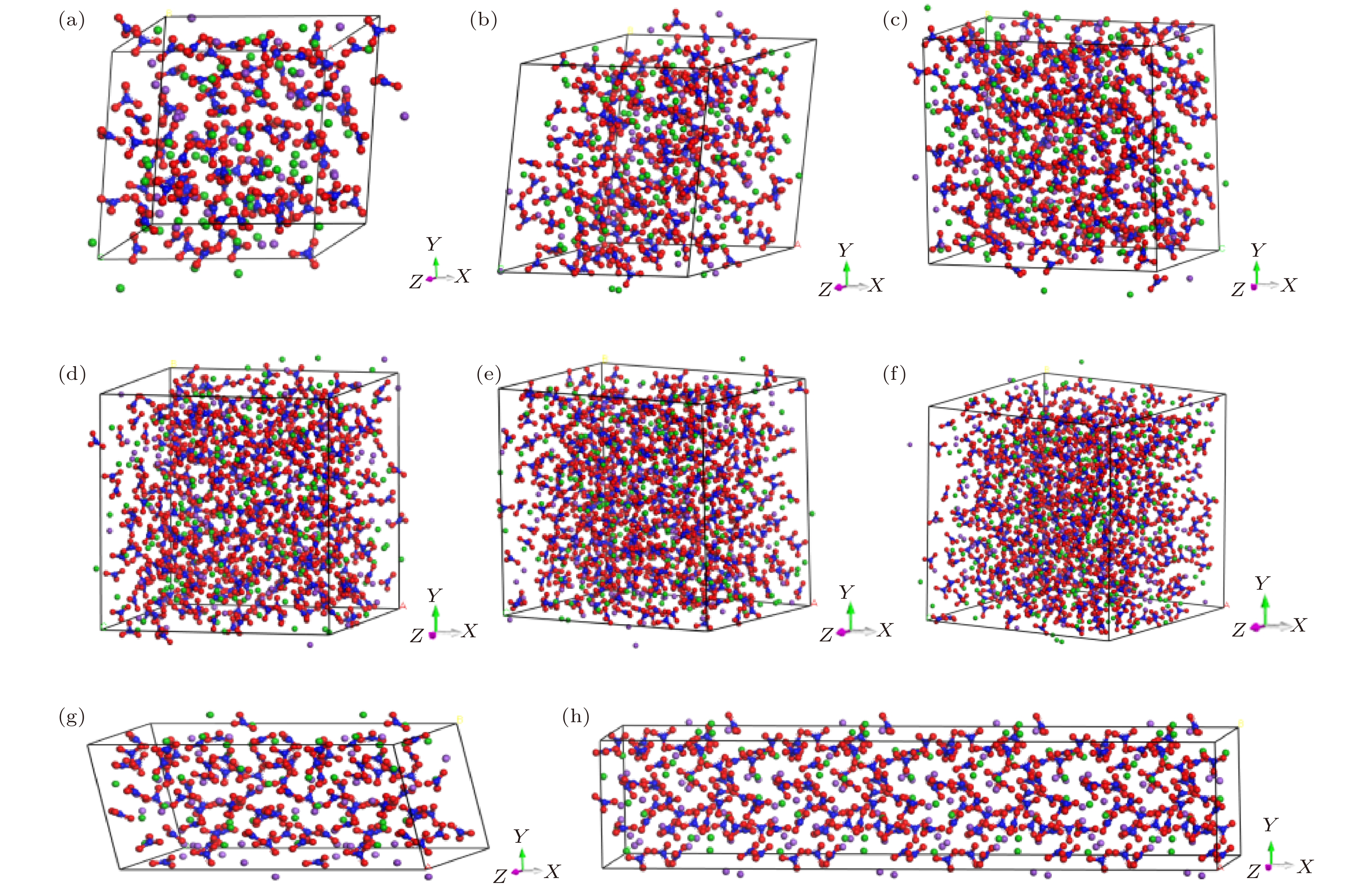
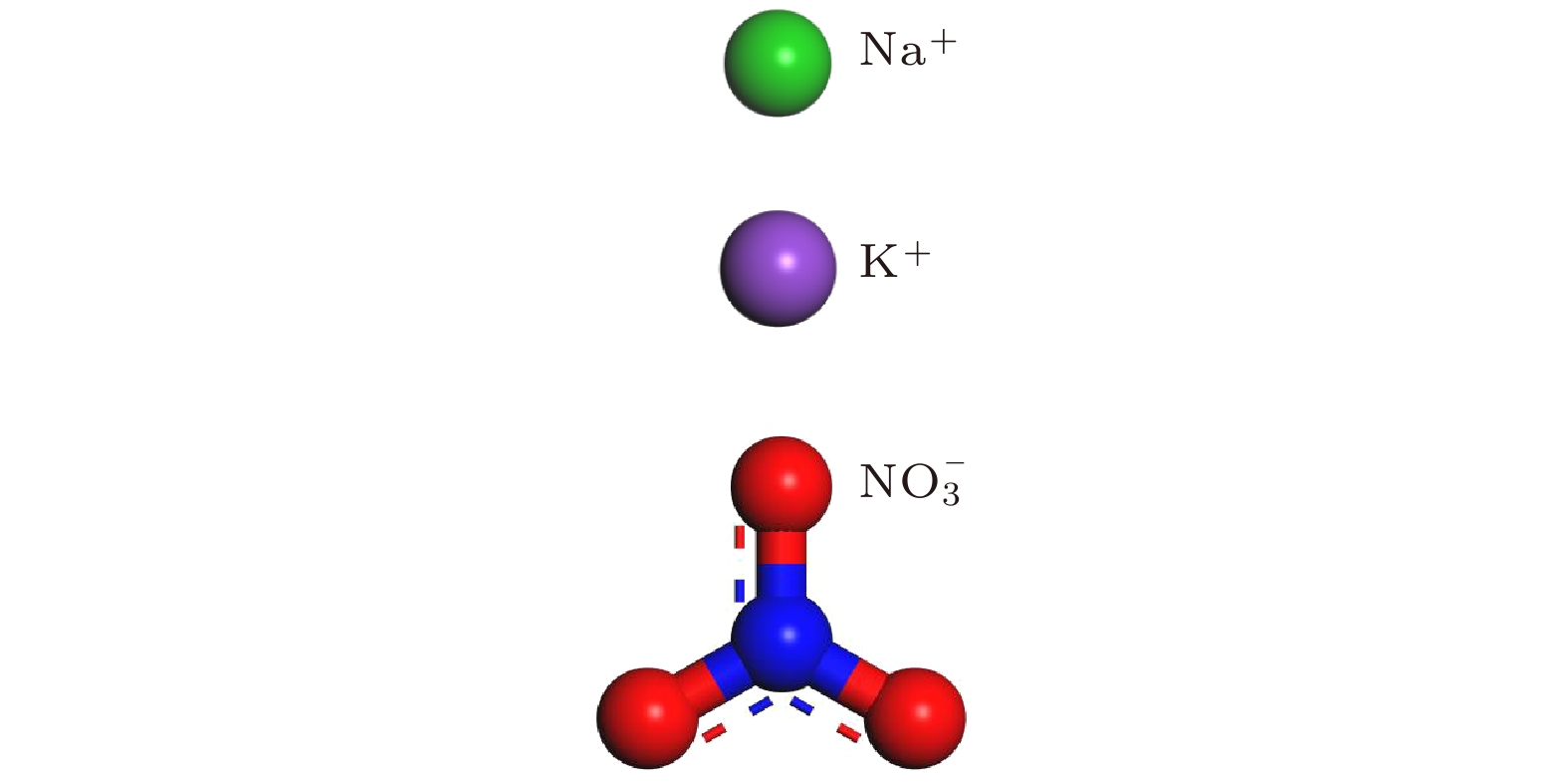
表 1 太阳盐(w (NaNO3)∶w (KNO3) = 6∶4)中NaNO3和KNO3的离子数[22]
Table 1. Ion numbers of NaNO3 and KNO3 in solar salts (w (NaNO3) : w (KNO3) = 6∶4)[22]
离子数 种类 尺度/nm Na+ K+ N– O2– 460 60 32 92 276 5 920 120 64 184 552 6—7 1380 180 96 276 828 7—8 1840 240 128 368 1104 8 2300 300 160 460 1380 9—10 2760 360 192 552 1656 10 Atom Q/e E/(10–3 eV) σ/Å Na 1.00 6.6373000 2.407 K 1.00 4.336000 3.188 N 0.95 4.017509 3.431 O –0.65 3.469129 3.285 表 3 模拟参数
Table 3. Simulation parameters.
原子数 460, 920, 1380, 1840, 2300, 2760 时间步长/fs 1 压强/(105 Pa) 1 系综 NPT 冷却速率/(K·ps–1) 0.1, 0.5 表 4 不同尺度下的太阳盐的相变温度
Table 4. Phase transition temperature of solar salts at different scales.
离子数 460 920 1380 1840 2300 2760 5520 11040 熔点/K 493 493 503 518 508 492 493 492 凝固点/K 463 483 473 503 490 483 478 476 过冷度/K 30 10 30 15 12 9 15 16 表 5 纳米线结构太阳盐的相变温度
Table 5. Phase transition temperature of nanostructured solar salts
离子数 460 920 熔点/K 528 548 凝固点/K 518 543 过冷度/K 10 5 表 6 不同尺度下的太阳盐的相变潜热
Table 6. Phase transition latent heat of solar salts at different scales.
离子数 460 920 1380 1840 2300 2760 相变潜热
/(kJ·kg–1)108.75 109.39 110.12 112.25 114.69 117.57 表 7 纳米线结构太阳盐的相变潜热
Table 7. Phase transition latent heat of nanostructured solar salts.
离子数 460 920 相变潜热/(kJ·kg–1) 141.39 150.64 -
[1] Jiang Z, Leng G, Ye F, Ge Z, Liu C, Wang L 2015 Energy Convers. Manage. 106 165
 Google Scholar
Google Scholar
[2] Alehosseini E, Jafari S. M. 2019 Trends Food Sci. Tech. 91 116
 Google Scholar
Google Scholar
[3] Umair M M, Zhang Y, Zhang S, Tang B 2019 Applied Energy 235 846
 Google Scholar
Google Scholar
[4] Chen X, Gao H, Yang M, Xing L, Dong W, Li A 2019 Energy Storage Mater. 18 349
 Google Scholar
Google Scholar
[5] 冯妍卉, 冯黛丽, 张欣欣 2019 介孔复合材料的相变及热输运特性 (北京: 科学出版社) 第110—113页
Feng Y H, Feng D L, Zhang X X 2019 Phase Transition and Heat Transport Properties of Mesoporous Composites (Beijing: Science Press) pp110–113 (in Chinese)
[6] Chen X, Tang Z, Chang Y, Gao H, Lv J 2020 iScience 23 101606
 Google Scholar
Google Scholar
[7] Qian T T, Li J H, Xin M, Fan B 2018 ACS Sustain. Chem. Eng. 6 897
 Google Scholar
Google Scholar
[8] Xiao, C, Hg A, Lx A, Wd A, Al A, Pc B 2019 Energy Storage Mater. 18 280
 Google Scholar
Google Scholar
[9] Huang, X, Liu Z, Xia W, Zou R, Han R P S 2014 J. Mater. Chem. A 3 1935
 Google Scholar
Google Scholar
[10] 袁思伟, 冯妍卉, 王鑫, 张欣欣 2014 63 014402
 Google Scholar
Google Scholar
Yuan S W, Feng Y H, Wang X, Zhang X X 2014 Acta Phys. Sin. 63 014402
 Google Scholar
Google Scholar
[11] Feng D, Feng Y, Qiu L, Li P, Zang Y, Zou H 2019 Renew. Sust. Energ. Rev. 109 578
 Google Scholar
Google Scholar
[12] Lewis L J, Jensen P, Barrat J L 1997 Mrs Proceedings 56.4 2248-2257
 Google Scholar
Google Scholar
[13] 毋志民, 王新强 2006 原子与分子 23 167
 Google Scholar
Google Scholar
Wu Z M, Wang X Q 2006 Journal of Atomic and Molecular Physics 23 167
 Google Scholar
Google Scholar
[14] Alavi S, Thompson D L 2006 J. Phys. Chem. A 110 1518
 Google Scholar
Google Scholar
[15] Kang J W, Hwang H J 2003 Comp. Mater. Sci. 27 305
 Google Scholar
Google Scholar
[16] Wen Y H, Zhu Z Z, Zhu R, Shao G F 2005 Physica E: Low-Dimensional Systems and Nanostructures 25 47
[17] Goitandia A M, Beobide G, Aranzabe E, Aranzabe A 2015 Sol. Energ. Mat. Sol. C. 134 318
 Google Scholar
Google Scholar
[18] Nakano K, Masuda Y, Daiguji H 2015 J. Phys. Chem. C 119 4769
 Google Scholar
Google Scholar
[19] Zou T, X Liang, Wang S, Gao X, Fang, Y 2020 Micropor. Mesopor. Mater. 305 110403
 Google Scholar
Google Scholar
[20] Zhang P, Xiao X, Ma Z W 2016 Appl. Energy 165 472
 Google Scholar
Google Scholar
[21] 赵亚溥 2012 表面与界面物理力学 (北京: 科学出版社) 第212—220页
Zhao Y P 2012 Physical Mechanics of Surfaces and Interfaces (Beijing: Science Press) pp212–220 (in Chinese)
[22] 何卓亚, 杨启容, 李昭莹, 毛蕊, 王力伟, 闫晨宣 2022 71 030503
 Google Scholar
Google Scholar
He Z Y, Yang Q R, Li Z Y, Mao R, Wang L W, Yan C X 2022 Acta Phys. Sin. 71 030503
 Google Scholar
Google Scholar
[23] Anagnostopoulos A, Alexiadis A, Ding Y 2019 Sol. Energ. Mater. Sol. C 200 109897
[24] Hu G J, Cao B Y 2013 J. Appl. Phys. 114 96
 Google Scholar
Google Scholar
[25] 吴晨光, 李蓓 2022 复合材料学报 32 1
 Google Scholar
Google Scholar
[26] Karasawa N, Goddard WA 1992 Macromolecules 25 7268
 Google Scholar
Google Scholar
[27] Pan G, Ding J, Wang W L 2016 Int. J. Heat Mass Tran. 103 417
 Google Scholar
Google Scholar
[28] Sheppard D, Terrell R, Henkelman G 2008 J. Chem. Phys. 128 385
 Google Scholar
Google Scholar
[29] Zhang C, Chen Y, Yang L, Shi M 2011 Int. J. Heat Mass Transfer 54 4770
 Google Scholar
Google Scholar
[30] Li P T, Yang Y Q, Zhang W, Luo X, Jin N, Liu G 2016 RSC Advances 6 54763
 Google Scholar
Google Scholar
[31] 李昌, 侯兆阳, 牛媛, 高全华, 王真, 王晋国, 邹鹏飞 2022 71 016101
 Google Scholar
Google Scholar
Li C, Hou Z Y, Niu Y, Gao Q H, Wang Z, Wang J G, Zou P F 2022 Acta Phys. Sin 71 016101
 Google Scholar
Google Scholar
[32] Nicole P, Thomas B, Claudia M, Markus E, Antje W 2015 Beilstein J. Nanotech. 6 1487
 Google Scholar
Google Scholar
[33] Danneman D M, Johansen J B, Furbo S 2016 Sol. Energ. Mater. Sol. C. 145 287
 Google Scholar
Google Scholar
[34] Kibria M A, Anisur M R, Mahfuz M H, Saidur R, Metselaar I 2015 Energy Convers. Manage. 95 69
 Google Scholar
Google Scholar
[35] Al-Shannaq R, Kurdi J, Al-Muhtaseb S, Dickinson M, Farid M 2015 Energy 87 654
[36] Song Z, Deng Y, Li J, Nian H 2018 Mater. Res. Bull. 102 203
 Google Scholar
Google Scholar
[37] Cao F, Bao Y 2014 Appl. Energy 113 1512
 Google Scholar
Google Scholar
[38] Fang G, Hui L, Fan Y, Xu L, Wu S 2009 Chem. Eng. J. 153 217
 Google Scholar
Google Scholar
[39] Wei L L, Kenichi, Ohsasa 2010 ISIJ Int. 50 1265
 Google Scholar
Google Scholar
[40] Sun J, Simon S L 2007 Thermochim. Acta 463 32
 Google Scholar
Google Scholar
[41] Wang W, Zhong Y, Li D, Wang P, Cai Y, Duan Z 2015 J. Electr. Mater. 44 4920
 Google Scholar
Google Scholar
[42] Saranprabhu M K, Rajan K S 2019 Renew. Energy 141 451
 Google Scholar
Google Scholar
[43] Pan K, Li Y, Zhao Q, Zhang S 2018 JOM 71 737
 Google Scholar
Google Scholar
[44] Li Y 2018 Ph. D. Dissertation (Zhengzhou: Zhengzhou University) (in Chinese) [李扬 2018 博士学位论文 (郑州: 郑州大学)]
[45] Eryürek M, Güven 2007 Physica A:Stat. Mech. Appl. 377 514
 Google Scholar
Google Scholar
计量
- 文章访问数: 7524
- PDF下载量: 72
- 被引次数: 0













 下载:
下载: