-
基于密度泛函理论的第一性原理方法, 计算了锂离子电池富锂锰基三元正极材料Li1.167Ni0.167Co0.167Mn0.5O2中的氧空位形成, 讨论了环境温度、压强以及点缺陷的存在对氧空位形成能的影响, 还讨论了氧空位对材料容量的影响. 结果表明, 氧空位的形成能随温度的升高而下降, 随氧分压的降低而降低. 对于带电氧空位(
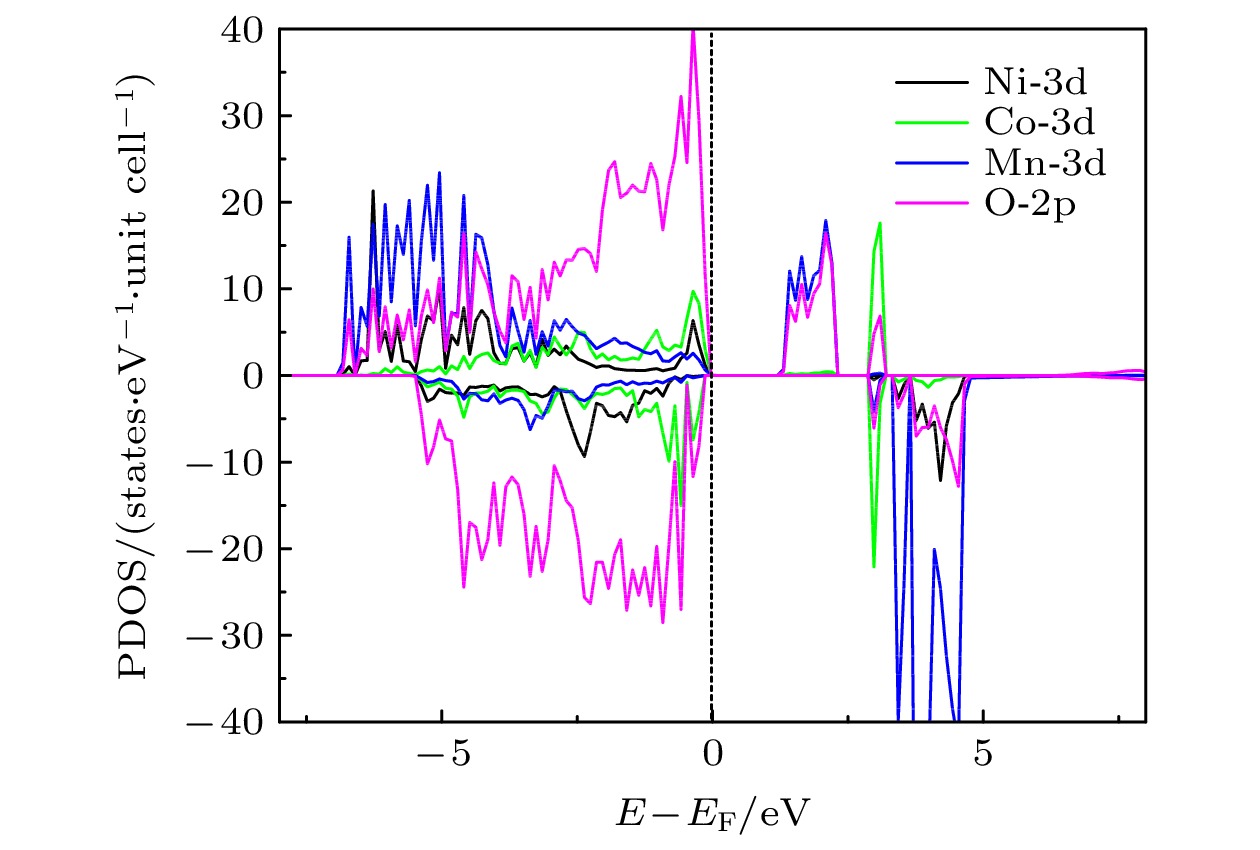
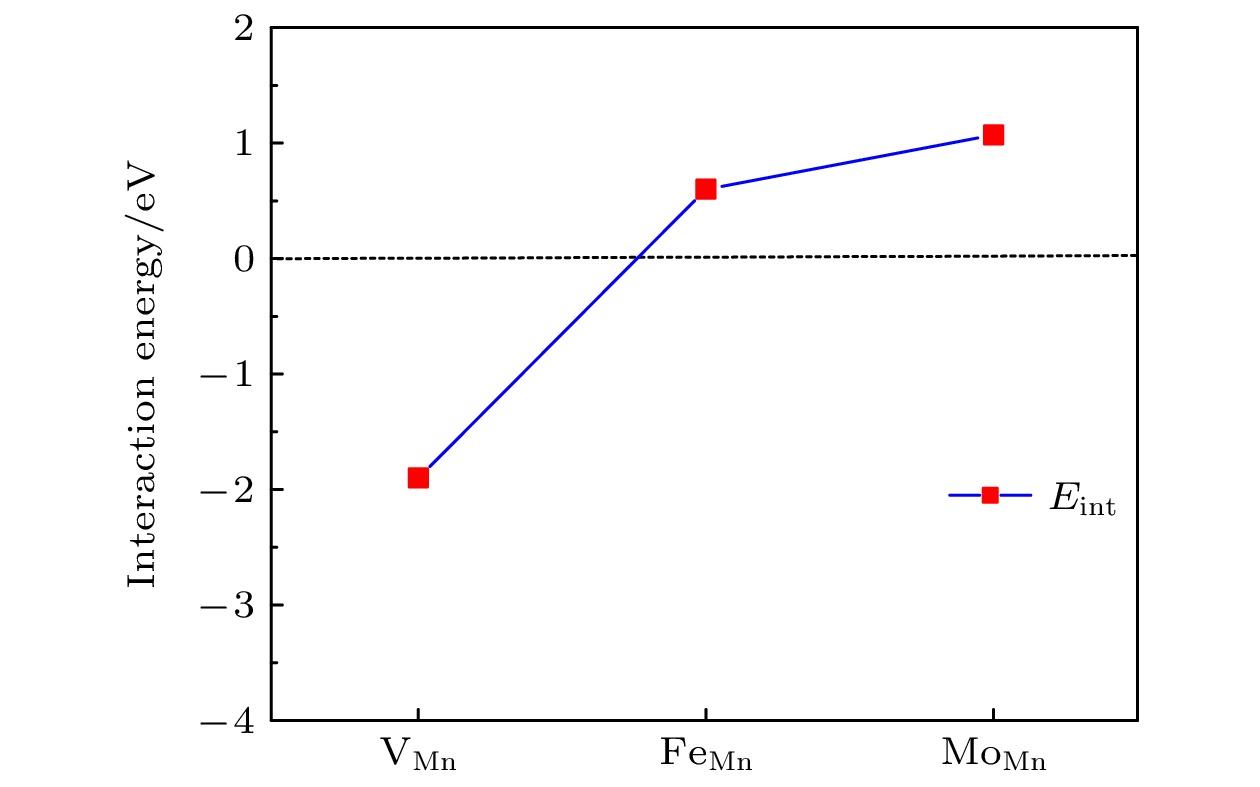
$ {\mathrm{V}}_{\mathrm{O}}^{+1} $ ,$ {\mathrm{V}}_{\mathrm{O}}^{+2} $ ), 空位形成能随着费米能级的升高而增加. 研究还表明, 氧空位的形成对Li1.167Ni0.167Co0.167Mn0.5O2材料中电荷密度分布的影响是相当局域的,$ {\mathrm{V}}_{\mathrm{O}}^{0} $ 氧空位形成后仅在氧空位附近的Mn离子周围出现明显的电荷密度的重新分布. 此外, 计算了氧空位附近存在阳离子空位以及替位点缺陷对氧空位形成能的影响. 结果显示, Mn空位的存在能够明显地促进氧空位的产生. 另外, 当Mn被Mo或Fe原子替位时, 氧空位的产生会受到抑制.Using the first-principles method based on the density functional theory, the oxygen vacancy formations in the lithium-rich manganese-based ternary cathode material Li1.167Ni0.167Co0.167Mn0.5O2 are calculated. The changes of oxygen vacancy formation energy with temperature, oxygen partial pressure and point defects in the material are discussed, meanwhile, the effect of oxygen vacancies on the capacity is also discussed. The calculation results show that the increase of temperature and the decrease of oxygen partial pressure can lead the formation energy of an oxygen vacancy to decline. For the charged oxygen vacancies ($ {\mathrm{V}}_{\mathrm{O}}^{+1} $ ,$ {\mathrm{V}}_{\mathrm{O}}^{+2} $ ), the formation energy of an O-vacancy increases with Fermi level increasing. It is also found that the presence of an oxygen vacancy will trigger off a very local charge density redistributions, mainly around the neighboring Mn ions next to the O-vacancy. Furthermore, the effects of point defects, including cation vacancies and substitutional defects in the vicinity of the O-vacancy, on the formation energy of O-vacancy are also calculated. The results show that the presence of Mn vacancy near the O-vacancy is beneficial to the formation of the O-vacancy. In addition, the formation of oxygen vacancy is suppressed when the Mn atoms near the O-vacancy are substituted by the Mo or Fe atoms.-
Keywords:
- Li-rich Mn-based ternary materials /
- oxygen vacancy /
- defect formation /
- first-principles calculations
[1] Dunn B, Kamath H, Tarascon J M 2011 Science 334 928
 Google Scholar
Google Scholar
[2] Goodenough J B, Kim Y 2010 Chem. Mater. 22 587
 Google Scholar
Google Scholar
[3] Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D 2011 Energy Environ. Sci. 4 3243
 Google Scholar
Google Scholar
[4] Nitta N, Wu F, Lee J T, Yushin G 2015 Mater. Today 18 252
 Google Scholar
Google Scholar
[5] Mizushima K, Jones P, Wiseman P, Goodenough J B 1981 Solid State Ionics 3 171
[6] Wang H, Jang Y I, Huang B Y, Sadoway D R, Chiang Y M 1999 J. Electrochem. Soc. 146 473
 Google Scholar
Google Scholar
[7] Huang H, Yin S C, Nazar L F 2001 Electrochem. Solid. St. 4 A170
 Google Scholar
Google Scholar
[8] Ouyang C Y, Shi S Q, Wang Z X, Huang X J, Chen L Q 2004 Phys. Rev. B 69 104303
 Google Scholar
Google Scholar
[9] Yuan L X, Wang Z H, Zhang W X, Hu X L, Chen J T, Huang Y H, Goodenough J B 2011 Energy Environ. Sci. 4 269
 Google Scholar
Google Scholar
[10] Liu J L, Hou M Y, Yi J, Guo S S, Wang C X, Xia Y Y 2014 Energy Environ. Sci. 7 705
 Google Scholar
Google Scholar
[11] Zuo W H, Luo M Z, Liu X S, Wu J, Liu H D, Li J, Winter M, Fu R Q, Yang W L, Yang Y 2020 Energy Environ. Sci. 13 4450
 Google Scholar
Google Scholar
[12] Koga H, Croguennec L, Mannessiez P, M Ménétrier, Delmas C 2012 J. Phys. Chem. C. 116 13497
 Google Scholar
Google Scholar
[13] He Z J, Wang Z X, Huang Z M, Chen H, Li X H, Guo H J 2015 J. Mater. Chem. A. 3 16817
 Google Scholar
Google Scholar
[14] Sung Nam Lim, Jung Yoon Seo, Dae Soo Jung 2015 J. Alloy. Compd. 623 55
 Google Scholar
Google Scholar
[15] Lai X W, Hu G R, Peng Z D, Tong H, Lu Y, Wang Y Z, Qi X Y, Xue Z C, Huang Y, Du K 2019 J. Power. Sources. 431 144
 Google Scholar
Google Scholar
[16] Qiu B, Zhang M H, Wu L J, Wang J, Xia Y G, Qian D N 2016 Nat. Commun. 7 1
[17] Zhang B, Wang L, Bai F, Xiao P, Zhang B, Chen X, Sun Jie, Yang W S 2019 Dalton. T. 48 3209
 Google Scholar
Google Scholar
[18] Kresse G, Furthmuller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[19] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[20] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[21] Dudarev S L, Botton G A, Savrasov S Y, Humphreys C J, Sutton A P 1998 Phys. Rev. B 57 1505
 Google Scholar
Google Scholar
[22] Zhou F, Cococcioni M, Marianetti C A, Morgan D, Ceder G 2004 Phys. Rev. B 70 235121
 Google Scholar
Google Scholar
[23] Xiao R J, Li H, Chen L Q 2012 Chem. Mater. 24 4242
 Google Scholar
Google Scholar
[24] Jain A, Hautier G, Ong S P, Moore C J, Fischer C C, Persson K A, Ceder G 2011 Phys. Rev. B 84 045115
 Google Scholar
Google Scholar
[25] Nakamura T, Ohta K, Hou X Y, Kimura Y, Tsuruta K, Tamenori Y, Aso R, Yoshida H, Amezawa K 2021 J. Mater. Chem. A 9 3657
 Google Scholar
Google Scholar
[26] Hu W, Wang H W, Luo W W, Xu B, Ouyang C Y 2020 Solid State Ionics 347 115257
 Google Scholar
Google Scholar
[27] Hashimoto T, Moriwake H 2008 Phys. Rev. B 78 092106
 Google Scholar
Google Scholar
[28] Reuter K, Scheffler M 2001 Phys. Rev. B 65 035406
 Google Scholar
Google Scholar
[29] Wu S Q, Cai N L, Zhu Z Z, Yang Y 2008 Electrochim. Acta 53 7915
 Google Scholar
Google Scholar
[30] Turner D E, Zhu Z Z, Chan C T, Ho K M 1997 Phys. Rev. B 55 13842
 Google Scholar
Google Scholar
[31] Zhang W, Hou Z F 2014 J. Appl. Phys. 115 124104
 Google Scholar
Google Scholar
-
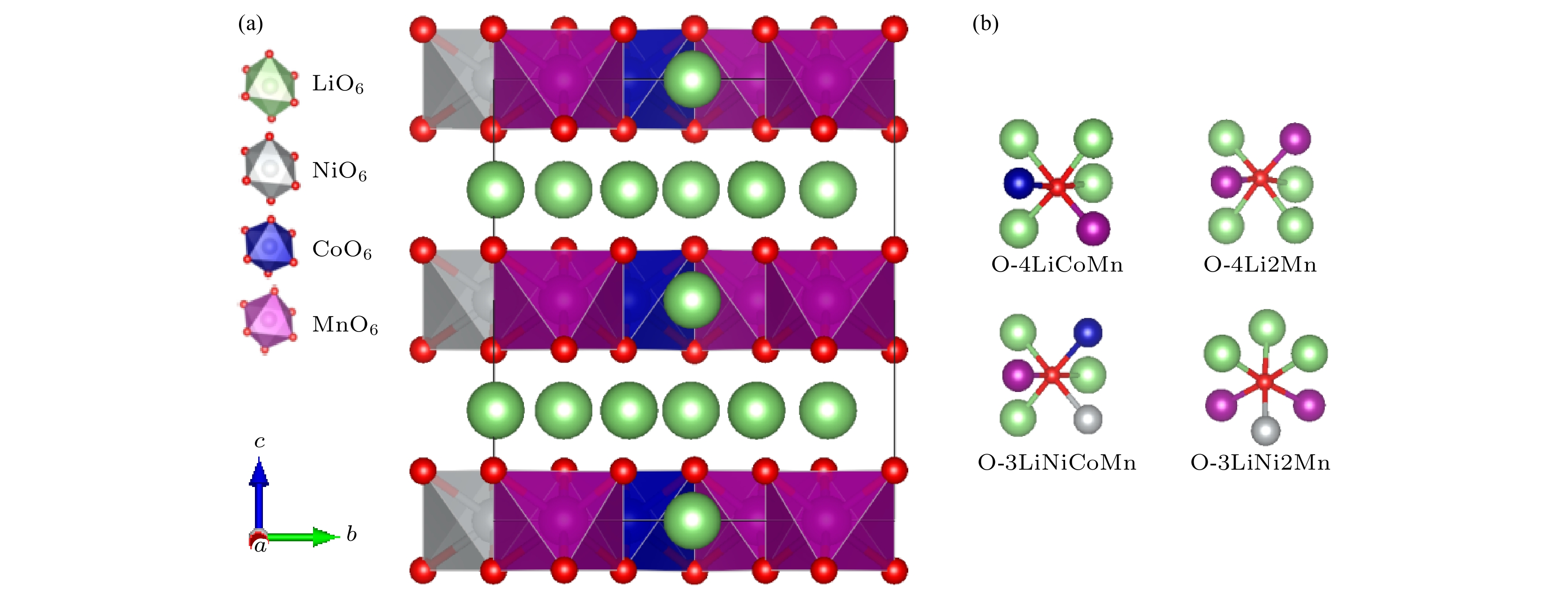
图 1 (a) Li1.167Ni0.167Co0.167Mn0.5O2的晶体结构(其中NiO6, CoO6和MnO6八面体分别标识为灰色、蓝色和紫色, 氧原子为红色, 锂离子为绿色); (b) 氧与周围配位原子的4种示意图
Fig. 1. (a) Crystal structure of Li1.167Ni0.167Co0.167Mn0.5O2 (NiO6, CoO6 and MnO6 octahedra are marked by gray, blue and purple, respectively; oxygen and lithium ions are denoted by red and green balls, respectively); (b) diagram of the four coordination pattern of oxygen and surrounding atoms.
图 4 P = 0.2 bar, 费米能级不同时的氧空位形成能随温度的变化 (a)
$ {E}_{\mathrm{F}}=0 $ ; (b)$ {E}_{\mathrm{F}}={E}_{\mathrm{g}\mathrm{a}\mathrm{p}} $ .Fig. 4. Formation energies of an oxygen vacancy with different Fermi level as a function of temperature at P = 0.2 bar: (a)
$ {E}_{\mathrm{F}}=0 $ ; (b)$ {E}_{\mathrm{F}}={E}_{\mathrm{g}\mathrm{a}\mathrm{p}} $ 图 5
${E}_{\mathrm{F}}={E}_{\mathrm{gap}}=1.10 $ eV, 温度不同时氧空位形成能随氧分压的变化 (a) T = 300 K; (b) T = 1000 KFig. 5. Formation energies of an oxygen vacancy as a function of oxygen partial pressure at different temperatures with
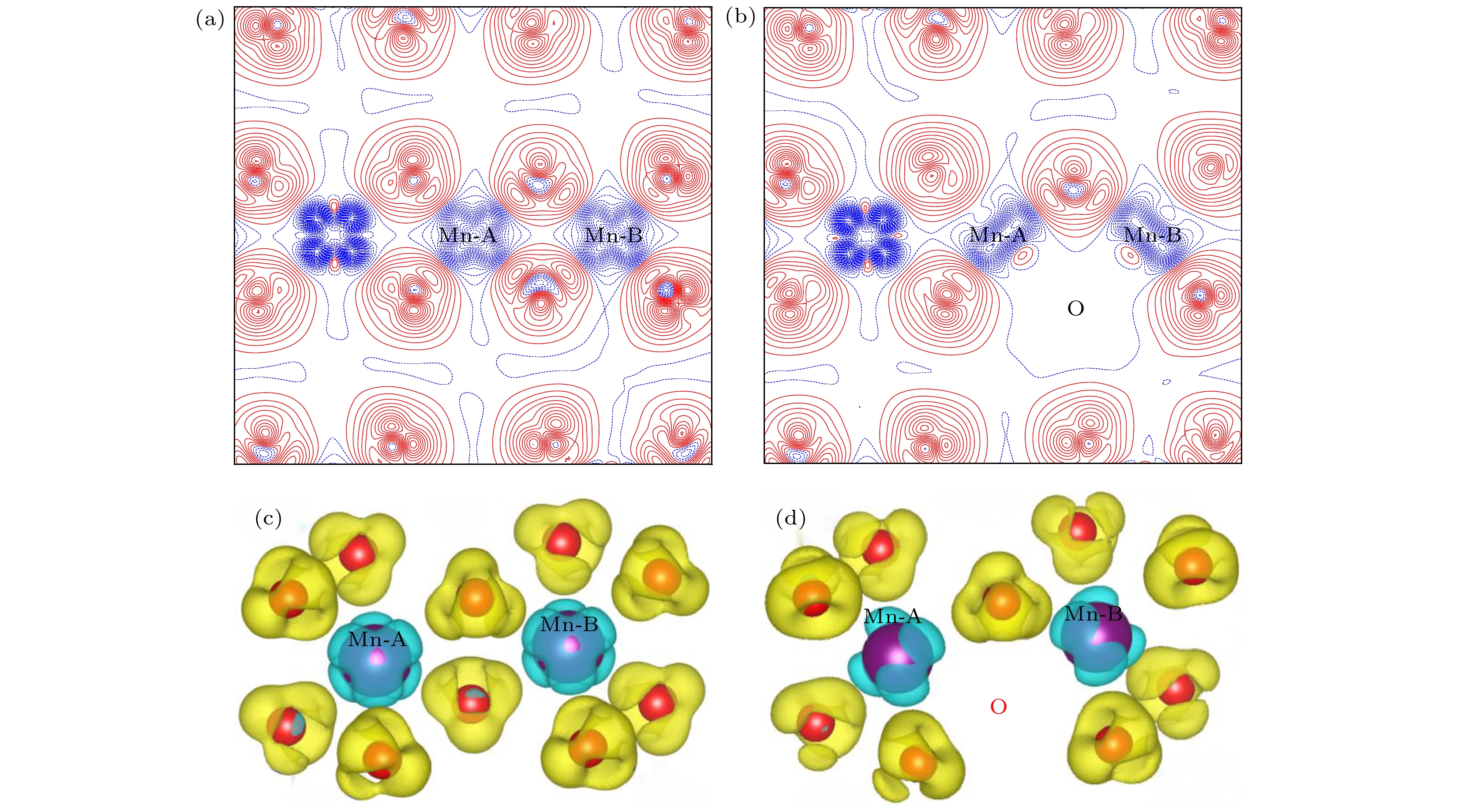
${E}_{\mathrm{F}}={E}_{\mathrm{gap}}=1.10 {\rm{eV}}$ : (a) T = 300 K; (b) T = 1000 K.图 7 Li1.167Ni0.167Co0.167Mn0.5O2的二维差分电荷密度 (a) 完整晶体, (b) 有氧空位(红色线表示该区域有电荷聚集, 而蓝色线表示该区域有电荷的移出); Li1.167Ni0.167Co0.167Mn0.5O2的三维差分电荷密度 (c) 完整晶体, (d) 有氧空位
Fig. 7. 2D charge density plots of Li1.167Ni0.167Co0.167Mn0.5O2: (a) Pristine; (b) with oxygen vacancy (The solid and dashed lines represent the accumulation and depletion of charges relative to the independent atoms, respectively); 3D charge density plots of Li1.167Ni0.167Co0.167Mn0.5O2: (c) pristine; (d) with oxygen vacancy.
表 1 Li1.167Ni0.167Co0.167Mn0.5O2材料中不同费米能级的3种带电荷态氧空位的形成能
Table 1. The calculated formation energies of non-equivalent oxygen vacancies at different charge states in the bulk Li1.167Ni0.167Co0.167Mn0.5O2 at different Fermi level.
不同配位环境的氧空位形成能/eV VO-4Li2Mn VO-4LiCoMn VO-3LiNiCoMn VO-3LiNi2Mn $ {\mathrm{V}}_{\mathrm{O}}^{0} $ 2.30 2.80 3.12 3.20 EF = 0 $ {\mathrm{V}}_{\mathrm{O}}^{+1} $ –1.30 –1.13 –0.95 –0.66 $ {\mathrm{V}}_{\mathrm{O}}^{+2} $ –5.02 –4.90 –4.42 –4.40 EF = Egap $ {\mathrm{V}}_{\mathrm{O}}^{0} $ 2.30 2.80 3.12 3.20 $ {\mathrm{V}}_{\mathrm{O}}^{+1} $ –0.20 –0.03 0.15 0.44 $ {\mathrm{V}}_{\mathrm{O}}^{+2} $ –2.82 –2.70 –2.22 –2.20 -
[1] Dunn B, Kamath H, Tarascon J M 2011 Science 334 928
 Google Scholar
Google Scholar
[2] Goodenough J B, Kim Y 2010 Chem. Mater. 22 587
 Google Scholar
Google Scholar
[3] Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D 2011 Energy Environ. Sci. 4 3243
 Google Scholar
Google Scholar
[4] Nitta N, Wu F, Lee J T, Yushin G 2015 Mater. Today 18 252
 Google Scholar
Google Scholar
[5] Mizushima K, Jones P, Wiseman P, Goodenough J B 1981 Solid State Ionics 3 171
[6] Wang H, Jang Y I, Huang B Y, Sadoway D R, Chiang Y M 1999 J. Electrochem. Soc. 146 473
 Google Scholar
Google Scholar
[7] Huang H, Yin S C, Nazar L F 2001 Electrochem. Solid. St. 4 A170
 Google Scholar
Google Scholar
[8] Ouyang C Y, Shi S Q, Wang Z X, Huang X J, Chen L Q 2004 Phys. Rev. B 69 104303
 Google Scholar
Google Scholar
[9] Yuan L X, Wang Z H, Zhang W X, Hu X L, Chen J T, Huang Y H, Goodenough J B 2011 Energy Environ. Sci. 4 269
 Google Scholar
Google Scholar
[10] Liu J L, Hou M Y, Yi J, Guo S S, Wang C X, Xia Y Y 2014 Energy Environ. Sci. 7 705
 Google Scholar
Google Scholar
[11] Zuo W H, Luo M Z, Liu X S, Wu J, Liu H D, Li J, Winter M, Fu R Q, Yang W L, Yang Y 2020 Energy Environ. Sci. 13 4450
 Google Scholar
Google Scholar
[12] Koga H, Croguennec L, Mannessiez P, M Ménétrier, Delmas C 2012 J. Phys. Chem. C. 116 13497
 Google Scholar
Google Scholar
[13] He Z J, Wang Z X, Huang Z M, Chen H, Li X H, Guo H J 2015 J. Mater. Chem. A. 3 16817
 Google Scholar
Google Scholar
[14] Sung Nam Lim, Jung Yoon Seo, Dae Soo Jung 2015 J. Alloy. Compd. 623 55
 Google Scholar
Google Scholar
[15] Lai X W, Hu G R, Peng Z D, Tong H, Lu Y, Wang Y Z, Qi X Y, Xue Z C, Huang Y, Du K 2019 J. Power. Sources. 431 144
 Google Scholar
Google Scholar
[16] Qiu B, Zhang M H, Wu L J, Wang J, Xia Y G, Qian D N 2016 Nat. Commun. 7 1
[17] Zhang B, Wang L, Bai F, Xiao P, Zhang B, Chen X, Sun Jie, Yang W S 2019 Dalton. T. 48 3209
 Google Scholar
Google Scholar
[18] Kresse G, Furthmuller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[19] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[20] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[21] Dudarev S L, Botton G A, Savrasov S Y, Humphreys C J, Sutton A P 1998 Phys. Rev. B 57 1505
 Google Scholar
Google Scholar
[22] Zhou F, Cococcioni M, Marianetti C A, Morgan D, Ceder G 2004 Phys. Rev. B 70 235121
 Google Scholar
Google Scholar
[23] Xiao R J, Li H, Chen L Q 2012 Chem. Mater. 24 4242
 Google Scholar
Google Scholar
[24] Jain A, Hautier G, Ong S P, Moore C J, Fischer C C, Persson K A, Ceder G 2011 Phys. Rev. B 84 045115
 Google Scholar
Google Scholar
[25] Nakamura T, Ohta K, Hou X Y, Kimura Y, Tsuruta K, Tamenori Y, Aso R, Yoshida H, Amezawa K 2021 J. Mater. Chem. A 9 3657
 Google Scholar
Google Scholar
[26] Hu W, Wang H W, Luo W W, Xu B, Ouyang C Y 2020 Solid State Ionics 347 115257
 Google Scholar
Google Scholar
[27] Hashimoto T, Moriwake H 2008 Phys. Rev. B 78 092106
 Google Scholar
Google Scholar
[28] Reuter K, Scheffler M 2001 Phys. Rev. B 65 035406
 Google Scholar
Google Scholar
[29] Wu S Q, Cai N L, Zhu Z Z, Yang Y 2008 Electrochim. Acta 53 7915
 Google Scholar
Google Scholar
[30] Turner D E, Zhu Z Z, Chan C T, Ho K M 1997 Phys. Rev. B 55 13842
 Google Scholar
Google Scholar
[31] Zhang W, Hou Z F 2014 J. Appl. Phys. 115 124104
 Google Scholar
Google Scholar
计量
- 文章访问数: 8533
- PDF下载量: 193
- 被引次数: 0


















 下载:
下载: