-
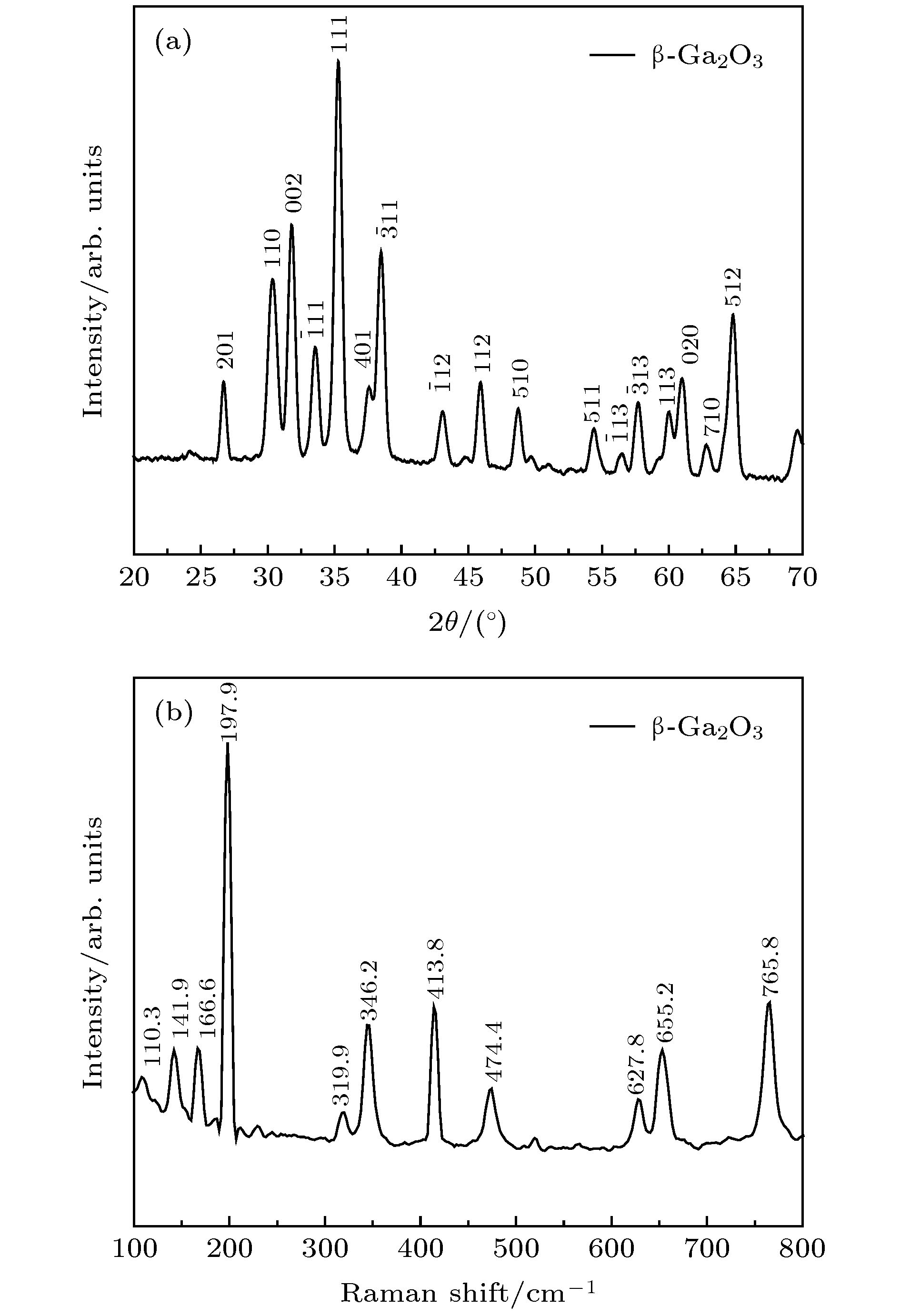
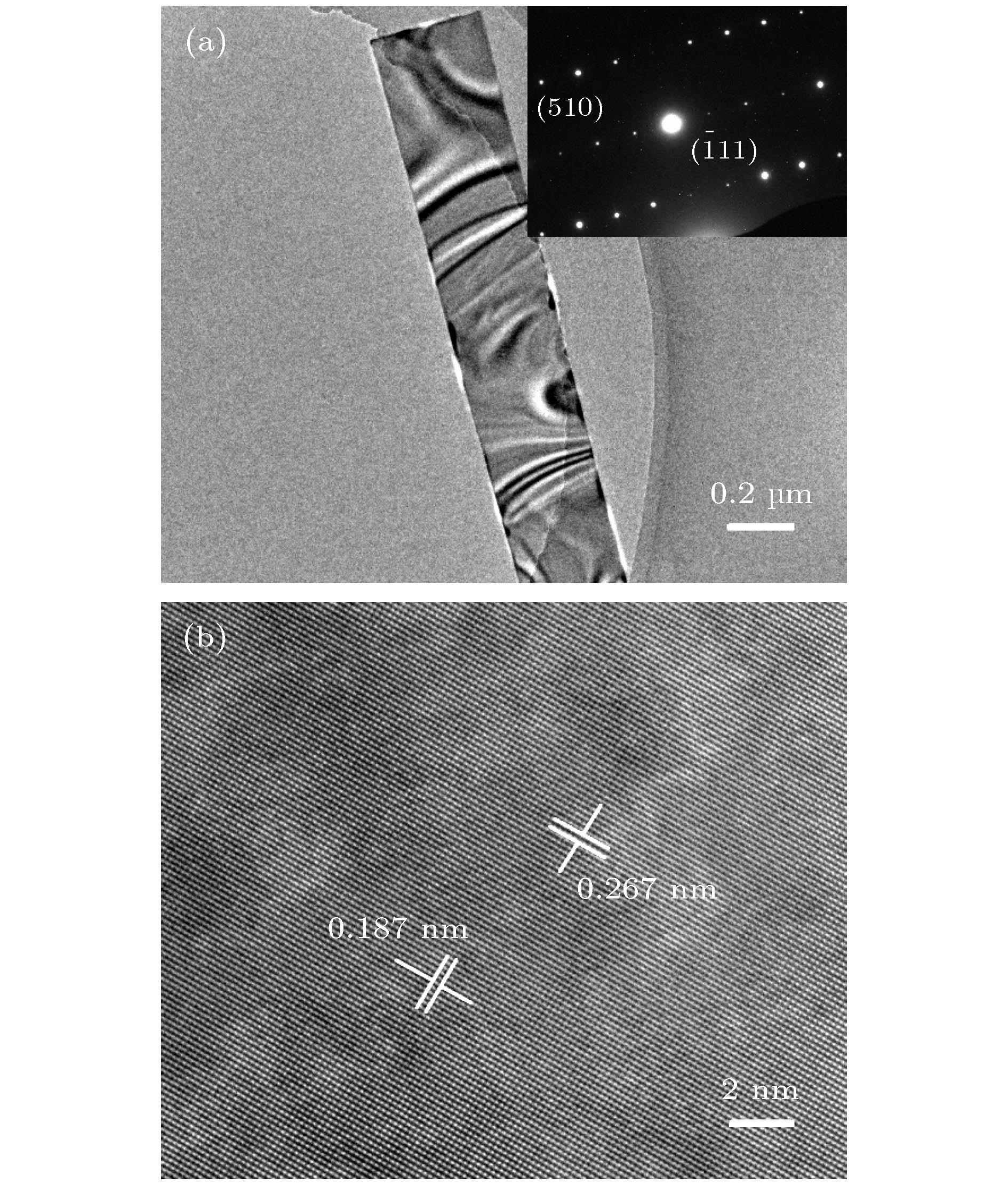
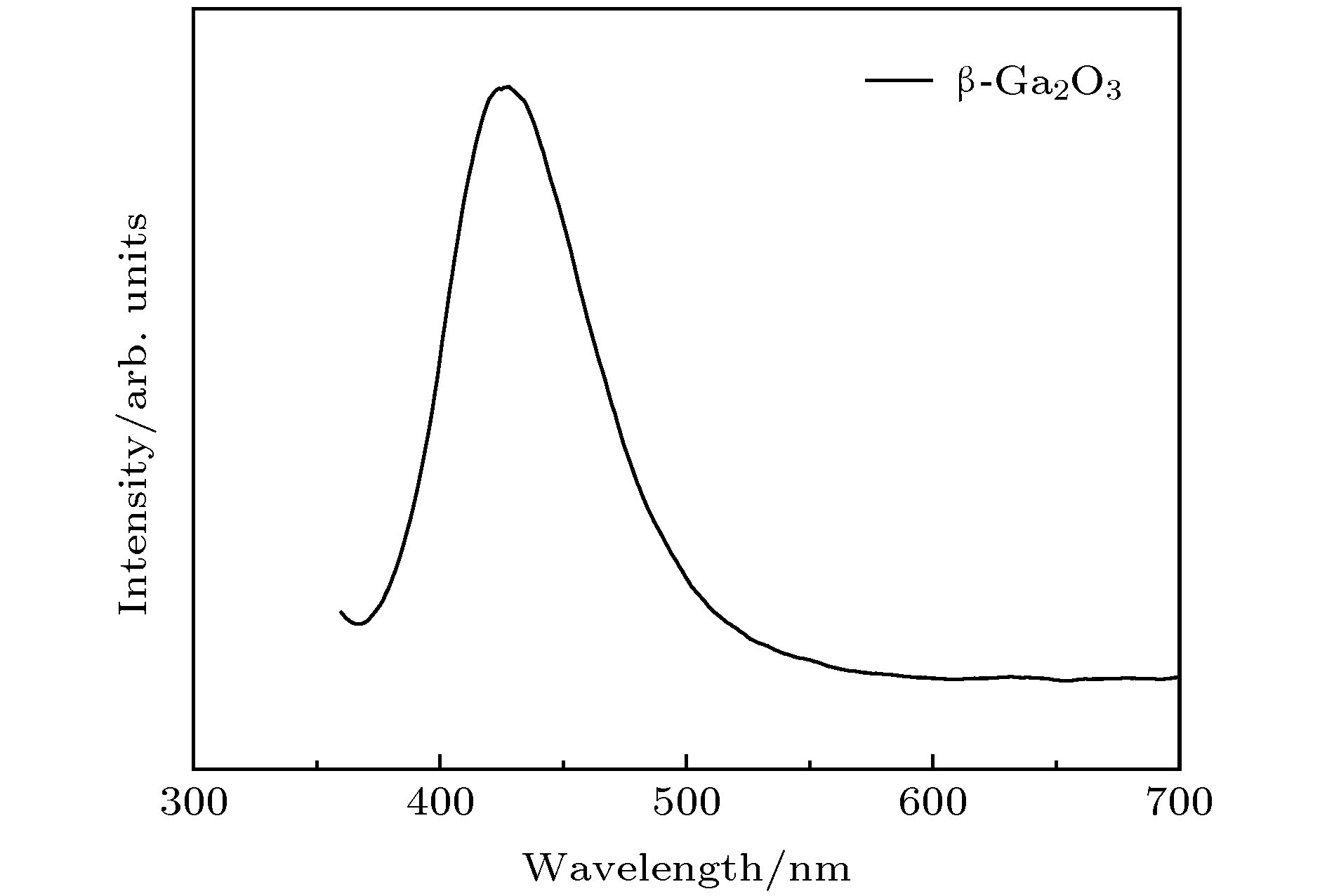
Gallium oxide (Ga2O3) single crystal nanoribbons have the potential applications in electronic devices due to their unique properties. However, the current small surface area makes the fabrication of device based on this nano-material very complex and challenging, and the introduction of catalyst also makes the growth process of Ga2O3 nanomaterial complicated and hard to control. Therefore, it is very important to study the growth method and physical mechanism of Ga2O3 nanoribbon with the larger surface area without catalyst. In this paper, the carbothermal reduction method is used to grow the Ga2O3 nanomaterial. In this paper, the gallium oxide powder mixes with the carbon nanotubes at a mass ratio of 1:1.5 without the catalyst, and then they are put into a high temperature diffusion furnace for the growth of Ga2O3 nanomaterials with different structures on silicon-based substrates by controlling the reaction temperature. In this paper, it is found that the reaction temperature directly affects the diameter and ratio of gallium oxide nanostructures. The reason is that the bonding energy of gallium oxide crystal is different in different crystal directions which leads to the different growth speed. The interface energy along the growth direction is the smallest and the growth speed is the fastest, while the growth speed along the vertical direction is slow. Finally, the crystal gradually grows into nanoriband, nanometer sheet and other structures. In addition, the ultra-wide β-Ga2O3 single crystal nanobelt up to the millimeter level was prepared in this paper. This nanobelt’s lateral dimension is observed to reach 44.3 μm under the scanning electron microscope (SEM), and the transmission electron microscope (TEM) is used to confirm that the nanoribbons have a single crystal structure. Further, Raman spectroscopy (Raman) shows that the β-Ga2O3 nanoribbons grown by this method have the smaller strain and the lower defect density. Additionally, the room temperature photoluminescence spectrum (PL) test shows that the gallium oxide nanoribbon emits a stable and high-brightness blue light at 425 nm at the excitation wavelength of 295 nm. This growth method can provide a useful way for the preparation of device-level gallium oxide nanoribbons in the future. -
Keywords:
- β-Ga2O3 /
- carbothermal reduction /
- crystal nanoribbons /
- defect density
[1] 冯秋菊, 李芳, 李彤彤, 李昀铮, 石博, 李梦轲, 梁红伟 2018 67 218101
 Google Scholar
Google Scholar
Feng Q J, Li F, Li T T, Li X Z, Shi B, Li M K, Liang H W 2018 Acta Phys. Sin. 67 218101
 Google Scholar
Google Scholar
[2] Guo D, Guo Q, Chen Z, Wu Z, Li P, Tang W 2019 Mater. Today Phys. 11 100157
 Google Scholar
Google Scholar
[3] Wang H, Wang Y, Gong S Y, Zhou X Y, Yang Z X, Yang J, Han N, Chen Y F 2019 Cryst. 9 155
 Google Scholar
Google Scholar
[4] Ma J W, Fan H Q, Zheng X K, Wang H, Zhao N, Zhang M C, Yadav A K, Wang W J, Dong W Q, Wang S R 2020 J. Hazard. Mater. 387 122017
 Google Scholar
Google Scholar
[5] 郑树文, 范广涵, 何苗, 赵灵智 2014 63 057102
 Google Scholar
Google Scholar
Zheng S W, Fan G H, He M, Zhao L Z 2014 Acta Phys. Sin. 63 057102
 Google Scholar
Google Scholar
[6] He T, Zhang X D, Ding X Y, Sun C, Zhao Y K, Yu Q, Ning J, Wang R X, Yu G H, Lu S L, Zhang K, Zhang X P, Zhang B S 2019 Adv. Opt. Mater. 7 1801563
 Google Scholar
Google Scholar
[7] Wu Z Y, Jiang Z X, Song P Y, Tian P F, Hu L G, Liu R, Fang Z L, Kang J Y, Zhang T Y 2019 Small 15 1900580
 Google Scholar
Google Scholar
[8] Afzal A 2019 J. Materiomics 5 542
 Google Scholar
Google Scholar
[9] Gundiah G, Govindaraj A, Rao C 2002 Chem. Phys. Lett. 351 189
 Google Scholar
Google Scholar
[10] Cha S Y, Ahn B G, Ka ng, H C, Lee S Y, Noh D Y 2018 Ceram. Int. 44 16470
 Google Scholar
Google Scholar
[11] Tang C C, Fan S S, Chapelle M L, Li P 2001 Chem. Phys. Lett. 333 12
 Google Scholar
Google Scholar
[12] Feng Q Y, Liu J Y, Yang Y Q, Pan D Z, Xing Y, Shi X C, Xia X C and Liang H 2016 J. Alloys Compd. 687 964
 Google Scholar
Google Scholar
[13] Alhalaili B, Bunk R, Vidu R, Islam M S 2019 Nanomaterials 9 1272
 Google Scholar
Google Scholar
[14] Fang J W, Fan H Q, Tian H L, Dong G Z 2015 Mater. Charact. 108 51
 Google Scholar
Google Scholar
[15] Kumar M, Kumar V, Singh R 2017 Scr. Mater. 138 75
 Google Scholar
Google Scholar
[16] Calestani D, Alabib A B, Coppede N, Villani M, Lazzarini L, Fabbri F, Salviati G, Zappettinia A 2017 J. Cryst. Growth 457 255
 Google Scholar
Google Scholar
[17] Feng Q J, Lia T T, Lia F, Lia Y Z, Shi B, Gao C, Wang D Y, Liang H W 2019 J. Cryst. Growth 509 91
 Google Scholar
Google Scholar
[18] Korbutowicz R, Stafiniak A, Serafinczuk J 2017 Mater. Sci-Poland 35 412
 Google Scholar
Google Scholar
[19] Wang S, Li Y W, Xiu X Q, Zhang L Y, Hua X M, Xie Z L, Tao T, Liu B, Chen P, Zhang R, Zheng Y D 2019 Chin. Phys. B 28 028104
 Google Scholar
Google Scholar
[20] Kumar M, Kumar V, Singh R 2017 Nano Res. Lett. 12 184
 Google Scholar
Google Scholar
[21] Li J, Fan H Q, Chen X P, Cao Z Y 2009 Colloid. Surf., A 349 202
 Google Scholar
Google Scholar
[22] Wang S L, Sun H L, Wang Z, Zeng X H, Ungar G, Guo D Y, Shen J Y, Li P G, Liu A P, Li C R, Tang W H 2019 J. Alloys Compd. 787 133
 Google Scholar
Google Scholar
[23] Gonzalo A, Nogales E, Lorenz K, Víllora E G, Shimamura K, Piqueras J, Méndez B 2017 J. Lumin. 191 56
 Google Scholar
Google Scholar
[24] Hu D Q, Zhuang S W, Dong X, Du G T, Zhang B L, Zhang Y T, Yin J Z 2018 Mater. Sci. Semicond. Process. 75 31
 Google Scholar
Google Scholar
[25] Dohy D, Lucazeau G 1982 J. Mol. Struct. 79 419
 Google Scholar
Google Scholar
[26] Alonso-Orts M, Sanchez A M, Lopez I, Nogales E, Piquerasa J, Mendeza B 2017 Cryst. Eng. Commun. 19 6217
 Google Scholar
Google Scholar
[27] Cheng J P, Zhang X B, Kong F Z, Ye Y, Tao X Y 2006 Rare Met. Mater. Eng. 35 1629
 Google Scholar
Google Scholar
[28] Binet L, Gourier D 1998 J. Phys. Chem. Solids 59 1241
 Google Scholar
Google Scholar
[29] Harwig T, Kellendonk F 1978 J. Solid State Chem. 24 255
 Google Scholar
Google Scholar
[30] Harwig T, Kellendonk F, Slappendel S 1978 J. Phys. Chem. Solids 39 675
 Google Scholar
Google Scholar
[31] Li J, Fan H Q, Jia X H, Chen J, Cao Z Y, Chen X P 2009 J. Alloys Compd. 481 735
 Google Scholar
Google Scholar
-
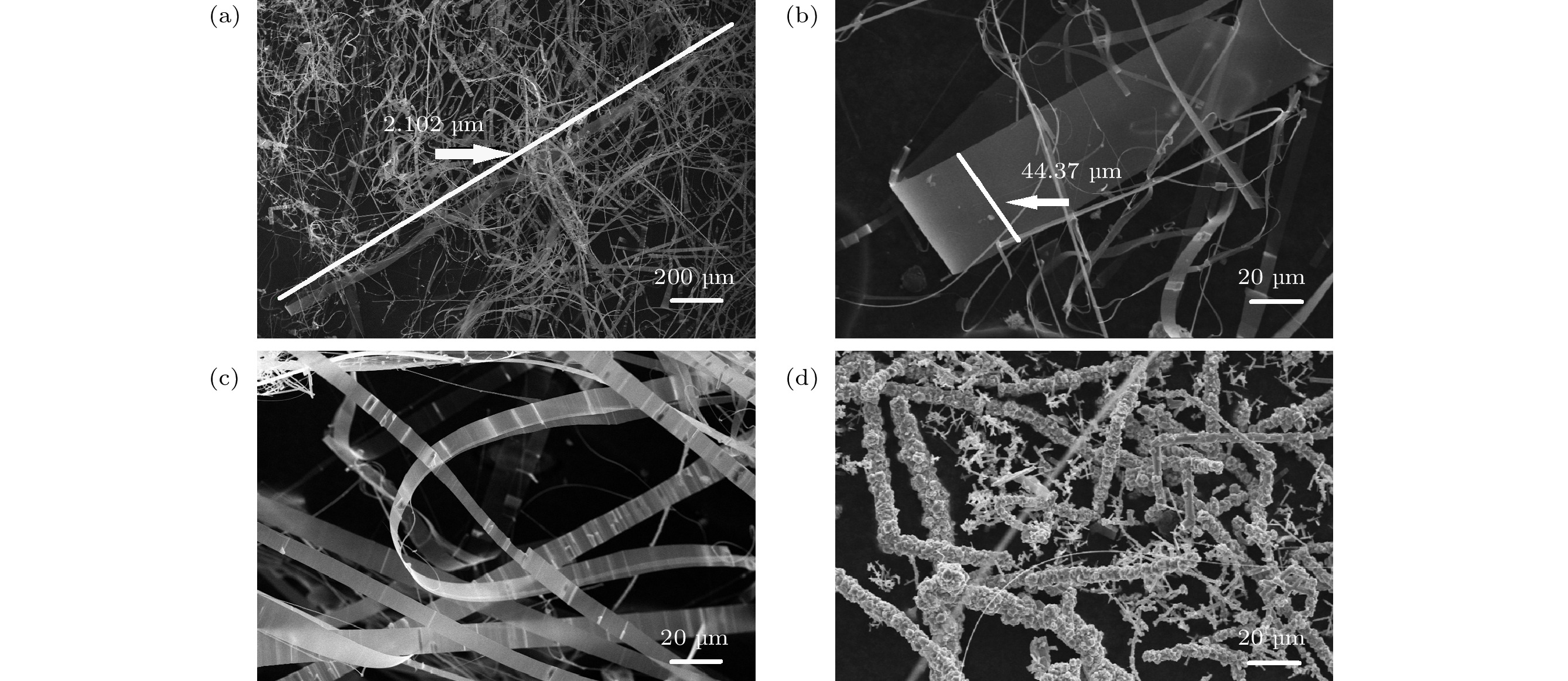
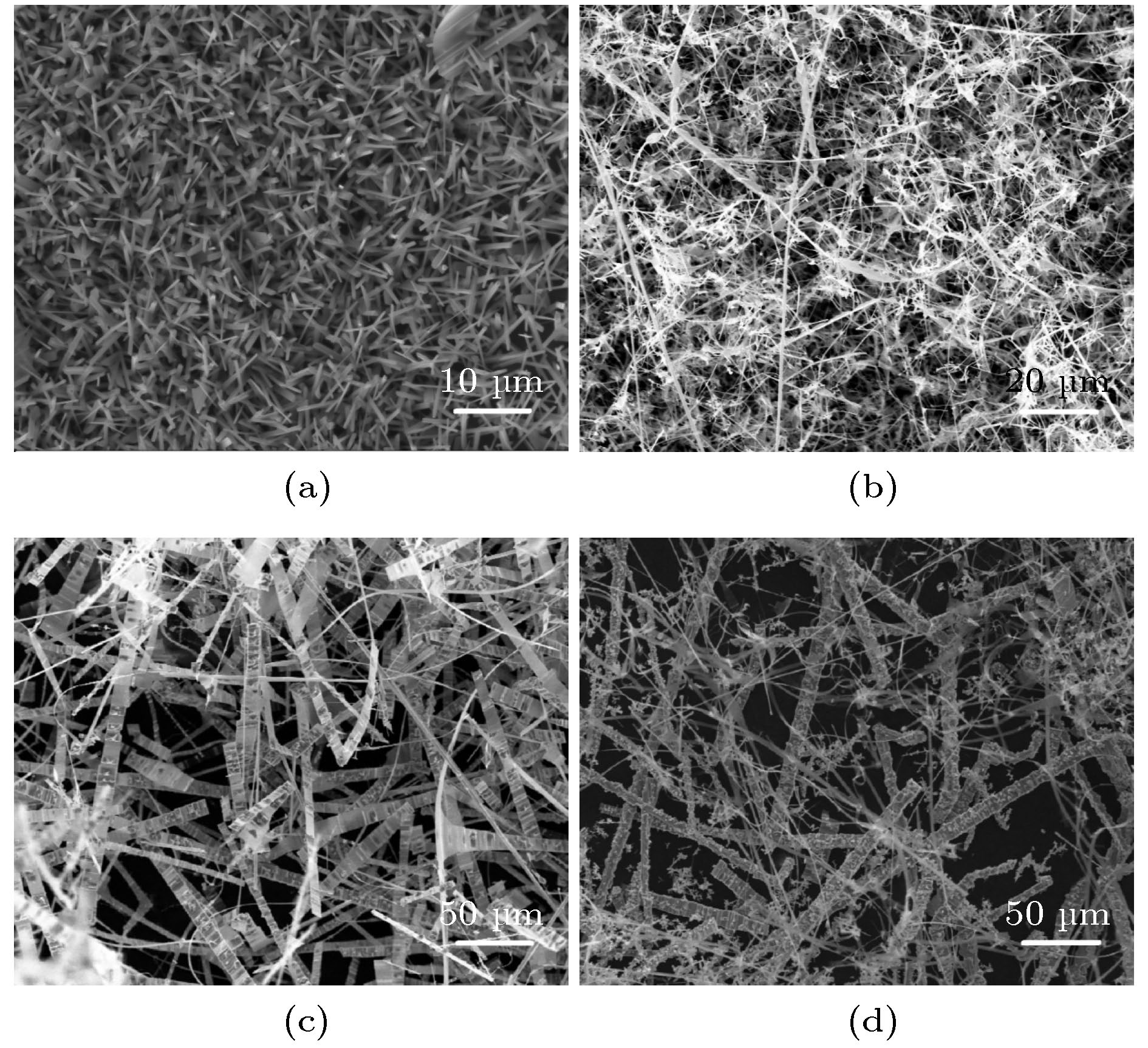
图 2 (a) 低倍镜下样品C超长的氧化镓纳米带; (b) 样品C高倍率下单个氧化镓纳米带; (c)互相缠绕弯曲的纳米带; (d) 高倍镜下样品D纳米带表面结块恶化
Figure 2. (a) The ultra-long gallium oxide nanoribbons of sample C under low magnification; (b) single gallium oxide nanoribbon at high magnification of sample C; (c) intertwined curved nanoribbons; (d) under high power, the agglomeration of the surface of the sample D nanoribbons deteriorates.
表 1 样品A—D在不同温度下的生长参数
Table 1. The growth parameters of samples A–D at different temperature.
Samples Ga2O3/g CNTs/g Growth temperature/℃ Times/min N2 carrier gas /sccm A 1 1.5 800 90 2 B 1 1.5 850 90 2 C 1 1.5 900 90 2 D 1 1.5 950 90 2 -
[1] 冯秋菊, 李芳, 李彤彤, 李昀铮, 石博, 李梦轲, 梁红伟 2018 67 218101
 Google Scholar
Google Scholar
Feng Q J, Li F, Li T T, Li X Z, Shi B, Li M K, Liang H W 2018 Acta Phys. Sin. 67 218101
 Google Scholar
Google Scholar
[2] Guo D, Guo Q, Chen Z, Wu Z, Li P, Tang W 2019 Mater. Today Phys. 11 100157
 Google Scholar
Google Scholar
[3] Wang H, Wang Y, Gong S Y, Zhou X Y, Yang Z X, Yang J, Han N, Chen Y F 2019 Cryst. 9 155
 Google Scholar
Google Scholar
[4] Ma J W, Fan H Q, Zheng X K, Wang H, Zhao N, Zhang M C, Yadav A K, Wang W J, Dong W Q, Wang S R 2020 J. Hazard. Mater. 387 122017
 Google Scholar
Google Scholar
[5] 郑树文, 范广涵, 何苗, 赵灵智 2014 63 057102
 Google Scholar
Google Scholar
Zheng S W, Fan G H, He M, Zhao L Z 2014 Acta Phys. Sin. 63 057102
 Google Scholar
Google Scholar
[6] He T, Zhang X D, Ding X Y, Sun C, Zhao Y K, Yu Q, Ning J, Wang R X, Yu G H, Lu S L, Zhang K, Zhang X P, Zhang B S 2019 Adv. Opt. Mater. 7 1801563
 Google Scholar
Google Scholar
[7] Wu Z Y, Jiang Z X, Song P Y, Tian P F, Hu L G, Liu R, Fang Z L, Kang J Y, Zhang T Y 2019 Small 15 1900580
 Google Scholar
Google Scholar
[8] Afzal A 2019 J. Materiomics 5 542
 Google Scholar
Google Scholar
[9] Gundiah G, Govindaraj A, Rao C 2002 Chem. Phys. Lett. 351 189
 Google Scholar
Google Scholar
[10] Cha S Y, Ahn B G, Ka ng, H C, Lee S Y, Noh D Y 2018 Ceram. Int. 44 16470
 Google Scholar
Google Scholar
[11] Tang C C, Fan S S, Chapelle M L, Li P 2001 Chem. Phys. Lett. 333 12
 Google Scholar
Google Scholar
[12] Feng Q Y, Liu J Y, Yang Y Q, Pan D Z, Xing Y, Shi X C, Xia X C and Liang H 2016 J. Alloys Compd. 687 964
 Google Scholar
Google Scholar
[13] Alhalaili B, Bunk R, Vidu R, Islam M S 2019 Nanomaterials 9 1272
 Google Scholar
Google Scholar
[14] Fang J W, Fan H Q, Tian H L, Dong G Z 2015 Mater. Charact. 108 51
 Google Scholar
Google Scholar
[15] Kumar M, Kumar V, Singh R 2017 Scr. Mater. 138 75
 Google Scholar
Google Scholar
[16] Calestani D, Alabib A B, Coppede N, Villani M, Lazzarini L, Fabbri F, Salviati G, Zappettinia A 2017 J. Cryst. Growth 457 255
 Google Scholar
Google Scholar
[17] Feng Q J, Lia T T, Lia F, Lia Y Z, Shi B, Gao C, Wang D Y, Liang H W 2019 J. Cryst. Growth 509 91
 Google Scholar
Google Scholar
[18] Korbutowicz R, Stafiniak A, Serafinczuk J 2017 Mater. Sci-Poland 35 412
 Google Scholar
Google Scholar
[19] Wang S, Li Y W, Xiu X Q, Zhang L Y, Hua X M, Xie Z L, Tao T, Liu B, Chen P, Zhang R, Zheng Y D 2019 Chin. Phys. B 28 028104
 Google Scholar
Google Scholar
[20] Kumar M, Kumar V, Singh R 2017 Nano Res. Lett. 12 184
 Google Scholar
Google Scholar
[21] Li J, Fan H Q, Chen X P, Cao Z Y 2009 Colloid. Surf., A 349 202
 Google Scholar
Google Scholar
[22] Wang S L, Sun H L, Wang Z, Zeng X H, Ungar G, Guo D Y, Shen J Y, Li P G, Liu A P, Li C R, Tang W H 2019 J. Alloys Compd. 787 133
 Google Scholar
Google Scholar
[23] Gonzalo A, Nogales E, Lorenz K, Víllora E G, Shimamura K, Piqueras J, Méndez B 2017 J. Lumin. 191 56
 Google Scholar
Google Scholar
[24] Hu D Q, Zhuang S W, Dong X, Du G T, Zhang B L, Zhang Y T, Yin J Z 2018 Mater. Sci. Semicond. Process. 75 31
 Google Scholar
Google Scholar
[25] Dohy D, Lucazeau G 1982 J. Mol. Struct. 79 419
 Google Scholar
Google Scholar
[26] Alonso-Orts M, Sanchez A M, Lopez I, Nogales E, Piquerasa J, Mendeza B 2017 Cryst. Eng. Commun. 19 6217
 Google Scholar
Google Scholar
[27] Cheng J P, Zhang X B, Kong F Z, Ye Y, Tao X Y 2006 Rare Met. Mater. Eng. 35 1629
 Google Scholar
Google Scholar
[28] Binet L, Gourier D 1998 J. Phys. Chem. Solids 59 1241
 Google Scholar
Google Scholar
[29] Harwig T, Kellendonk F 1978 J. Solid State Chem. 24 255
 Google Scholar
Google Scholar
[30] Harwig T, Kellendonk F, Slappendel S 1978 J. Phys. Chem. Solids 39 675
 Google Scholar
Google Scholar
[31] Li J, Fan H Q, Jia X H, Chen J, Cao Z Y, Chen X P 2009 J. Alloys Compd. 481 735
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 10514
- PDF Downloads: 245
- Cited By: 0














 DownLoad:
DownLoad: