-
采用第一原理方法计算了两种不同镍含量的锂离子电池富锂锰基三元正极材料Li1.2Ni0.32Co0.04Mn0.44O2 (空间群为
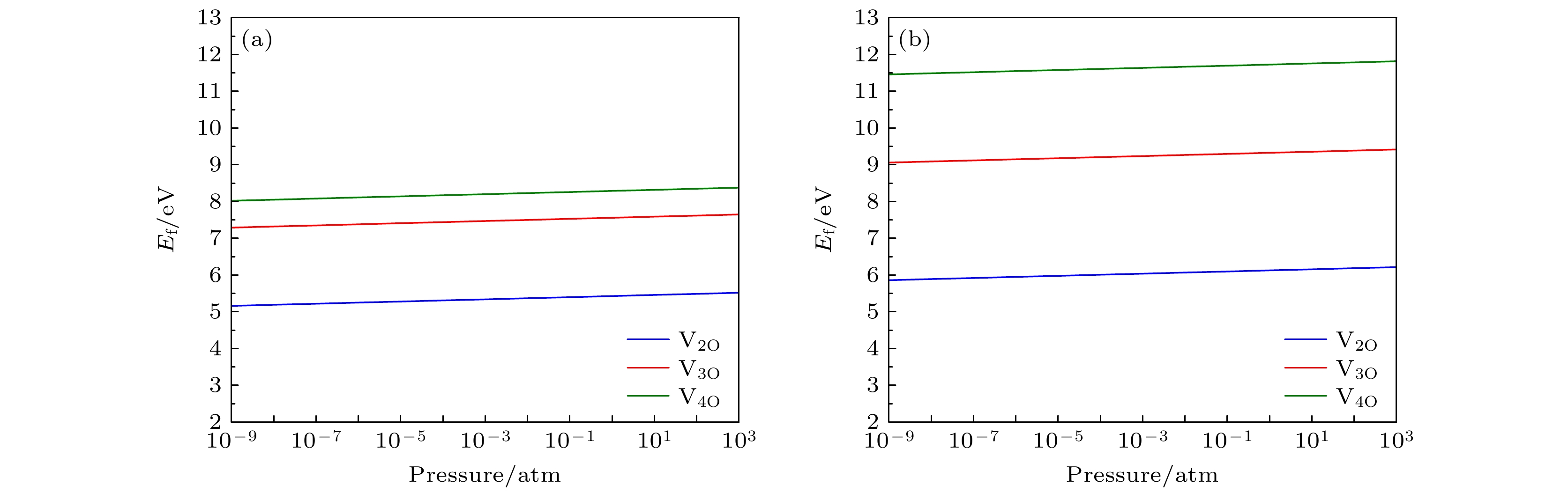
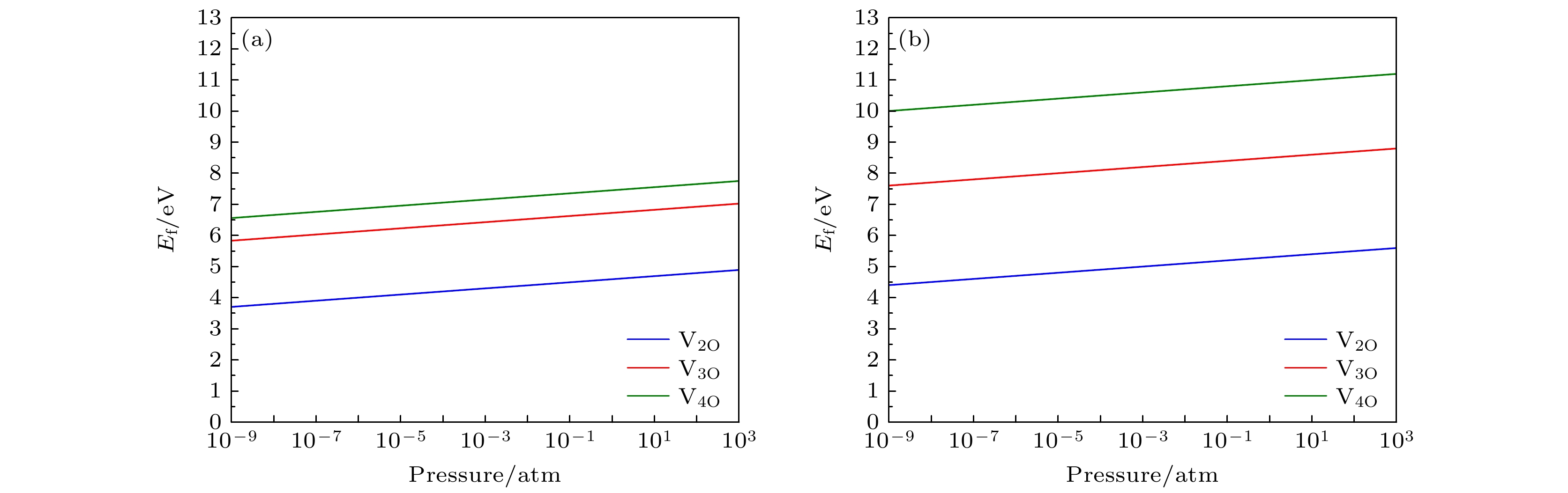
$ R\bar{3}m $ ) 和Li1.167Ni0.167Co0.167Mn0.5O2 (空间群为C2/m)中氧空位簇的形成能. 结果表明, 含镍量较少的Li1.167Ni0.167Co0.167Mn0.5O2正极材料中氧空位簇的形成能总是高于含镍量较多的Li1.2Ni0.32Co0.04Mn0.44O2材料中的氧空位簇形成能, 这说明含镍量较高的正极材料中氧空位簇更容易形成. 无论是含镍量较高的富锂锰基材料, 还是含镍量较少的同类材料, 过渡金属边上的氧空位簇的形成能总是大于锂离子附近空位簇的形成能, 说明氧的脱去更趋向于在Li离子附近发生. 较低的温度和较高的氧分压会使氧空位簇的形成能增加, 从而抑制氧空位簇的形成. 此外, 还计算了空位簇边上的过渡金属原子被其它过渡金属原子(Ti 和Mo)替位后的氧空位簇形成能. 结果表明, 除了Li1.2Ni0.32Co0.04Mn0.44O2材料中双氧空位V2O-Li 附近的Ni元素被Ti替位外, 其余情况下过渡金属Ni和Mn分别被Ti或Mo替位后均能够增大VnO-Li空位簇的形成能, 故替位点缺陷的掺杂有抑制氧的损失和提高材料的结构稳定性的作用.Using the first-principles method, the formation energy values of O-vacancy clusters of two Li-rich Mn-based ternary cathode materials of lithium ion battery with different amounts of nickel , i.e. Li1.2Ni0.32Co0.04Mn0.44O2 (space group$R\bar{3}m)$ and Li1.167Ni0.167Co0.167Mn0.5O2 (space group C2/m), are calculated. Results show that the formation energy of oxygen vacancy cluster of the material with less nickel content Li1.167Ni0.167Co0.167Mn0.5O2 can be always higher than that of the material Li1.2Ni0.32Co0.04Mn0.44O2 with higher nickel content. This indicates that the oxygen vacancy clusters are more likely to form in cathode material with higher nickel content. The formation energy of the oxygen vacancy cluster near the transition metal is always greater than that near the lithium ion, indicating that the removal of oxygen tends to occur near the Li ion. Lower temperature and higher partial pressure can increase the formation energy of oxygen vacancy cluster, and therefore inhibit the formation of oxygen vacancy cluster. In addition, the formation energy values of oxygen vacancy clusters with the transition metals in the materials replaced by other transition metals (i.e., Ti and Mo) are also calculated. The results show that, in addition to the case of Ni replaced by Ti near the double oxygen vacancies near the Li-ion in Li1.2Ni0.32Co0.04Mn0.44O2, all the remaining cases of the transition metals Ni or Mn replaced by Ti or Mo always increase the formation energy of the O-vacancy cluster. Therefore, the doping should be able to inhibit the loss of oxygen and improve the structural stability of material.-
Keywords:
- Li-rich Mn-based ternary materials /
- oxygen vacancy cluster /
- formation energy /
- first-principles calculations
[1] Dunn B, Kamath H, Tarascon J M 2011 Science 334 928
 Google Scholar
Google Scholar
[2] Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D 2011 Energy Environ. Sci. 4 3243
 Google Scholar
Google Scholar
[3] Nitta N, Wu F, Lee J T, Yushin G 2015 Mater. Today 18 252
 Google Scholar
Google Scholar
[4] Yuan L X, Wang Z H, Zhang W X, Hu X L, Chen J T, Huang Y H, Goodenough J B 2011 Energy Environ. Sci. 4 269
 Google Scholar
Google Scholar
[5] Liu J L, Hou M Y, Yi J, Guo S S, Wang C X, Xia Y Y 2014 Energy Environ. Sci. 7 705
 Google Scholar
Google Scholar
[6] Csernica P M, Kalirai S S, Gent W E, Lim K, Yu Y S, Liu Y, Ahn S J, Kaeli E, Xu X, Stone K H, Marshall A F, Sinclair R, Shapiro D A, Toney M F, Chueh W C 2021 Nat. Energy 6 642
 Google Scholar
Google Scholar
[7] Hu E Y, Yu X Q, Lin R Q, Bi X X, Lu J, Bak S M, Nam K W, Xin H L, Jaye C, Fischer D A, Amine K, Yang X Q 2018 Nat. Energy 3 690
 Google Scholar
Google Scholar
[8] Zhu Z, Yu D W, Yang Y, Su C, Huang Y M, Dong Y H, Waluyo I, Wang B M, Hunt A, Yao X H, Lee J, Xue W J, Li J 2019 Nat. Energy 4 1049
 Google Scholar
Google Scholar
[9] Yan P, Zheng J, Tang Z K, Devaraj A, Chen G, Amine K, Zhang J G, Liu L M, Wang C 2019 Nat. Nanotechnol. 14 602
 Google Scholar
Google Scholar
[10] Lee E, Persson K A 2014 Adv. Energy Mater. 4 1400498
 Google Scholar
Google Scholar
[11] Hoang K 2015 Phys. Rev. Appl. 3 024013
 Google Scholar
Google Scholar
[12] 史晓红, 陈京金, 曹昕睿, 吴顺情, 朱梓忠 2022 71 178202
 Google Scholar
Google Scholar
Shi X H, Chen J J, Cao X R, Wu S Q, Zhu ZZ 2022 Acta Phy. Sin. 71 178202
 Google Scholar
Google Scholar
[13] Michaud-Rioux V, Zhang L, Guo H 2016 J. Comput. Phys. 307 593
 Google Scholar
Google Scholar
[14] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[15] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[16] Dudarev S L, Botton G A, Savrasov S Y, Humphreys C J, Sutton A P 1998 Phys. Rev. B 57 1505
 Google Scholar
Google Scholar
[17] Xiao R, Li H, Chen L 2012 Chem. Mater. 24 4242
 Google Scholar
Google Scholar
[18] Jain A, Hautier G, Ong S P, Moore C J, Fischer C C, Persson K A, Ceder G 2011 Phys. Rev. B 84 045115
 Google Scholar
Google Scholar
[19] Chen Y F, Jiang X, Li Y Y, Li P, Liu Q C, Fu G T, Xu L, Sun D M, Tang Y W 2018 Adv. Mater. Interfaces 5 1701015
 Google Scholar
Google Scholar
[20] Zheng H F, Hu Z Y, Liu P F, Xu W J, Xie Q S, He W, Luo Q, Wang L S, Gu D D, Qu B H, Zhu Z Z, Peng D L 2020 Energy Storage Mater. 25 76
 Google Scholar
Google Scholar
[21] Nakamura T, Ohta K, Hou X Y, Kimura Y, Tsuruta K, Tamenori Y, Aso R, Yoshida H, Amezawa K 2021 J. Mater. Chem. A 9 3657
 Google Scholar
Google Scholar
[22] Limpijumnong S, Van de Walle C G 2004 Phys. Rev. B 69 035207
 Google Scholar
Google Scholar
[23] Ouyang C Y, Šljivančanin Ž, Baldereschi A 2009 Phys. Rev. B 79 235410
 Google Scholar
Google Scholar
[24] Reuter K, Scheffler M 2001 Phys. Rev. B 65 035406
 Google Scholar
Google Scholar
[25] Hu W, Wang H W, Luo W W, Xu B, Ouyang C Y 2020 Solid State Ionics 347 115257
 Google Scholar
Google Scholar
[26] Park J H, Lim J, Yoon J, K S, Gim J, Song J, Park H, Im D, Park M, Ahn D, Paik Y, Kim J 2012 Dalton Trans. 41 3053
-
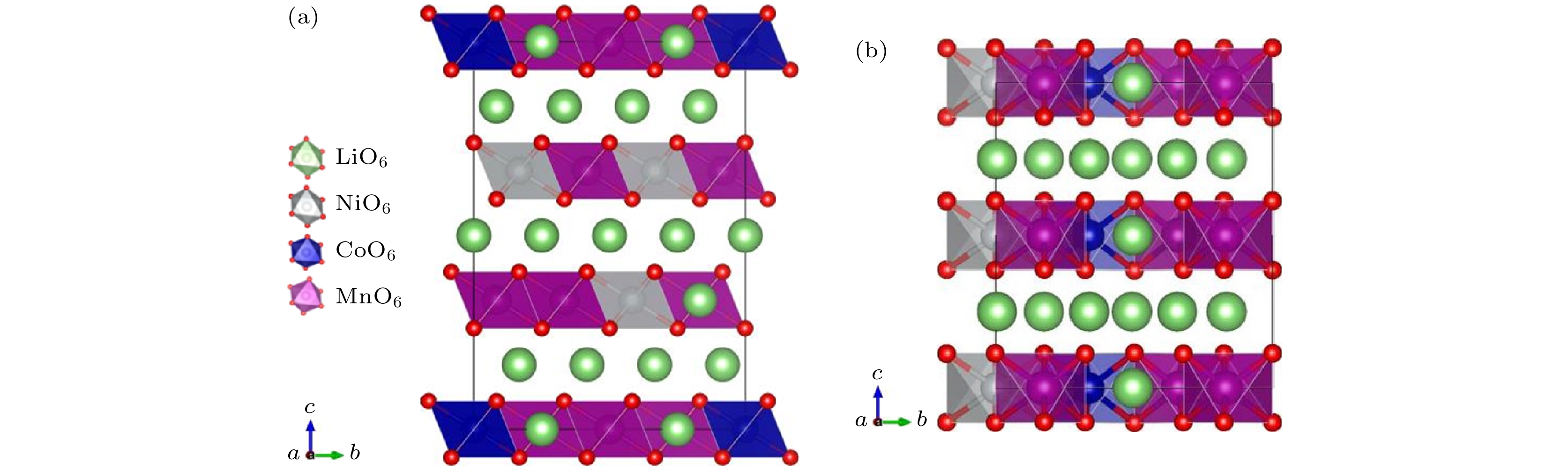
图 1 两种富锂锰基三元正极材料的结构对比 (a) Li1.2Ni0.32Co0.04Mn0.44O2的晶体结构图; (b) Li1.167Ni0.167Co0.167Mn0.5O2的晶体结构图. 不同颜色的八面体分别表示: NiO6灰色、CoO6蓝色、MnO6紫色. 氧为红色小球, 锂为绿色的球
Fig. 1. Comparison of the structures for two Li-rich Mn-based ternary cathode materials. Crystal structures of (a) Li1.2Ni0.32Co0.04Mn0.44O2 and (b) Li1.167Ni0.167Co0.167Mn0.5O2. The octahedra are denoted by different colors: NiO6 gray, CoO6 blue, and MnO6 purple. Oxygen is given by red balls, lithium is shown by green balls.
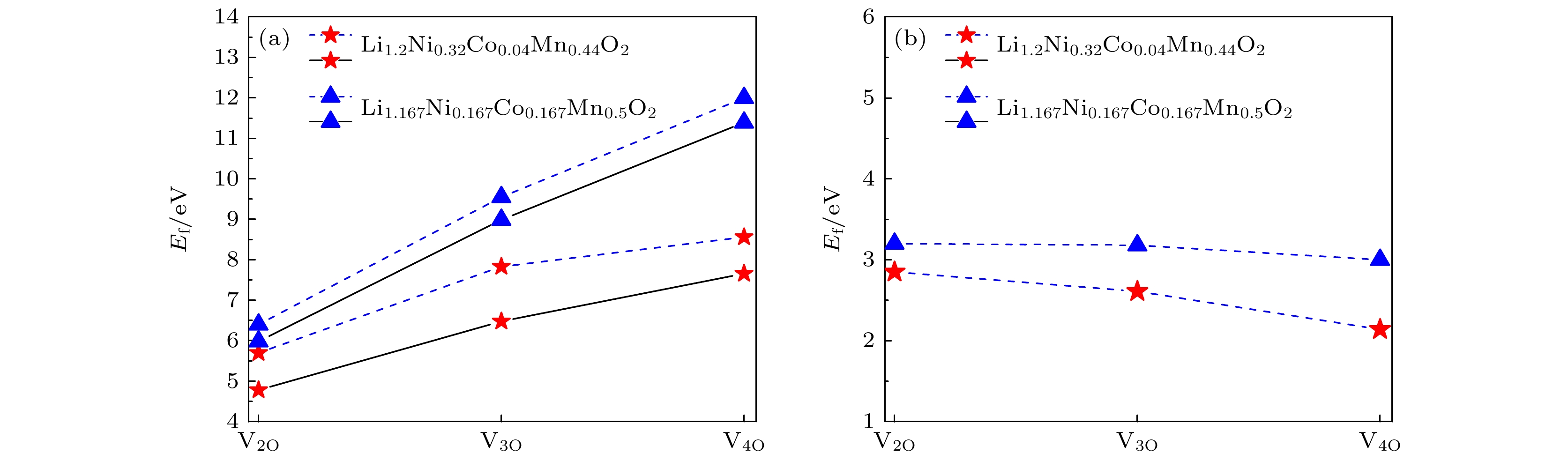
图 2 两种三元材料中 (a) 双空位、三空位和四空位(V2O, V3O和V4O)的形成能. 虚线表示同时拿掉n个氧的空位族形成能, 实线表示分别一个一个拿掉氧的空位族形成能; (b) 双空位、三空位和四空位平均到形成一个氧空位的形成能
Fig. 2. Formation energies of oxygen of (a) bivacancy, trivacancy and quadruvacancy (V2O, V3O and V4O) in two materials. The dashed line represents the vacancy formation energy of n oxygens detached at the same time, and the solid line represents the formation energy of oxygen detached one by one. (b) The formation energies of the double, triple and quadruple vacancy averaged to a single oxygen.
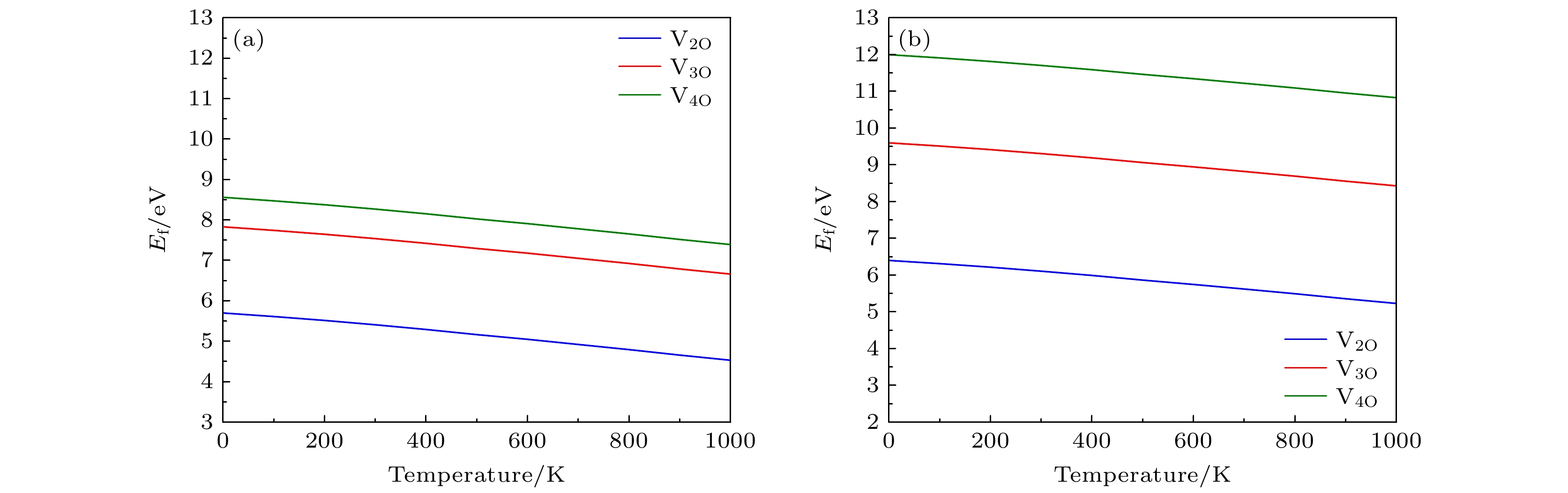
图 3 (a) Li1.2Ni0.32Co0.04Mn0.44O2 和 (b) Li1.167Ni0.167Co0.167Mn0.5O2材料中氧空位簇的形成能随温度的变化关系(氧分压为P = 0.2 bar (1 bar=1×105 Pa)
Fig. 3. Formation energy of oxygen vacancy clusters versus temperatures in (a) Li1.2Ni0.32Co0.04Mn0.44O2 and (b) Li1.167Ni0.167Co0.167Mn0.5O2 (oxygen partial pressure is P = 0.2 bar(1 bar=1×105 Pa).
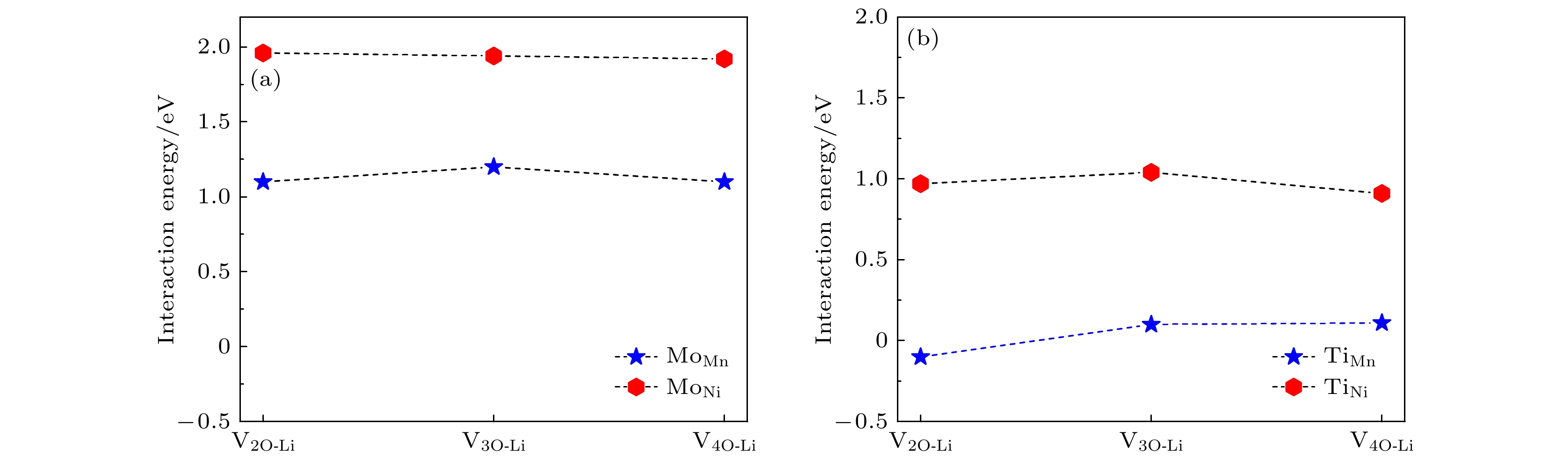
图 7 Li1.2Ni0.32Co0.04Mn0.44O2材料中, V2O-Li, V3O-Li和V4O-Li空位簇与替位点缺陷的相互作用能 (a) 过渡金属Ni或Mn被Mo替位; (b) 过渡金属Ni或Mn被Ti替位
Fig. 7. Interaction energies between the V2O-Li, V3O-Li, V4O-Li vacancy clusters and the substitutional defects in Li1.2Ni0.32Co0.04Mn0.44O2: (a) Transition metal Ni or Mn substituted by Mo; (b) transition metal Ni or Mn substituted by Ti.
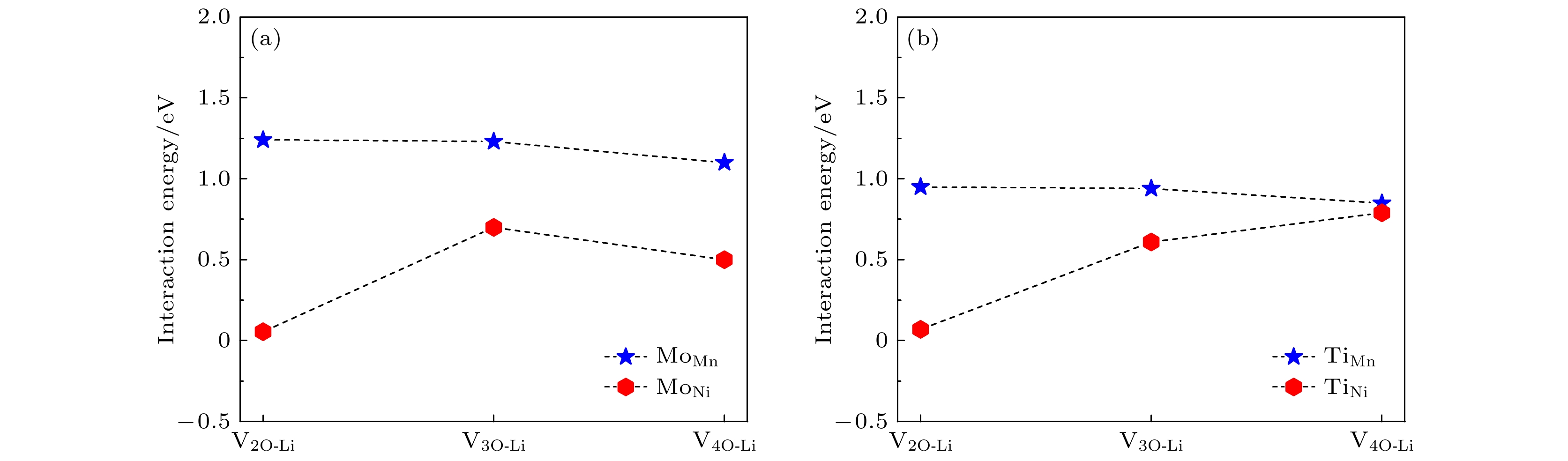
图 8 Li1.167Ni0.167Co0.167Mn0.5O2材料中, V2O-Li, V3O-Li和V4O-Li空位簇与替位点缺陷的相互作用能 (a) 过渡金属Ni或Mn被Mo替位; (b) 过渡金属Ni或Mn被Ti替位
Fig. 8. Interaction energies between the V2O-Li, V3O-Li, V4O-Li vacancy clusters and the substitutional defects in Li1.167Ni0.167Co0.167Mn0.5O2: (a) Transition metal Ni or Mn substituted by Mo; (b) transition metal Ni or Mn substituted by Ti.
表 1 Li1.2Ni0.32Co0.04Mn0.44O2和Li1.167Ni0.167Co0.167Mn0.5O2三元材料中不等价单氧空位的形成能
Table 1. Formation energy of a single oxygen vacancy in Li1.2Ni0.32Co0.04Mn0.44O2 and Li1.167Ni0.167Co0.167Mn0.5O2 ternary materials.
单个氧空位的形成能 /eV Li1.2Ni0.32Co0.04Mn0.44O2 Li1.167Ni0.167Co0.167Mn0.5O2 VO1 VO2 VO3 VO4 VO5 VO6 VO7 VO8 VO1 VO2 VO3 VO4 2.0 3.31 2.17 4.43 4.15 3.03 2.86 4.27 2.3 2.80 3.12 3.20 表 2 Li1.2Ni0.32Co0.04Mn0.44O2和Li1.167Ni0.167Co0.167Mn0.5O2三元材料中氧空位族中的氧原子被一个一个分别脱去时的形成能
Table 2. Formation energy of an oxygen vacancy in vacancy clusters in Li1.2Ni0.32Co0.04Mn0.44O2 and Li1.167Ni0.167Co0.167Mn0.5O2 ternary materials, when the oxygen atoms are extracted one by one, respectively.
各个单氧空位的形成能 /eV 氧空位的序号(第n个氧) 1 2 3 4 Li1.2Ni0.32Co0.04Mn0.44O2 2.00 2.78 1.70 1.14 Li1.167Ni0.167Co0.167Mn0.5O2 2.80 3.17 3.02 2.40 -
[1] Dunn B, Kamath H, Tarascon J M 2011 Science 334 928
 Google Scholar
Google Scholar
[2] Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D 2011 Energy Environ. Sci. 4 3243
 Google Scholar
Google Scholar
[3] Nitta N, Wu F, Lee J T, Yushin G 2015 Mater. Today 18 252
 Google Scholar
Google Scholar
[4] Yuan L X, Wang Z H, Zhang W X, Hu X L, Chen J T, Huang Y H, Goodenough J B 2011 Energy Environ. Sci. 4 269
 Google Scholar
Google Scholar
[5] Liu J L, Hou M Y, Yi J, Guo S S, Wang C X, Xia Y Y 2014 Energy Environ. Sci. 7 705
 Google Scholar
Google Scholar
[6] Csernica P M, Kalirai S S, Gent W E, Lim K, Yu Y S, Liu Y, Ahn S J, Kaeli E, Xu X, Stone K H, Marshall A F, Sinclair R, Shapiro D A, Toney M F, Chueh W C 2021 Nat. Energy 6 642
 Google Scholar
Google Scholar
[7] Hu E Y, Yu X Q, Lin R Q, Bi X X, Lu J, Bak S M, Nam K W, Xin H L, Jaye C, Fischer D A, Amine K, Yang X Q 2018 Nat. Energy 3 690
 Google Scholar
Google Scholar
[8] Zhu Z, Yu D W, Yang Y, Su C, Huang Y M, Dong Y H, Waluyo I, Wang B M, Hunt A, Yao X H, Lee J, Xue W J, Li J 2019 Nat. Energy 4 1049
 Google Scholar
Google Scholar
[9] Yan P, Zheng J, Tang Z K, Devaraj A, Chen G, Amine K, Zhang J G, Liu L M, Wang C 2019 Nat. Nanotechnol. 14 602
 Google Scholar
Google Scholar
[10] Lee E, Persson K A 2014 Adv. Energy Mater. 4 1400498
 Google Scholar
Google Scholar
[11] Hoang K 2015 Phys. Rev. Appl. 3 024013
 Google Scholar
Google Scholar
[12] 史晓红, 陈京金, 曹昕睿, 吴顺情, 朱梓忠 2022 71 178202
 Google Scholar
Google Scholar
Shi X H, Chen J J, Cao X R, Wu S Q, Zhu ZZ 2022 Acta Phy. Sin. 71 178202
 Google Scholar
Google Scholar
[13] Michaud-Rioux V, Zhang L, Guo H 2016 J. Comput. Phys. 307 593
 Google Scholar
Google Scholar
[14] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[15] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[16] Dudarev S L, Botton G A, Savrasov S Y, Humphreys C J, Sutton A P 1998 Phys. Rev. B 57 1505
 Google Scholar
Google Scholar
[17] Xiao R, Li H, Chen L 2012 Chem. Mater. 24 4242
 Google Scholar
Google Scholar
[18] Jain A, Hautier G, Ong S P, Moore C J, Fischer C C, Persson K A, Ceder G 2011 Phys. Rev. B 84 045115
 Google Scholar
Google Scholar
[19] Chen Y F, Jiang X, Li Y Y, Li P, Liu Q C, Fu G T, Xu L, Sun D M, Tang Y W 2018 Adv. Mater. Interfaces 5 1701015
 Google Scholar
Google Scholar
[20] Zheng H F, Hu Z Y, Liu P F, Xu W J, Xie Q S, He W, Luo Q, Wang L S, Gu D D, Qu B H, Zhu Z Z, Peng D L 2020 Energy Storage Mater. 25 76
 Google Scholar
Google Scholar
[21] Nakamura T, Ohta K, Hou X Y, Kimura Y, Tsuruta K, Tamenori Y, Aso R, Yoshida H, Amezawa K 2021 J. Mater. Chem. A 9 3657
 Google Scholar
Google Scholar
[22] Limpijumnong S, Van de Walle C G 2004 Phys. Rev. B 69 035207
 Google Scholar
Google Scholar
[23] Ouyang C Y, Šljivančanin Ž, Baldereschi A 2009 Phys. Rev. B 79 235410
 Google Scholar
Google Scholar
[24] Reuter K, Scheffler M 2001 Phys. Rev. B 65 035406
 Google Scholar
Google Scholar
[25] Hu W, Wang H W, Luo W W, Xu B, Ouyang C Y 2020 Solid State Ionics 347 115257
 Google Scholar
Google Scholar
[26] Park J H, Lim J, Yoon J, K S, Gim J, Song J, Park H, Im D, Park M, Ahn D, Paik Y, Kim J 2012 Dalton Trans. 41 3053
计量
- 文章访问数: 8603
- PDF下载量: 201
- 被引次数: 0















 下载:
下载: