-
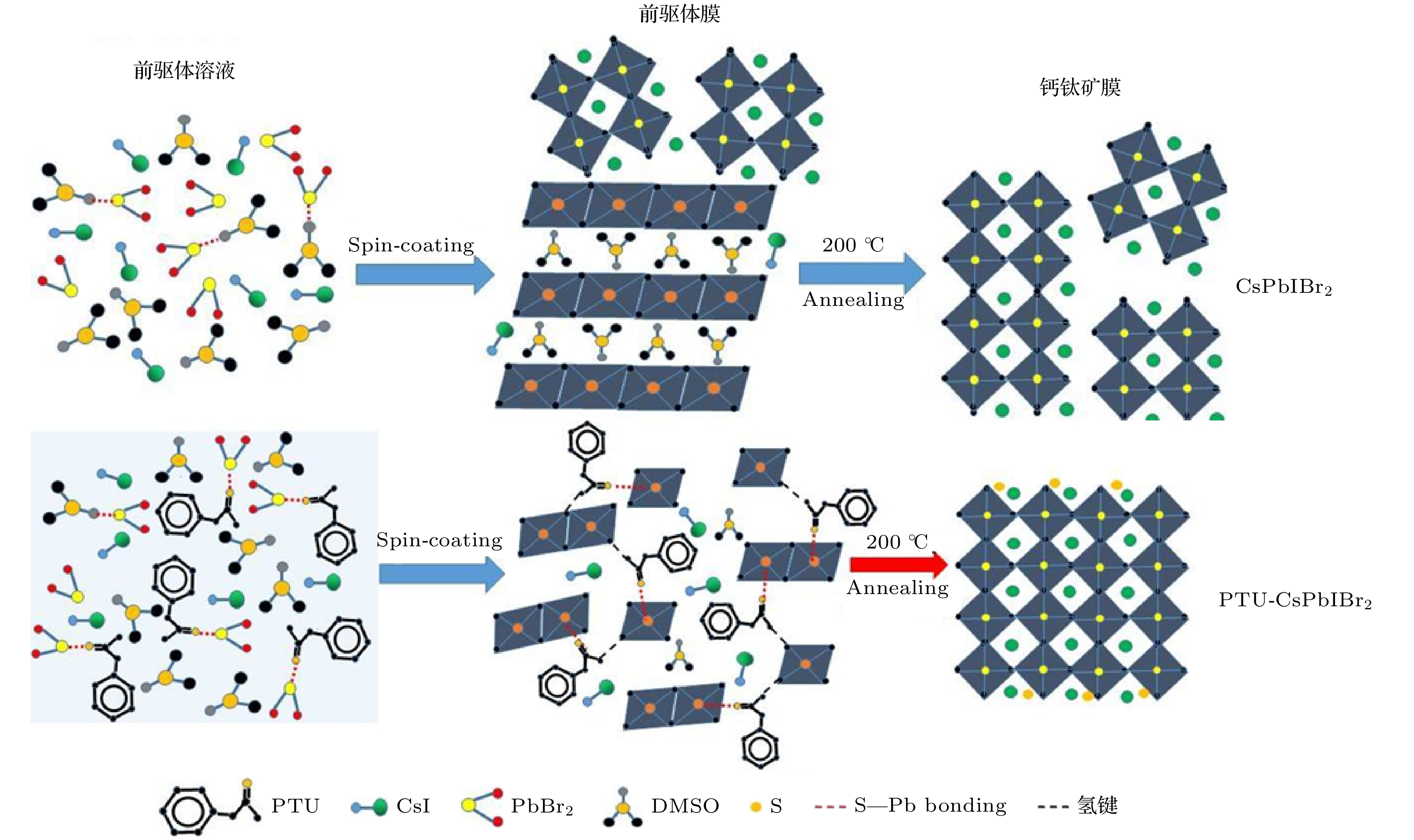
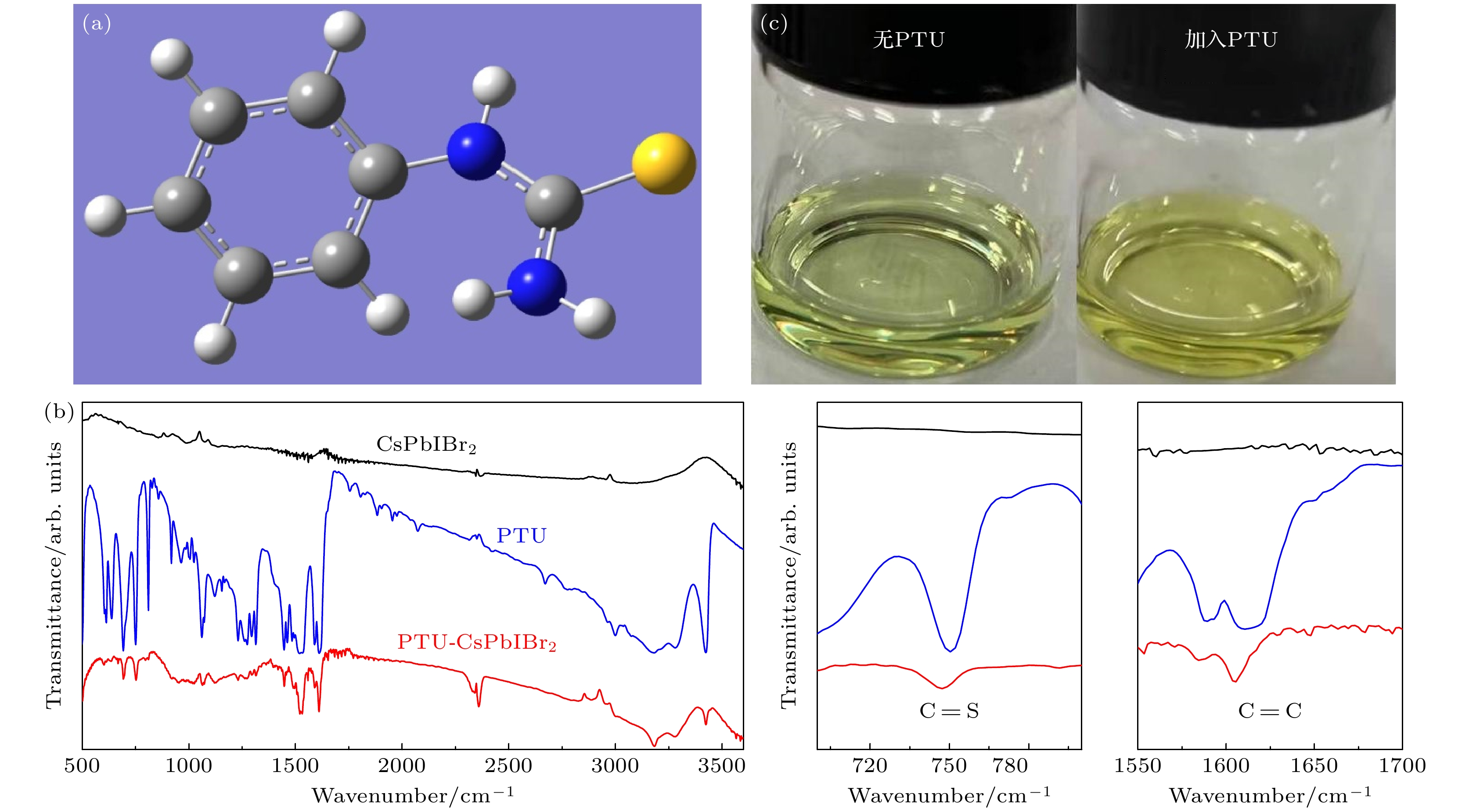
Inorganic CsPbIBr2 perovskite has been considered as a promising light-absorbing material for solar cells due to its high stability and suitable bandgap. Although the remarkable improvement of CsPbIBr2 PSC has been achieved, the efficiency of this cell is still lower than those of other analogues and far below its theoretical limit. This is mainly due to the serious charge recombination in the as-fabricated CsPbIBr2 cells derived from the poor-quality CsPbIBr2 perovskite film with a large quantity of defects and numerous grain boundaries. Therefore, fabricating high-quality CsPbIBr2 perovskite film is a key factor for the further efficiency improvement of CsPbIBr2 PSCs. Herein, phenylthiourea (PTU) additive is introduced into the CsPbIBr2 precursor to tailor the crystallization of CsPbIBr2 perovskite for fabricating high-quality CsPbIBr2 perovskite. The C=S group of PTU can coordinate with PbBr2 in the precursor owing to the lone-pair electrons on S and the empty orbits of Pb2+. The strong interaction between PTU and the CsPbIBr2 precursor components can form PTU·Pb···Br(I) intermediate phase in the precursor upon PTU introduction. The PTU·Pb···Br(I) intermediate phase can reduce the nucleation rate of perovskite and modulate the perovskite crystal growth because the extra energy is required to break the strong coordination bond in the intermediate phase, resulting in a low crystallization rate of CsPbIBr2 perovskite. Such a retardation of perovskite crystallization is conducive to the formation of high-crystallinity perovskite film with smooth surface, large crystal grains, high crystallization, and low density of defect. Meanwhile, the decomposition of PTU during thermal annealing makes the S2– inserted into interstitial of CsPbIBr2 crystal lattice, which greatly enhance the stability of CsPbIBr2 perovskite. The carbon-based PSCs with a normal n-i-p structure of FTO/compact-TiO2 layer/meso-TiO2 layer/perovskite film/carbon layer are fabricated, and their photovoltaic performances are measured under a simulated AM1.5 illumination (100 mW·cm–2). The PSC based on PTU-CsPbIBr2 perovskite delivers a high power conversion efficiency of 10.09%, which is much higher than that of the control device. This great improvement of photovoltaic performance can be attributed to the largely promoted perovskite quality, which enhances the charge collection and suppresses the charge recombination in the device. In addition, the unencapsulated device preserves 82% of the initial efficiency after being stored under ambient condition for 35 days, suggesting excellent stability. Therefore, this work provides an effective complementary strategy for effectively improving the performance of inorganic PSCs.
[1] Jia X, Zou C T, Tao S X, Sun K. Zhao Y X, Yang S F, Chen M, Wang M K, Yuan Y B, Gao F, Wei Z H, Zhang L J, Yip H, Liu M Z, Shen Q, Yin L W, Tan H R, Jin Z W, Ding L M 2019 Sci. Bull. 64 1532
 Google Scholar
Google Scholar
[2] Tai Q, Tang K C, Yan F 2019 Energy Environ. Sci. 12 2375
 Google Scholar
Google Scholar
[3] Li B, Fu L, Li S, Chang B H, Li H, Yin L W 2019 J. Mater. Chem. A 7 20494
 Google Scholar
Google Scholar
[4] Faheem M B, Khan B, Feng C, Raziq F, Li Y B, Yao Y Q 2020 ACS Energy Lett. 5 290
 Google Scholar
Google Scholar
[5] Xiang W C, Tress W 2019 Adv. Mater. 31 1902851
 Google Scholar
Google Scholar
[6] Du Y C, Tian Q W, Chang X M, Fan J J, He X L, Ren X D, Zhao K, Liu S Z 2022 Adv. Mater. 34 2106750
 Google Scholar
Google Scholar
[7] Yu B C, Shi J J, Tan S, Zhao W Y, Hu H J, Luo Y H, Li D M, Meng Q B 2021 Angew. Chem. Int. Ed. 60 13436
 Google Scholar
Google Scholar
[8] Yoon S M, Min H, Kim J B, Lee S K, Seok S I 2021 Joule 5 183
 Google Scholar
Google Scholar
[9] Duan J L, Zhao Y Y, Yang X Y, Wang Y D, He B L, Tang Q W 2018 Adv. Energy Mater. 8 1802386
 Google Scholar
Google Scholar
[10] 马书鹏, 林飞宇, 罗媛, 朱刘, 郭学益, 杨英 2022 71 158101
 Google Scholar
Google Scholar
Ma S P, Lin F Y, Luo Y, Zhu L, Guo X Y, Yang Y 2022 Acta Phys. Sin. 71 158101
 Google Scholar
Google Scholar
[11] Montecucco R, Quadrivi E, Po R, Grancini G 2021 Adv. Energy Mater. 11 2100672
 Google Scholar
Google Scholar
[12] Subhani W S, Wang K, Du M Y, Wang X L, Liu S Z 2019 Adv. Energy Mater. 9 1803785
 Google Scholar
Google Scholar
[13] Guo Y X, Yin X T, Liu J, Yu Y T, Que W X 2019 Sol. RRL 3 1900135
 Google Scholar
Google Scholar
[14] Du J, Duan J L, Yang X Y, Duan Y Y, Zhou Q Z, Tang Q W 2021 Angew. Chem. Int. Ed. 60 10608
 Google Scholar
Google Scholar
[15] Wang H X, Li H Y, Cao S L, Chen J Z, Zang Z G 2020 Sol. RRL 4 2000226
 Google Scholar
Google Scholar
[16] Zhang W H, Xiong J, Li J H, Daoud W A 2020 Small 16 2001535
 Google Scholar
Google Scholar
[17] Wang G Q, Liu J Q, Lei M, Zhang W, Zhu G 2020 Electrochim. Acta 349 136354
 Google Scholar
Google Scholar
[18] Chen Z, Wang Q, Xu Y Y, Zhang L, Huang Y, Lyu M, Zhu J 2021 ACS Appl. Mater. Interfaces 13 24654
 Google Scholar
Google Scholar
[19] Wang R, Xue J J, Wang K L, Wang Z K, Luo Y, Fenning D, Huang T Y, Zhao Y P, Yang J L, Zhu J, Tan S, Houk K N, Yang Y 2019 Science 366 1509
 Google Scholar
Google Scholar
[20] Zhang W Y, Liu X J, He B L, Li X K, Chen H Y, Duang Y Y, Tang Q W 2020 ACS Appl. Mater. Interfaces 12 36092
 Google Scholar
Google Scholar
[21] Wang J P, Chen L B, Qian Z Y, Ren G Q, Wu J, Zhang H 2020 J. Mater. Chem. A 8 25336
 Google Scholar
Google Scholar
[22] Wang X, Ran X, Liu X, Xia Y, Chen Y, Huang W 2020 Angew. Chem. Int. Ed. 132 13456
 Google Scholar
Google Scholar
[23] Wang S, Ma Z R, Liu B B, Wu W C, Zhu Y, Ma R X, Wang C G 2018 Sol. RRL 2 1800034
 Google Scholar
Google Scholar
[24] Li X D, Zhang W X, Guo X M, Lu C Y, Wei J Y, Fang J F 2022 Science 375 434
 Google Scholar
Google Scholar
[25] Hu C, Bai Y, Xiao S, Ng W K, Cheung S H, So S K, Chen Q, Yang S H 2020 Sol. RRL 4 2000270
 Google Scholar
Google Scholar
[26] Chen W J, Li X Q, Li Y W, Li Y F 2020 Energy Environ. Sci. 13 1971
 Google Scholar
Google Scholar
[27] Gong J, Yang M J, Rebollar D, Rucinski J, Zhu K, Xu T 2018 Adv. Mater. 30 1800973
 Google Scholar
Google Scholar
[28] Wang Z, Baranwal A K, Kamarudin M K, Ng C H, Pandey M, Ma T L, Hayase S 2019 Nano Energy 59 258
 Google Scholar
Google Scholar
[29] Yan F R, Duan J L, Guo Q Y, Zhang Q Y, Yang P Z, Tang Q W 2023 Sci. Chin. Mater. 66 485
 Google Scholar
Google Scholar
-
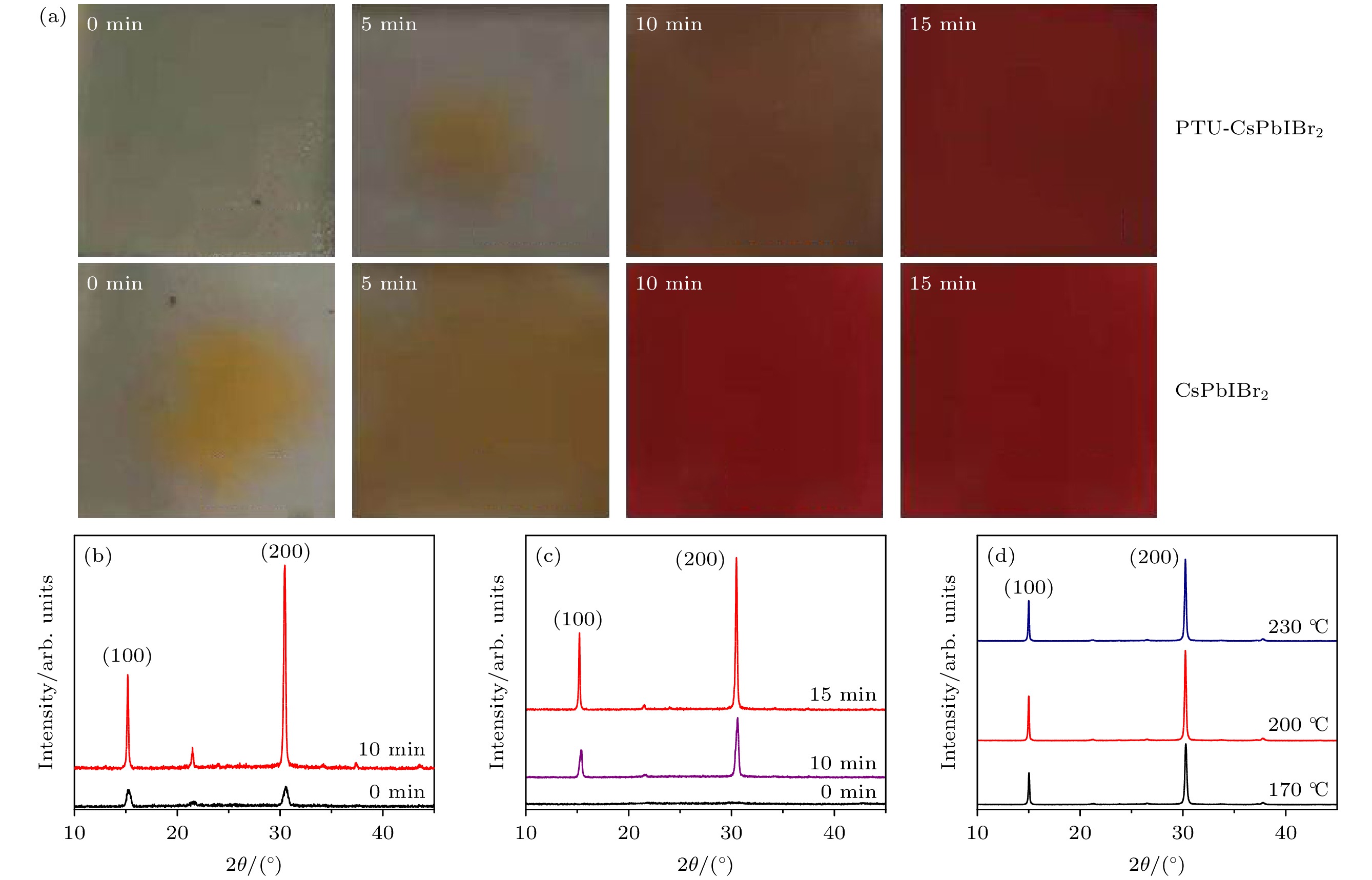
图 2 (a) 前驱体膜在200 ℃热处理不同时间点的照片; (b) CsPbIBr2前驱体膜及在200 ℃处理10 min的XRD曲线; (c) PTU-CsPbIBr2前驱体膜及在200 ℃处理10 min和15 min的XRD曲线; (d) 不同温度热处理 PTU-CsPbIBr2前驱体膜的XRD曲线
Figure 2. (a) Photos of precursor film annealed at 200 ℃ with different time; (b) XRD patterns of CsPbIBr2 precursor film annealed at 200 ℃ with different time; (c) XRD patterns of PTU-CsPbIBr2 precursor film annealed at 200 ℃ with different time; (d) XRD patterns of PTU-CsPbIBr2 precursor annealed at different temperatures.
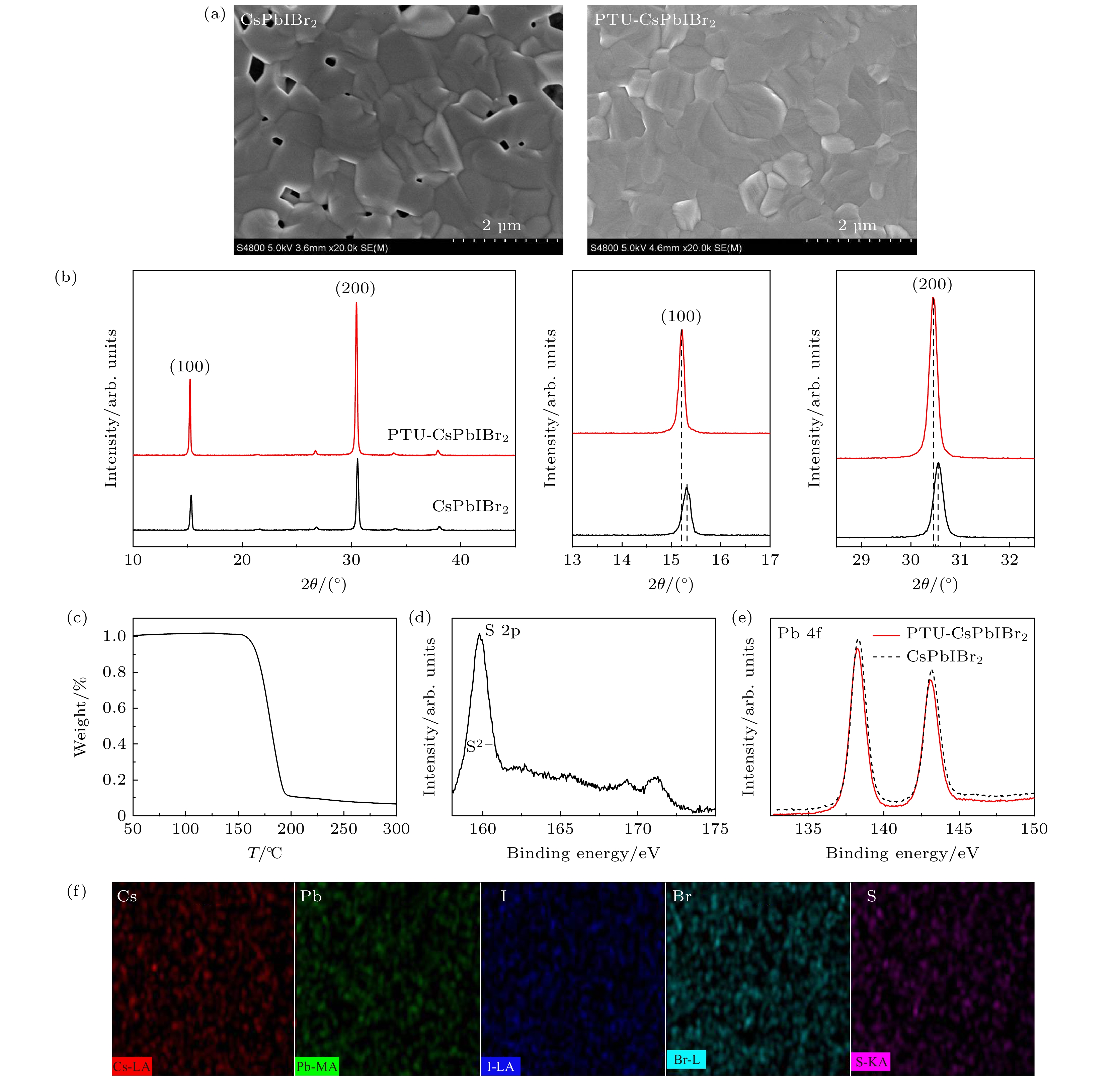
图 3 (a) CsPbIBr2和PTU-CsPbIBr2钙钛矿膜的SEM照片; (b) CsPbIBr2和PTU-CsPbIBr2钙钛矿膜的XRD曲线; (c) PTU的热重曲线; (d) PTU-CsPbIBr2钙钛矿膜的XPS S 2p峰; (e) CsPbIBr2和PTU-CsPbIBr2钙钛矿膜的XPS Pb 4f峰; (f) PTU-CsPbIBr2钙钛矿膜的EDX扫描照片
Figure 3. (a) SEM images of CsPbIBr2 and PTU-CsPbIBr2 perovskite films; (b) XRD curves of CsPbIBr2 and PTU-CsPbIBr2 perovskite films; (c) thermogravimetry curve of PTU; (d) XPS S 2p peak of PTU-CsPbIBr2 perovskite; (e) XPS Pb 4f peak of CsPbIBr2 and PTU-CsPbIBr2 perovskite; (f) EDX mapping image of PTU-CsPbIBr2 perovskite.
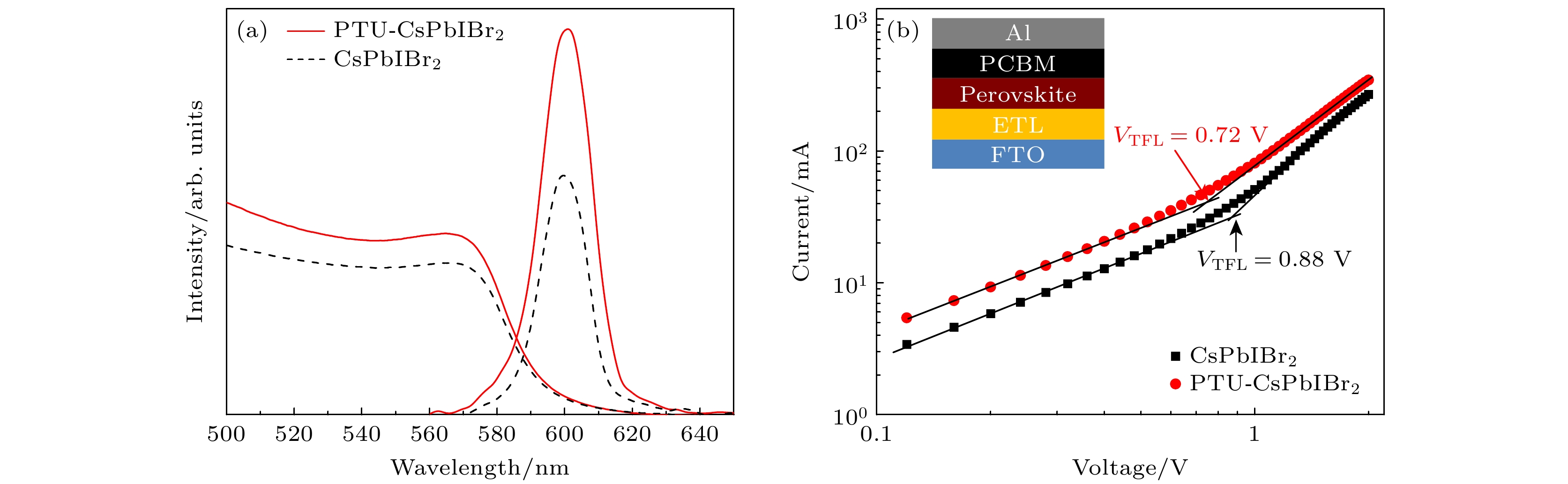
图 6 (a) CsPbIBr2和PTU-CsPbIBr2钙钛矿膜的紫外-可见吸收光谱和稳态PL光谱; (b) CsPbIBr2和PTU-CsPbIBr2钙钛矿组成的单电子器件(插图)的空间电荷限制电流图
Figure 6. (a) UV-vis absorption spectra and steady-state PL of CsPbIBr2 and PTU-CsPbIBr2 perovskite films; (b) SCLC curves based on the electron-only device (the inset) with CsPbIBr2 and PTU-CsPbIBr2 perovskite.
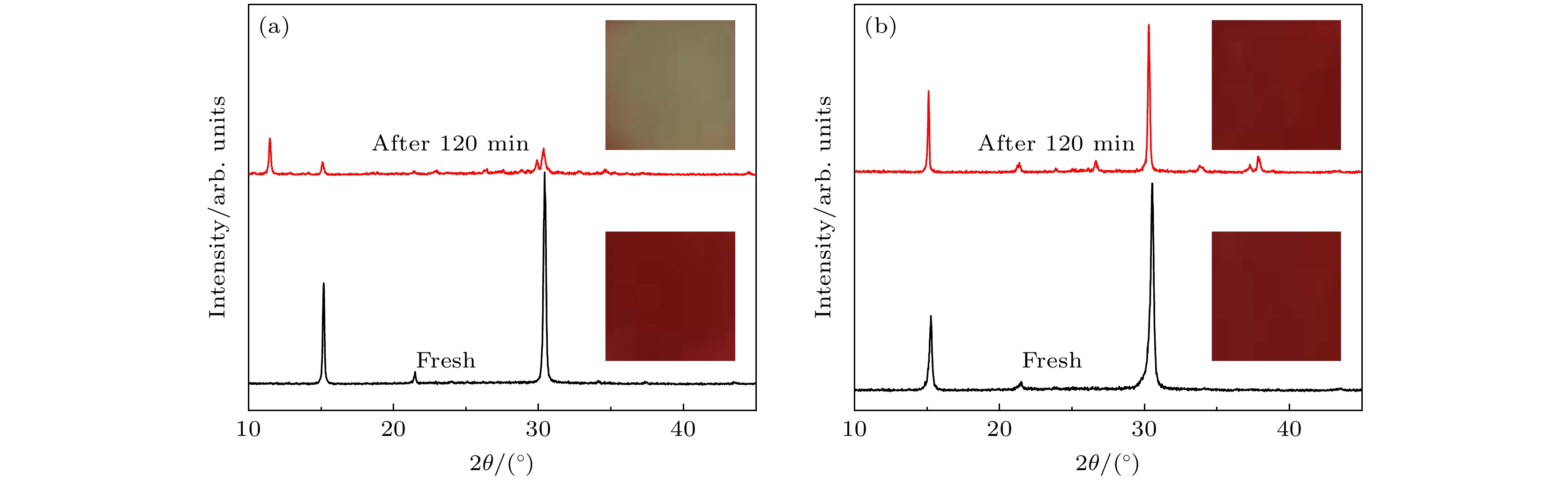
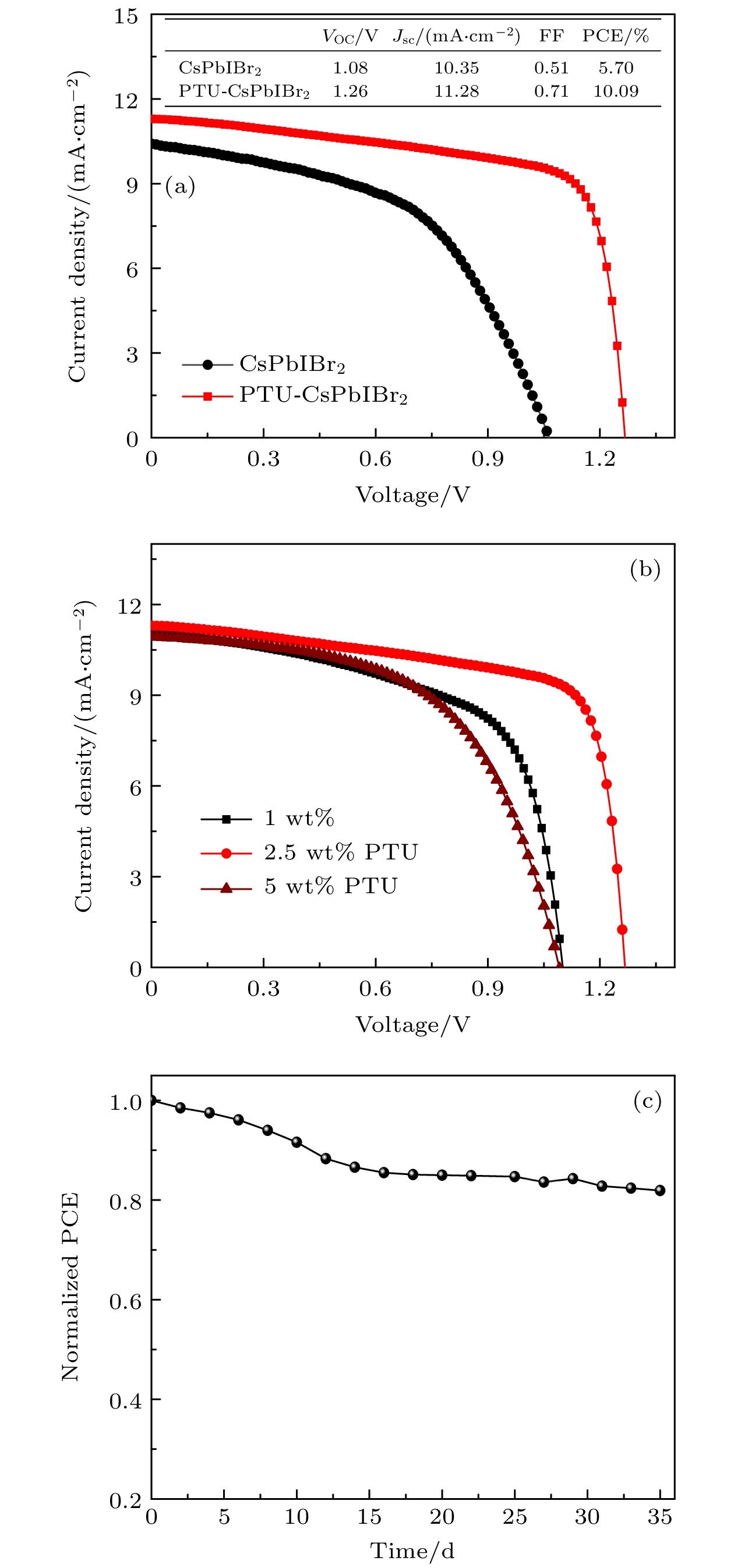
图 7 (a)碳基钙钛矿太阳能电池的光电流密度-电压曲线; (b) CsPbIBr2前驱体中加入不同量PTU所组装的钙钛矿太阳能电池的光电性能曲线; (c)未密封PTU-CsPbIBr2钙钛矿电池在空气环境中效率随时间的变化
Figure 7. (a) Current density-voltage curves of carbon-based PSCs with CsPbIBr2 and PTU-CsPbIBr2 perovskite; (b) current density-voltage curves of PSCs based on PTU-CsPbIBr2 with different amounts of PTU; (c) PCE variation of unencapsulated device stored in ambient condition.
-
[1] Jia X, Zou C T, Tao S X, Sun K. Zhao Y X, Yang S F, Chen M, Wang M K, Yuan Y B, Gao F, Wei Z H, Zhang L J, Yip H, Liu M Z, Shen Q, Yin L W, Tan H R, Jin Z W, Ding L M 2019 Sci. Bull. 64 1532
 Google Scholar
Google Scholar
[2] Tai Q, Tang K C, Yan F 2019 Energy Environ. Sci. 12 2375
 Google Scholar
Google Scholar
[3] Li B, Fu L, Li S, Chang B H, Li H, Yin L W 2019 J. Mater. Chem. A 7 20494
 Google Scholar
Google Scholar
[4] Faheem M B, Khan B, Feng C, Raziq F, Li Y B, Yao Y Q 2020 ACS Energy Lett. 5 290
 Google Scholar
Google Scholar
[5] Xiang W C, Tress W 2019 Adv. Mater. 31 1902851
 Google Scholar
Google Scholar
[6] Du Y C, Tian Q W, Chang X M, Fan J J, He X L, Ren X D, Zhao K, Liu S Z 2022 Adv. Mater. 34 2106750
 Google Scholar
Google Scholar
[7] Yu B C, Shi J J, Tan S, Zhao W Y, Hu H J, Luo Y H, Li D M, Meng Q B 2021 Angew. Chem. Int. Ed. 60 13436
 Google Scholar
Google Scholar
[8] Yoon S M, Min H, Kim J B, Lee S K, Seok S I 2021 Joule 5 183
 Google Scholar
Google Scholar
[9] Duan J L, Zhao Y Y, Yang X Y, Wang Y D, He B L, Tang Q W 2018 Adv. Energy Mater. 8 1802386
 Google Scholar
Google Scholar
[10] 马书鹏, 林飞宇, 罗媛, 朱刘, 郭学益, 杨英 2022 71 158101
 Google Scholar
Google Scholar
Ma S P, Lin F Y, Luo Y, Zhu L, Guo X Y, Yang Y 2022 Acta Phys. Sin. 71 158101
 Google Scholar
Google Scholar
[11] Montecucco R, Quadrivi E, Po R, Grancini G 2021 Adv. Energy Mater. 11 2100672
 Google Scholar
Google Scholar
[12] Subhani W S, Wang K, Du M Y, Wang X L, Liu S Z 2019 Adv. Energy Mater. 9 1803785
 Google Scholar
Google Scholar
[13] Guo Y X, Yin X T, Liu J, Yu Y T, Que W X 2019 Sol. RRL 3 1900135
 Google Scholar
Google Scholar
[14] Du J, Duan J L, Yang X Y, Duan Y Y, Zhou Q Z, Tang Q W 2021 Angew. Chem. Int. Ed. 60 10608
 Google Scholar
Google Scholar
[15] Wang H X, Li H Y, Cao S L, Chen J Z, Zang Z G 2020 Sol. RRL 4 2000226
 Google Scholar
Google Scholar
[16] Zhang W H, Xiong J, Li J H, Daoud W A 2020 Small 16 2001535
 Google Scholar
Google Scholar
[17] Wang G Q, Liu J Q, Lei M, Zhang W, Zhu G 2020 Electrochim. Acta 349 136354
 Google Scholar
Google Scholar
[18] Chen Z, Wang Q, Xu Y Y, Zhang L, Huang Y, Lyu M, Zhu J 2021 ACS Appl. Mater. Interfaces 13 24654
 Google Scholar
Google Scholar
[19] Wang R, Xue J J, Wang K L, Wang Z K, Luo Y, Fenning D, Huang T Y, Zhao Y P, Yang J L, Zhu J, Tan S, Houk K N, Yang Y 2019 Science 366 1509
 Google Scholar
Google Scholar
[20] Zhang W Y, Liu X J, He B L, Li X K, Chen H Y, Duang Y Y, Tang Q W 2020 ACS Appl. Mater. Interfaces 12 36092
 Google Scholar
Google Scholar
[21] Wang J P, Chen L B, Qian Z Y, Ren G Q, Wu J, Zhang H 2020 J. Mater. Chem. A 8 25336
 Google Scholar
Google Scholar
[22] Wang X, Ran X, Liu X, Xia Y, Chen Y, Huang W 2020 Angew. Chem. Int. Ed. 132 13456
 Google Scholar
Google Scholar
[23] Wang S, Ma Z R, Liu B B, Wu W C, Zhu Y, Ma R X, Wang C G 2018 Sol. RRL 2 1800034
 Google Scholar
Google Scholar
[24] Li X D, Zhang W X, Guo X M, Lu C Y, Wei J Y, Fang J F 2022 Science 375 434
 Google Scholar
Google Scholar
[25] Hu C, Bai Y, Xiao S, Ng W K, Cheung S H, So S K, Chen Q, Yang S H 2020 Sol. RRL 4 2000270
 Google Scholar
Google Scholar
[26] Chen W J, Li X Q, Li Y W, Li Y F 2020 Energy Environ. Sci. 13 1971
 Google Scholar
Google Scholar
[27] Gong J, Yang M J, Rebollar D, Rucinski J, Zhu K, Xu T 2018 Adv. Mater. 30 1800973
 Google Scholar
Google Scholar
[28] Wang Z, Baranwal A K, Kamarudin M K, Ng C H, Pandey M, Ma T L, Hayase S 2019 Nano Energy 59 258
 Google Scholar
Google Scholar
[29] Yan F R, Duan J L, Guo Q Y, Zhang Q Y, Yang P Z, Tang Q W 2023 Sci. Chin. Mater. 66 485
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 6071
- PDF Downloads: 136
- Cited By: 0














 DownLoad:
DownLoad: