-
Recently, layered oxychalcogenide has attracted significant scientific attention because of its intriguing electronic properties, intrinsically low thermal conductivity and, correspondingly, outstanding thermoelectric properties, of which the BiCuSeO possesses the best thermoelectric performance ever reported. For instance, the optimized zT value of BiCuSeO system reaches 1.5 at 873 K through dual-doping approach. Such a zT value is comparable to those of the state-of-art p-type lead chalcogenide thermoelectric materials. However, comparing with BiCuSeO compound, little effort has been devoted to the isomorphic analogue BiCuTeO. On the one hand, the BiCuTeO has a pretty small band gap (0.4 eV) which limits its working temperature range. On the other hand, numerous intrinsic Cu vacancies are present in BiCuTeO due to the weak Cu-Te chemical bonding, leading to an excessive carrier concentration. Thus, further increasing carrier concentration through doping will lead to a deterioration of electrical transport properties and thus reduce the zT value. Herein, we choose Se and partially substitute it for Te in the BiCuTeO to enlarge the band gap and reduce intrinsic Cu vacancies by strengthening the chemical bonding in the conductive layers. By combining solid-phase reaction with hot-pressed sintering, the BiCuTe1–xSexO (x = 0, 0.1, 0.2, 0.3, 0.4) bulk thermoelectric materials are prepared, and their microscopic morphology and thermoelectric transport properties are systematically investigated. Our experimental results show that the substitution of Se for part of Te results in strengthening chemical bonding in the conducting layer, enlarging the band gap, increasing the carrier effective mass, reducing the carrier concentration, and enhancing the carrier scattering. Therefore, the electrical conductivity dramatically decreases but the Seebeck coefficient significantly increases with Se content increasing, leading to the decrease of thermoelectric power factor. Furthermore, a slight reduction of the total thermal conductivity is realized by Se alloying due to the decrease of the electronic thermal conductivity. Consequently, the dimensionless figure of merit zT decreases with the Se content increasing because electrical transport properties are deteriorated seriously. Finally, the zT value of 0.3 at room temperature and 0.7 at 723 K are achieved for the sample with x = 0.1, indicating that the Se substituted BiCuTeO sample can still maintain comparative zT values in a wide temperature range. Considering that the effective mass of BiCuTeO is significantly increased by Se alloying, the thermoelectric performance of BiCuTe1–xSexO compound might be further improved by optimizing the carrier concentration.
-
Keywords:
- BiCuTeO /
- BiCuSeO /
- band structure /
- thermoelectric properties
[1] Snyder G J, Toberer E S 2008 Nat. Mater. 7 105
 Google Scholar
Google Scholar
[2] He J, Tritt T M 2017 Science 357 eaak9997
 Google Scholar
Google Scholar
[3] Luo J, You L, Zhang J Y, Guo K, Zhu H T, Gu L, Yang Z Z, Li X, Yang J, Zhang W Q 2017 ACS Appl. Mater. Interfaces 9 8729
 Google Scholar
Google Scholar
[4] Pei Y Z, Shi X Y, LaLonde A, Wang H, Chen L D, Snyder G J 2011 Nature 473 66
 Google Scholar
Google Scholar
[5] Heremans J P, Jovovic V, Toberer E S, Saramat A, Kurosaki K, Charoenphakdee A, Yamanaka S, Snyder G J 2008 Science 321 554
 Google Scholar
Google Scholar
[6] Biswas K, He J Q, Blum I D, Wu C I, Hogan T P, Seidman D N, Dravid V P, Kanatzidis M G 2012 Nature 489 414
 Google Scholar
Google Scholar
[7] You L, Liu Y F, Li X, Nan P F, Ge B H, Jiang Y, Luo P F, Pan S S, Pei Y Z, Zhang W Q, Snyder G J, Yang J, Zhang J Y, Luo J 2018 Energy Environ. Sci. 11 1848
 Google Scholar
Google Scholar
[8] Zhou W X, Chen K Q 2015 Carbon 85 24
 Google Scholar
Google Scholar
[9] Chen X K, Xie Z X, Zhou W X, Tang L M, Chen K Q 2016 Appl. Phys. Lett. 109 023101
 Google Scholar
Google Scholar
[10] Chen X K, Liu J, Peng Z H, Du D, Chen K Q 2017 Appl. Phys. Lett. 110 091907
 Google Scholar
Google Scholar
[11] Lin S Q, Li W, Li S S, Zhang X Y, Chen Z W, Xu Y D, Chen Y, Pei Y Z 2017 Joule 1 816
 Google Scholar
Google Scholar
[12] Li J, Sui J H, Pei Y L, Barreteau C, Berardan D, Dragoe N, Cai W, He J Q, Zhao L D 2012 Energy Environ. Sci. 5 8543
 Google Scholar
Google Scholar
[13] Barreteau C, Berardan D, Amzallag E, Zhao L D, Dragoe N 2012 Chem. Mater. 24 3168
 Google Scholar
Google Scholar
[14] Pei Y L, He J Q, Li J F, Li F, Liu Q J, Pan W, Barreteau C, Berardan D, Dragoe N, Zhao L D 2013 NPG Asia Mater. 5 e47
 Google Scholar
Google Scholar
[15] Li J, Sui J H, Barreteau C, Berardan D, Dragoe N, Cai W, Pei Y L, Zhao L D 2013 J. Alloys Compd. 551 649
 Google Scholar
Google Scholar
[16] Lan J L, Liu Y C, Zhan B, Lin Y H, Zhang B P, Yuan X, Zhang W Q, Xu W, Nan C W 2013 Adv. Mater. 25 5086
 Google Scholar
Google Scholar
[17] Liu Y C, Zheng Y H, Zhan B, Chen K, Butt S, Zhang B P, Lin Y H 2015 J. Eur. Ceram. Soc. 35 845
 Google Scholar
Google Scholar
[18] Pei Y L, Wu H J, Wu D, Zheng F S, He J Q 2014 J. Am. Chem. Soc. 136 13902
 Google Scholar
Google Scholar
[19] Sui J H, Li J, He J Q, Pei Y L, Berardan D, Wu H J, Dragoe N, Cai W, Zhao L D 2013 Energy Environ. Sci. 6 2916
 Google Scholar
Google Scholar
[20] Liu Y, Lan J L, Xu W, Liu Y C, Pei Y L, Cheng B, Liu D B, Lin Y H, Zhao L D 2013 Chem. Commun. 49 8075
 Google Scholar
Google Scholar
[21] Pan L, Lang Y D, Zhao L, Berardan D, Amzallag E, Xu C, Gu Y F, Chen C C, Zhao L D, Shen X D, Lyu Y N, Lu C H, Wang Y F 2018 J. Mater. Chem. A 6 13340
 Google Scholar
Google Scholar
[22] Liu Y, Zhao L D, Zhu Y, Liu Y, Li F, Yu M, Liu D B, Xu W, Lin Y H, Nan C W 2016 Adv. Energy Mater. 6 1502423
 Google Scholar
Google Scholar
[23] Vaqueiro P, Guelou G, Stec M, Guilmeau E, Powell A V 2013 J. Mater. Chem. A 1 520
 Google Scholar
Google Scholar
[24] An T H, Lim Y S, Choi H S, Seo W S, Park C H, Kim G R, Park C, Lee C H, Shim J H 2014 J. Mater. Chem. A 2 19759
 Google Scholar
Google Scholar
[25] An T H, Lim Y S, Seo W S, Park C H, Yoo M D, Park C, Lee C H, Shim J H 2017 J. Electron. Mater. 46 2717
 Google Scholar
Google Scholar
[26] Barreteau C, Berardan D, Zhao L D, Dragoe N 2013 J. Mater. Chem. A 1 2921
 Google Scholar
Google Scholar
[27] Pichanusakorn P, Bandaru P 2010 Mater. Sci. Eng., R 67 19
 Google Scholar
Google Scholar
[28] Ren G K, Wang S Y, Zhu Y C, Ventura K J, Tan X, Xu W, Lin Y H, Yang J H, Nan C W 2017 Energy Environ. Sci. 10 1590
 Google Scholar
Google Scholar
[29] Lee C, An T H, Gordon E E, Ji H S, Park C, Shim J H, Lim Y S, Whangbo M H 2017 Chem. Mater. 29 2348
 Google Scholar
Google Scholar
[30] Ul Islam A K M F, Helal M A, Liton M N H, Kamruzzaman M, Islam H M T 2017 Indian J. Phys. 91 403
 Google Scholar
Google Scholar
[31] Wang H, LaLonde A D, Pei Y Z, Snyder G J 2013 Adv. Funct. Mater. 23 1586
 Google Scholar
Google Scholar
-
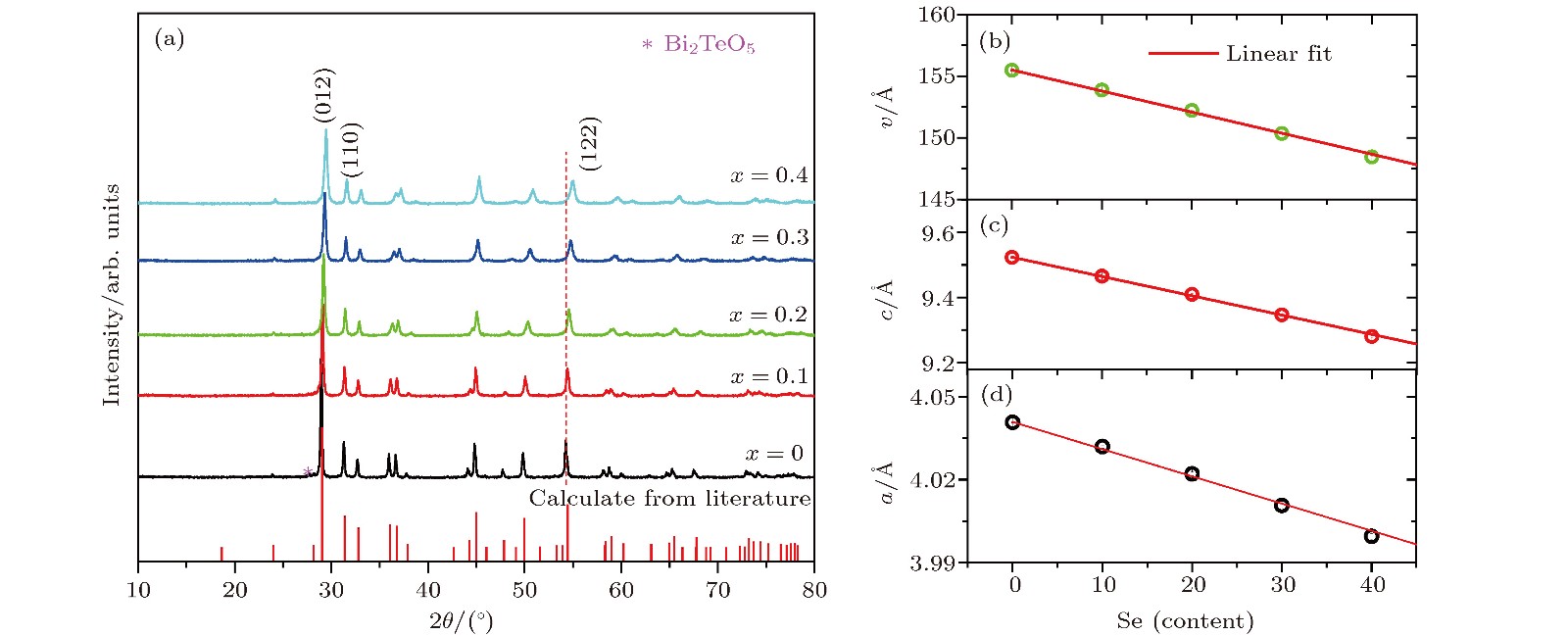
图 1 BiCuTe1–xSexO (x = 0, 0.1, 0.2, 0.3和0.4) 热压样品的 (a) XRD图谱; (b)晶胞体积V; (c) 晶格常数c; (d) 晶格常数a. 图(a) 中提供的BiCuTeO标准图谱为文献精修数据计算所得[23]; 图(b)—图(d) 中实线为线性拟合结果
Figure 1. (a) XRD patterns of the hot-pressed BiCuTe1–xSexO (x = 0, 0.1, 0.2, 0.3, 0.4) samples; (b) cell volume V; (c) lattice parameter c; (d) lattice parameter a as a function of the Se content. The standard XRD pattern of BiCuTeO in (a) is calculated from the Rietveld refinement data given by literature[23]. The solid lines in (b)−(d) are the linear fitting results of the experiment data.
图 3 BiCuTe1-xSexO样品 (x = 0, 0.1, 0.2, 0.3和0.4) 的 (a) 电导率σ; (b) Seebeck系数S; (c) 功率因子S2σ随温度的变化曲线; (d) 样品的载流子浓度n和迁移率
${\mu}$ 随Se含量的变化曲线. 图(a)—(c) 中短划线和点划线分别为文献中未掺杂的BiCuTeO[23]与BiCuSeO[14]的电输运性能Figure 3. Temperature dependent electrical transport properties of BiCuTe1-xSexO (x = 0, 0.1, 0.2, 0.3, 0.4) samples: (a) Electrical conductivity σ; (b) Seebeck coefficients S; (c) power factor S2σ. (d) Se cotent dependence of room temperature hole concentration n and mobility
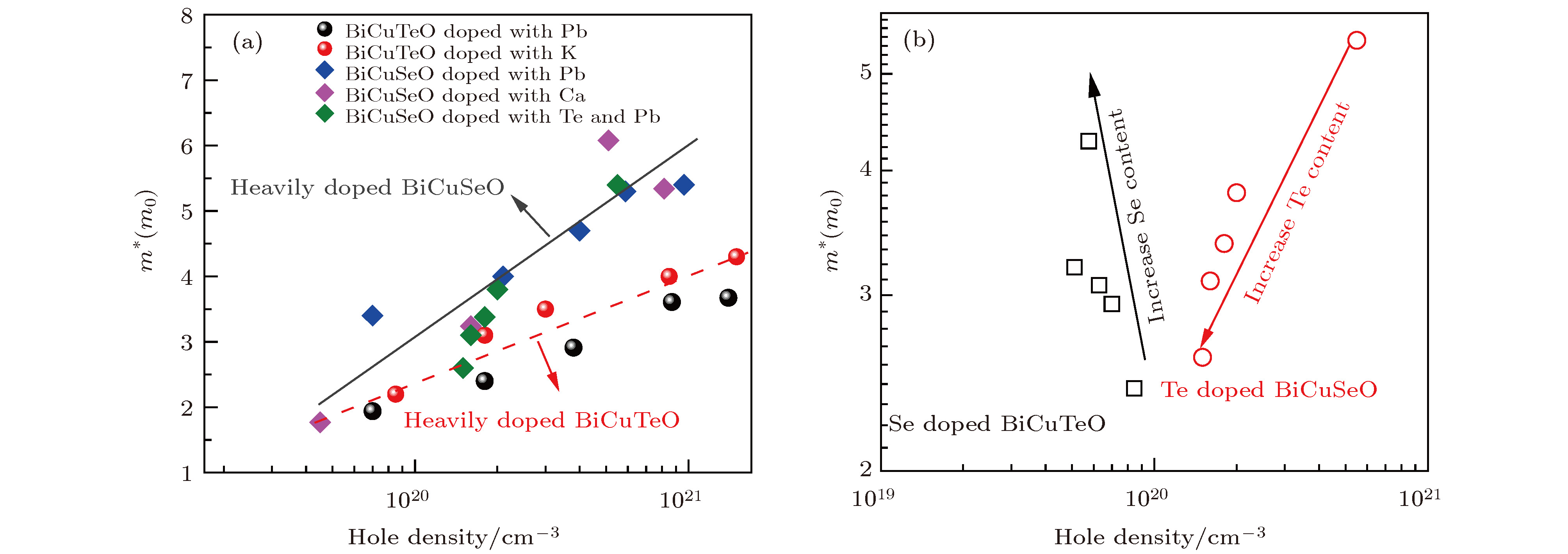
${\mu}$ . The dashed and dotted lines in (a)−(c) represent the electrical transport properties of undoped BiCuSeO[14] and BiCuTeO[23], respectively.图 4 (a) 重掺杂BiCuSeO[14,16,28]和BiCuTeO[24,25]的载流子有效质量m*随载流子浓度n的变化曲线; (b) Se掺杂BiCuTeO(本工作)和Te掺杂BiCuSeO[28]的载流子有效质量m*随载流子浓度n的变化曲线
Figure 4. Hole concentration n dependent effective mass m* of (a) Heavily doped BiCuSeO[14,16,28] and BiCuTeO[24,25]; (b) Se doped BiCuTeO (this work) and Te doped BiCuSeO[28].
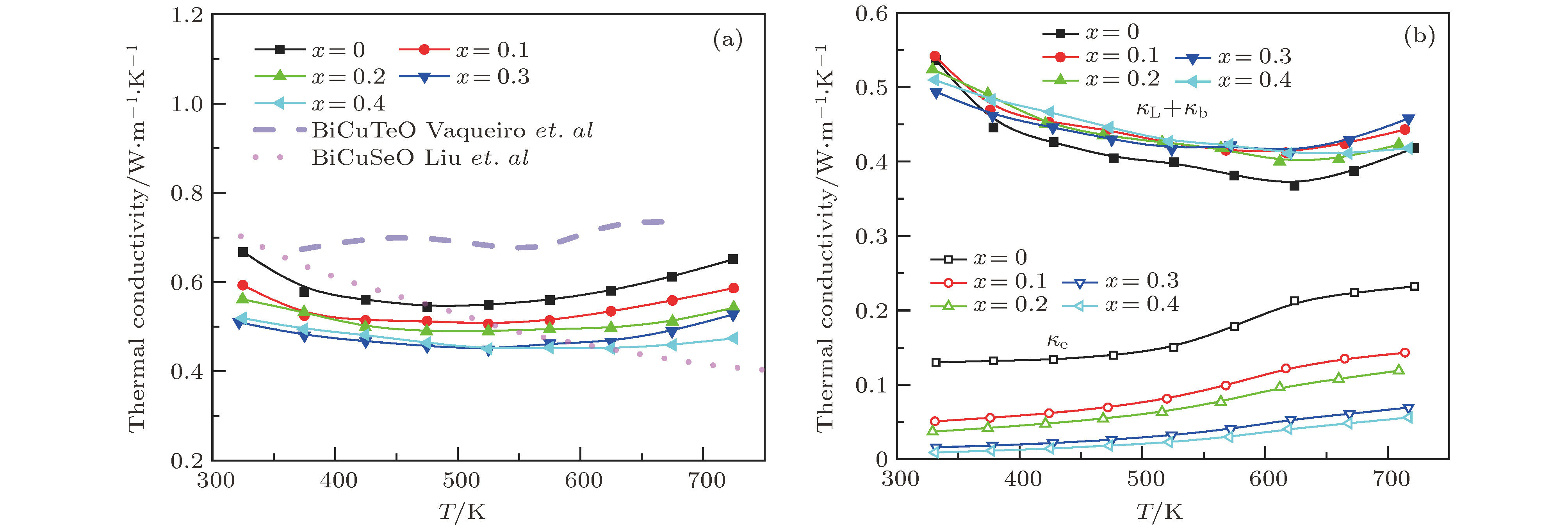
图 5 BiCuTe1–xSexO (x = 0, 0.1, 0.2, 0.3和0.4) 样品的(a) 总热导率和(b) 电子热导率、晶格热导率与双极扩散热导率之和随温度的变化曲线. 图(a)中短划线和点划线分别为未掺杂的BiCuTeO[23]与BiCuSeO[14]的热输运性能
Figure 5. Temperature dependent thermal transport properties of BiCuTe1–xSexO (x = 0, 0.1, 0.2, 0.3, 0.4) samples: (a) Total thermal conductivity; (b) the combination of lattice and bipolar thermal conductivity, and the electronic thermal conductivity. The dashed and dotted lines in (a) represent the total thermal conductivities of undoped BiCuTeO[23] and BiCuSeO[14], respectively.
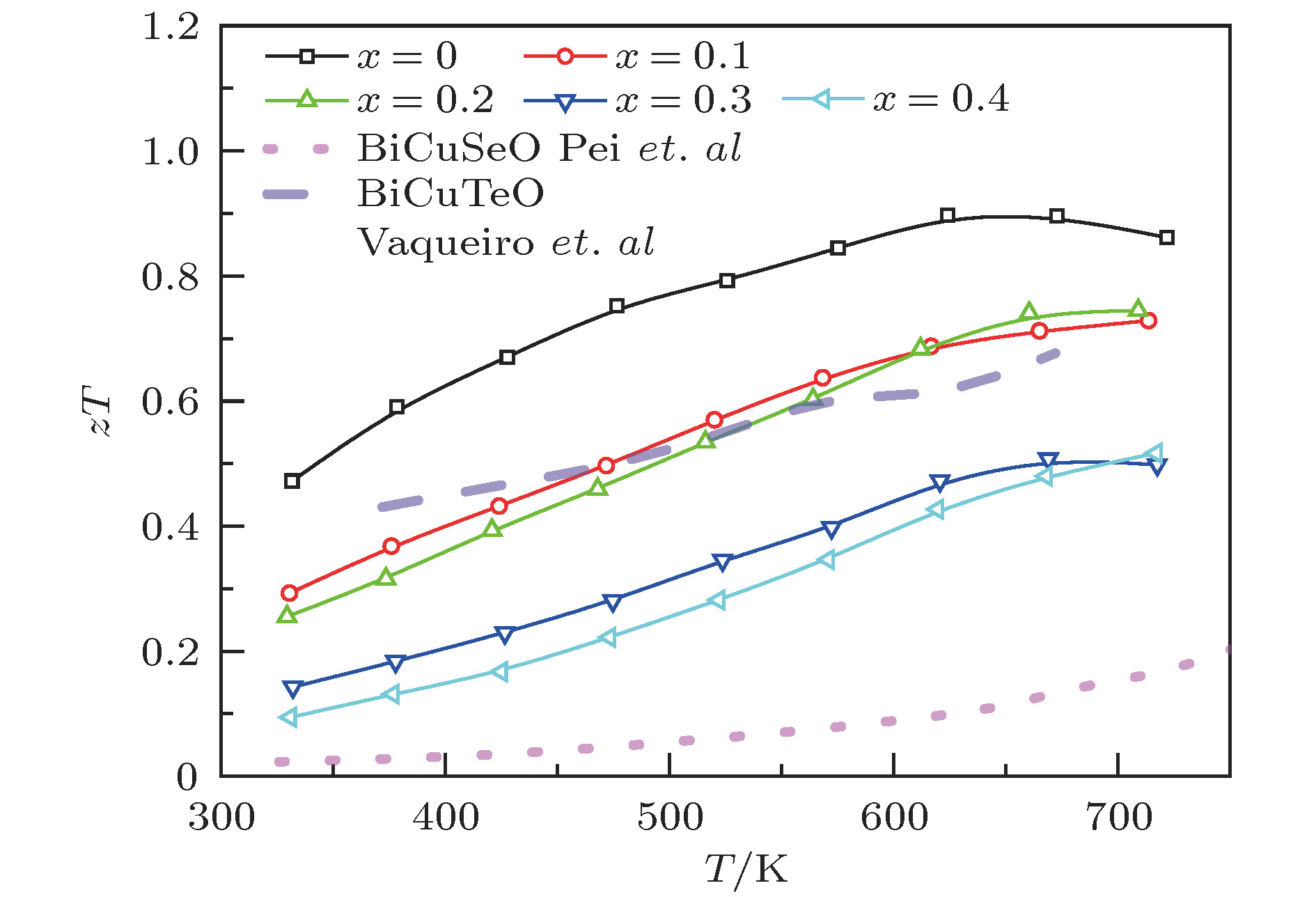
图 6 BiCuTe1–xSexO (x = 0, 0.1, 0.2, 0.3和0.4)样品的zT值随温度的变化曲线. 短划线和点划线分别为未掺杂的BiCuTeO[23]与BiCuSeO[14]的zT值
Figure 6. Temperature dependent zT values of BiCuTe1–xSexO (x = 0, 0.1, 0.2, 0.3, 0.4) samples. The dashed and dotted lines represent the zT values of undoped BiCuTeO[23] and BiCuSeO[14], respectively.
表 1 室温下BiCuTe1–xSexO (x = 0, 0.1, 0.2, 0.3和0.4) 样品的载流子浓度 (n)、迁移率 (
${\mu}$ )、电导率 (σ) 以及载流子有效质量 (m*)Table 1. Room temperature carrier concentrations (n), Hall mobilities (
${\mu}$ ), electrical conductivities (σ) and effective masses of BiCuTe1–xSexO (x = 0, 0.1, 0.2, 0.3, 0.4) samples.样品 载流子浓度n/cm–3 迁移率${\mu}$/cm2·V–1·s–1 电导率σ/Sm–1 m*/m0 BiCuTeO 8.4 × 1019 28.2 3.81 × 104 2.42 BiCuTe0.9Se0.1O 7.0 × 1019 11.8 1.32 × 104 2.94 BiCuTe0.8Se0.2O 6.3 × 1019 7.9 7.89 × 103 3.07 BiCuTe0.7Se0.3O 5.1 × 1019 4.0 3.31 × 103 3.2 BiCuTe0.6Se0.4O 5.8 × 1019 1.9 1.73 × 103 4.28 -
[1] Snyder G J, Toberer E S 2008 Nat. Mater. 7 105
 Google Scholar
Google Scholar
[2] He J, Tritt T M 2017 Science 357 eaak9997
 Google Scholar
Google Scholar
[3] Luo J, You L, Zhang J Y, Guo K, Zhu H T, Gu L, Yang Z Z, Li X, Yang J, Zhang W Q 2017 ACS Appl. Mater. Interfaces 9 8729
 Google Scholar
Google Scholar
[4] Pei Y Z, Shi X Y, LaLonde A, Wang H, Chen L D, Snyder G J 2011 Nature 473 66
 Google Scholar
Google Scholar
[5] Heremans J P, Jovovic V, Toberer E S, Saramat A, Kurosaki K, Charoenphakdee A, Yamanaka S, Snyder G J 2008 Science 321 554
 Google Scholar
Google Scholar
[6] Biswas K, He J Q, Blum I D, Wu C I, Hogan T P, Seidman D N, Dravid V P, Kanatzidis M G 2012 Nature 489 414
 Google Scholar
Google Scholar
[7] You L, Liu Y F, Li X, Nan P F, Ge B H, Jiang Y, Luo P F, Pan S S, Pei Y Z, Zhang W Q, Snyder G J, Yang J, Zhang J Y, Luo J 2018 Energy Environ. Sci. 11 1848
 Google Scholar
Google Scholar
[8] Zhou W X, Chen K Q 2015 Carbon 85 24
 Google Scholar
Google Scholar
[9] Chen X K, Xie Z X, Zhou W X, Tang L M, Chen K Q 2016 Appl. Phys. Lett. 109 023101
 Google Scholar
Google Scholar
[10] Chen X K, Liu J, Peng Z H, Du D, Chen K Q 2017 Appl. Phys. Lett. 110 091907
 Google Scholar
Google Scholar
[11] Lin S Q, Li W, Li S S, Zhang X Y, Chen Z W, Xu Y D, Chen Y, Pei Y Z 2017 Joule 1 816
 Google Scholar
Google Scholar
[12] Li J, Sui J H, Pei Y L, Barreteau C, Berardan D, Dragoe N, Cai W, He J Q, Zhao L D 2012 Energy Environ. Sci. 5 8543
 Google Scholar
Google Scholar
[13] Barreteau C, Berardan D, Amzallag E, Zhao L D, Dragoe N 2012 Chem. Mater. 24 3168
 Google Scholar
Google Scholar
[14] Pei Y L, He J Q, Li J F, Li F, Liu Q J, Pan W, Barreteau C, Berardan D, Dragoe N, Zhao L D 2013 NPG Asia Mater. 5 e47
 Google Scholar
Google Scholar
[15] Li J, Sui J H, Barreteau C, Berardan D, Dragoe N, Cai W, Pei Y L, Zhao L D 2013 J. Alloys Compd. 551 649
 Google Scholar
Google Scholar
[16] Lan J L, Liu Y C, Zhan B, Lin Y H, Zhang B P, Yuan X, Zhang W Q, Xu W, Nan C W 2013 Adv. Mater. 25 5086
 Google Scholar
Google Scholar
[17] Liu Y C, Zheng Y H, Zhan B, Chen K, Butt S, Zhang B P, Lin Y H 2015 J. Eur. Ceram. Soc. 35 845
 Google Scholar
Google Scholar
[18] Pei Y L, Wu H J, Wu D, Zheng F S, He J Q 2014 J. Am. Chem. Soc. 136 13902
 Google Scholar
Google Scholar
[19] Sui J H, Li J, He J Q, Pei Y L, Berardan D, Wu H J, Dragoe N, Cai W, Zhao L D 2013 Energy Environ. Sci. 6 2916
 Google Scholar
Google Scholar
[20] Liu Y, Lan J L, Xu W, Liu Y C, Pei Y L, Cheng B, Liu D B, Lin Y H, Zhao L D 2013 Chem. Commun. 49 8075
 Google Scholar
Google Scholar
[21] Pan L, Lang Y D, Zhao L, Berardan D, Amzallag E, Xu C, Gu Y F, Chen C C, Zhao L D, Shen X D, Lyu Y N, Lu C H, Wang Y F 2018 J. Mater. Chem. A 6 13340
 Google Scholar
Google Scholar
[22] Liu Y, Zhao L D, Zhu Y, Liu Y, Li F, Yu M, Liu D B, Xu W, Lin Y H, Nan C W 2016 Adv. Energy Mater. 6 1502423
 Google Scholar
Google Scholar
[23] Vaqueiro P, Guelou G, Stec M, Guilmeau E, Powell A V 2013 J. Mater. Chem. A 1 520
 Google Scholar
Google Scholar
[24] An T H, Lim Y S, Choi H S, Seo W S, Park C H, Kim G R, Park C, Lee C H, Shim J H 2014 J. Mater. Chem. A 2 19759
 Google Scholar
Google Scholar
[25] An T H, Lim Y S, Seo W S, Park C H, Yoo M D, Park C, Lee C H, Shim J H 2017 J. Electron. Mater. 46 2717
 Google Scholar
Google Scholar
[26] Barreteau C, Berardan D, Zhao L D, Dragoe N 2013 J. Mater. Chem. A 1 2921
 Google Scholar
Google Scholar
[27] Pichanusakorn P, Bandaru P 2010 Mater. Sci. Eng., R 67 19
 Google Scholar
Google Scholar
[28] Ren G K, Wang S Y, Zhu Y C, Ventura K J, Tan X, Xu W, Lin Y H, Yang J H, Nan C W 2017 Energy Environ. Sci. 10 1590
 Google Scholar
Google Scholar
[29] Lee C, An T H, Gordon E E, Ji H S, Park C, Shim J H, Lim Y S, Whangbo M H 2017 Chem. Mater. 29 2348
 Google Scholar
Google Scholar
[30] Ul Islam A K M F, Helal M A, Liton M N H, Kamruzzaman M, Islam H M T 2017 Indian J. Phys. 91 403
 Google Scholar
Google Scholar
[31] Wang H, LaLonde A D, Pei Y Z, Snyder G J 2013 Adv. Funct. Mater. 23 1586
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 9525
- PDF Downloads: 96
- Cited By: 0














 DownLoad:
DownLoad: