-
为了加快镁离子电池的开发与应用, 寻找合适的镁离子电池阳极材料势在必行. 此外, 具有较低摩尔质量的阳极材料有利于获得较高的理论存储容量. 因此, 本文采用基于密度泛函理论的第一性原理计算系统地研究了BeB2单层材料作为镁离子电池阳极的潜力. 计算结果表明, 基于声子谱检测, BeB2结构展现了优异的动力学稳定性. 此外, 从BeB2的能带结构可以看到清晰的狄拉克锥, 表明其具有良好的导电性能. BeB2可以稳定吸附镁离子, 并且镁离子在该材料上表现了较低的扩散势垒 (0.04 eV), 这意味着更快的充放电速率. 重要的是, BeB2展现了超高的理论容量 (5250 mA·h·g–1)、较低平均开路电压 (0.33 V)以及较小的体积膨胀 (2%). 此外, Mg离子在双层BeB2结构中的吸附能为–1.38 — –2.24 eV, 扩散势垒为0.134 — 0.84 eV. 综合以上性能, 我们相信BeB2可以作为一种优秀的镁离子电池阳极材料.
Rechargeable lithium-ion batteries as the main energy storage equipment should possess high power density, excellent reversible capacity, and long cycle life. However, due to the high cost and dendrite growth of Li, searching for non-Li-ion batteries is urgent. Compared with lithium, magnesium has abundant resources, small ionic radius, and high energy density. Therefore, magnesium-ion batteries (MIBs) can serve as the next generation metal-ion batteries. Two-dimensional materials based on Be or B element acting as the anode of metal-ion batteries always exhibit high theoretical storage capacity. Using first-principles calculations, we systematically explore the potential of BeB2 as MIBs anode. The optimized BeB2 monolayer structure shown in Fig. (a) consists of two atomic layers, where each Be atom is coordinated with six B atoms, and each B atom is coordinated with three Be atoms. The lattice constants are a = b = 3.037 Å with a thickness of 0.554 Å. From the phonon spectrum calculations, the absence of imaginary modes indicates the dynamic stability of BeB2 monolayer. The presence of a Dirac cone further suggests the excellent conductivity (Fig.(b)). Three stable adsorption sites (Be1: top of Be atoms; Be2 and B2: bottom of Be and B atoms) are labeled in Fig. (a). Taking symmetry into account, we consider three pathways to evaluate the migration of Mg atom on BeB2 monolayer (Fig.(c)). The corresponding lowest diffusion energy barrier is 0.04 eV along Path III. The stable configuration with the maximum adsorption Mg concentration is shown in Fig.(d), which generates a theoretical capacity of 5250 mA·h·g–1. The calculated average open-circuit voltage is 0.33 V. Based on ab initio molecular dynamics simulations, the total energy of BeB2, with Mg adsorbed, fluctuates within a narrow range, suggesting that BeB2 can sustain structural stability after storing Mg at room temperature (Fig.(e)). Finally, for practical application, we investigate the adsorption and diffusion behavior of Mg on bilayer BeB2. Three configurations are considered: AA stacking (overlapping of Be atoms in upper layer with Be atoms in lower layer), AB stacking (overlapping of Be atoms in upper layer with B atoms in lower layer), and AC stacking (overlapping of Be atoms in upper layer with B—B bonds in lower layer). The most stable configuration is AB stacking (shown in Fig.(f)) with the interlayer spacing of 3.12 Å and the binding energy of –120.97 meV/atom. Comparing with the BeB2 monolayer structure, the adsorption energy of Mg is –2.24 eV for Be1, –1.38 eV for B5 site, and –1.90 eV for B4 site, while the lowest diffusion energy barrier is 0.13 eV along the path of B5-Be3-B5. Therefore, according to the above-mentioned properties, we believe that BeB2 monolayer can serve as an excellent MIBs anode material. [1] Perveen T, Siddiq M, Shahzad N, Ihsan R, Ahmad A, Shahzad M I 2020 Renewable Sustainable Energy Rev. 119 109549
 Google Scholar
Google Scholar
[2] Ma Y, Doeff M M, Visco S J, Jonghe L C D 1993 J. Electrochem. Soc. 140 2726
 Google Scholar
Google Scholar
[3] Hwang J Y, Myung S T, Sun Y K 2017 Chem. Soc. Rev. 46 3529
 Google Scholar
Google Scholar
[4] Rajagopalan R, Tang Y G, Ji X B, Jia C K, Wang H Y 2020 Adv. Funct. Mater. 30 1909486
 Google Scholar
Google Scholar
[5] Lin J Y, Yu T, Han F J J, Yang G C 2020 Wiley Interdiscip. Rev. Comput. Mol. Sci. 10 e1473
 Google Scholar
Google Scholar
[6] Mortazavi B, Rahaman O, Ahzi S, Rabczuk T 2017 Appl. Mater. Today 8 60
 Google Scholar
Google Scholar
[7] Yeoh K H, Chew K H, Chu Y Z, Yoon T L, Rusi, Ong D S 2019 J. Appl. Phys. 126 125302
 Google Scholar
Google Scholar
[8] Ullah S, Denis P A, Sato F 2017 Appl. Mater. Today 9 333
 Google Scholar
Google Scholar
[9] Ye X J, Gao Q, Cao H B, Wang X H, Liu C S 2023 Appl. Phys. Lett. 122 223902
 Google Scholar
Google Scholar
[10] Wan M Q, Zhao S Q, Zhang Z Y, Zhou N G 2022 J. Phys. Chem. C 126 9642
 Google Scholar
Google Scholar
[11] Wu Y, Hou J 2022 Phys. Chem. Chem. Phys. 24 14953
 Google Scholar
Google Scholar
[12] Segall M D, Lindan P J D, Probert M J, Pickard C J, Hasnip P J, Clark S J, Payne M C 2002 J. Phys. Condens. Matter 14 2717
 Google Scholar
Google Scholar
[13] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[14] Zhang G X, Tkatchenko A, Paier J, Appel H, Scheffler M 2011 Phys. Rev. Lett. 107 245501
 Google Scholar
Google Scholar
[15] Tkatchenko A, Scheffler M 2009 Phys. Rev. Lett. 102 073005
 Google Scholar
Google Scholar
[16] Govind N, Petersen M, Fitzgerald G, King-Simith D 2003 Comput. Mater. Sci. 28 250
 Google Scholar
Google Scholar
[17] Henkelman G, Jónsson H 2000 J. Chem. Phys. 113 9978
 Google Scholar
Google Scholar
[18] Gao Q, Ye X J, Liu C S 2023 Phys. Chem. Chem. Phys. 25 6519
 Google Scholar
Google Scholar
[19] Panigrahi P, Mishra S B, Hussain T, Nanda B. R. K, Ahuja Rajeev 2020 ACS Appl. Nano Mater. 3 9055
 Google Scholar
Google Scholar
[20] Xiao C, Tang X Q, Peng J F, Ding Y H 2021 Appl. Surf. Sci. 563 150278
 Google Scholar
Google Scholar
[21] Khan A A, Muhammad I, Ahmad R, Ahmad L 2021 Ionics 27 4819
 Google Scholar
Google Scholar
[22] Wu D H, Yang B C, Zhang S R, Ruckenstein E Chen H Y 2021 J. Colloid Interface Sci. 597 401
 Google Scholar
Google Scholar
[23] Shakerzadeh E, Kazemimoghadam F 2021 Appl. Surf. Sci. 538 148060
 Google Scholar
Google Scholar
[24] Xiong F Y, Jiang Y L, Cheng L, Yu R H, Tan S S, Tang C, Zuo C L, An Q Y, Zhao Y L, Gaumet J J, Mai L Q 2022 Interdiscip. Mater. 1 140
 Google Scholar
Google Scholar
[25] Zhang Z H, Song M J, Si C H, Cui W R, Wang Y 2023 eScience 3 100070
 Google Scholar
Google Scholar
[26] Malyi O. I, Tan T. L, Manzhos S 2013 J. Power Sources 233 341
 Google Scholar
Google Scholar
[27] Wang L, Welborn S S, Kumar H, Li M N, Wang Z Y, Shenoy V B, Detsi E 2019 Adv. Energy Mater. 9 1902086
 Google Scholar
Google Scholar
[28] Shao Y Y, Gu M, Li X Y, Nie Z M, Zuo P J, Li G S, Liu T B, Xiao J Cheng Y W, Wang C M, Zhang J G, Liu J 2014 Nano Lett. 14 255
 Google Scholar
Google Scholar
[29] God C, Bitschnau B, Kapper K, Lenardt C, Schmuck M , Mautner F, Koller S 2017 RSC Adv. 7 14168
[30] Penki T R, Valurouthu G, Shivakumara S, Sethuraman V A, Munichandraiah N 2018 New J. Chem. 42 5996
 Google Scholar
Google Scholar
-
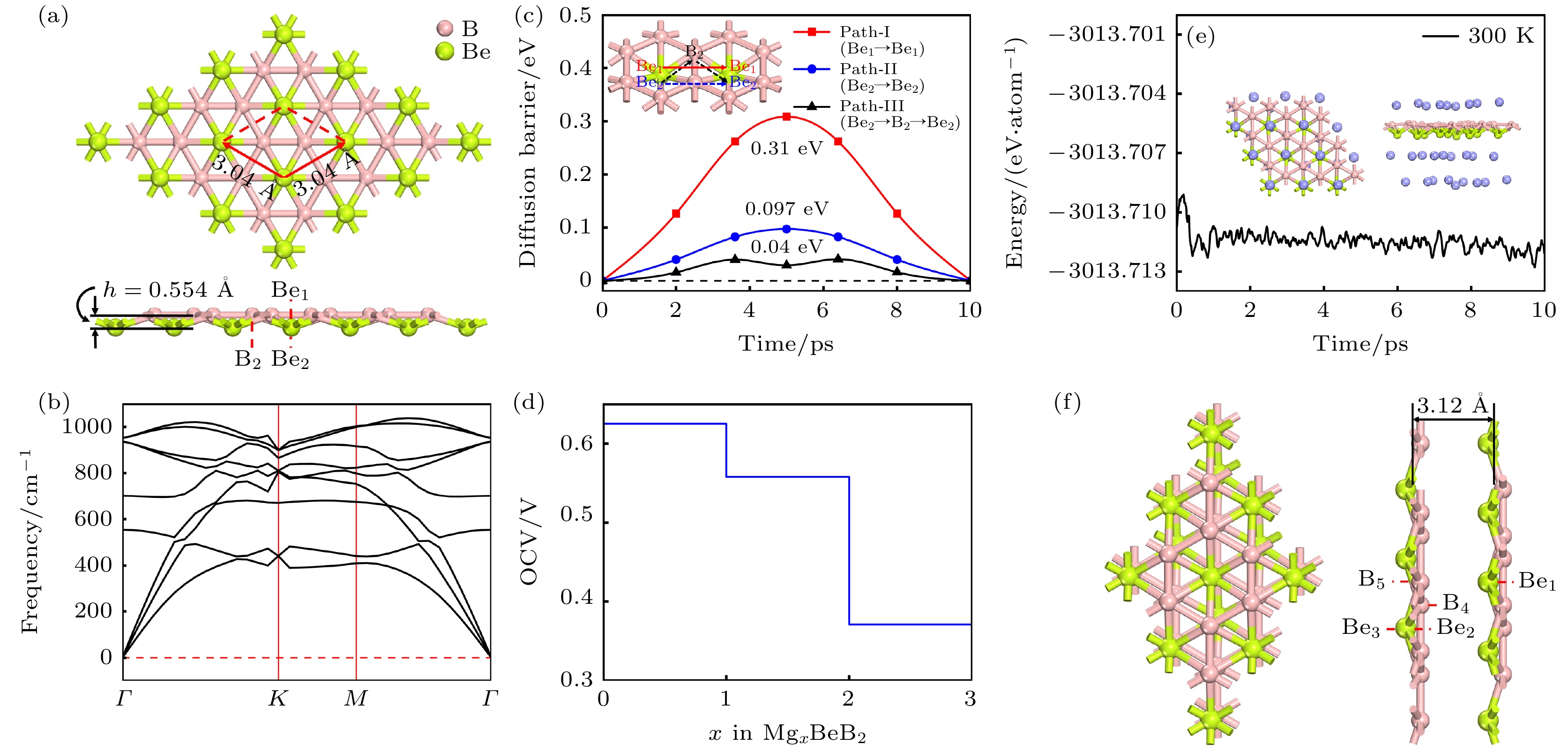
图 1 (a) BeB2单层结构的俯视图及侧视图, 红色框架展示了BeB2单层的初级原胞结构, h表示 BeB2单层的厚度; BeB2单层的声子谱(b)、能带结构(c)以及分态密度图(d)
Fig. 1. (a) Top and side views of BeB2 monolayer. Red frame represents the primary cell structure of BeB2, and h represents the thickness of BeB2 monolayer. (b) Phonon spectra, (c) electronic band structures, (d) partial density of states of BeB2 monolayer.
图 4 Mg2+在 BeB2表面的扩散路径俯视图, 分别对应路径 Ⅰ (a), II (c), Ⅲ (e); 对应路径的扩散势垒 路径Ⅰ (b), II (d), Ⅲ (f). 插图为 Mg2+扩散的侧视图
Fig. 4. (a), (c), (e) Top views of Mg2+ diffusion paths on BeB2 surface, corresponding to path Ⅰ, II, and Ⅲ, respectively; (b), (d), (e) diffusion barriers of path Ⅰ, II, and Ⅲ. Insets are side views of Mg2+ diffusion pathways.
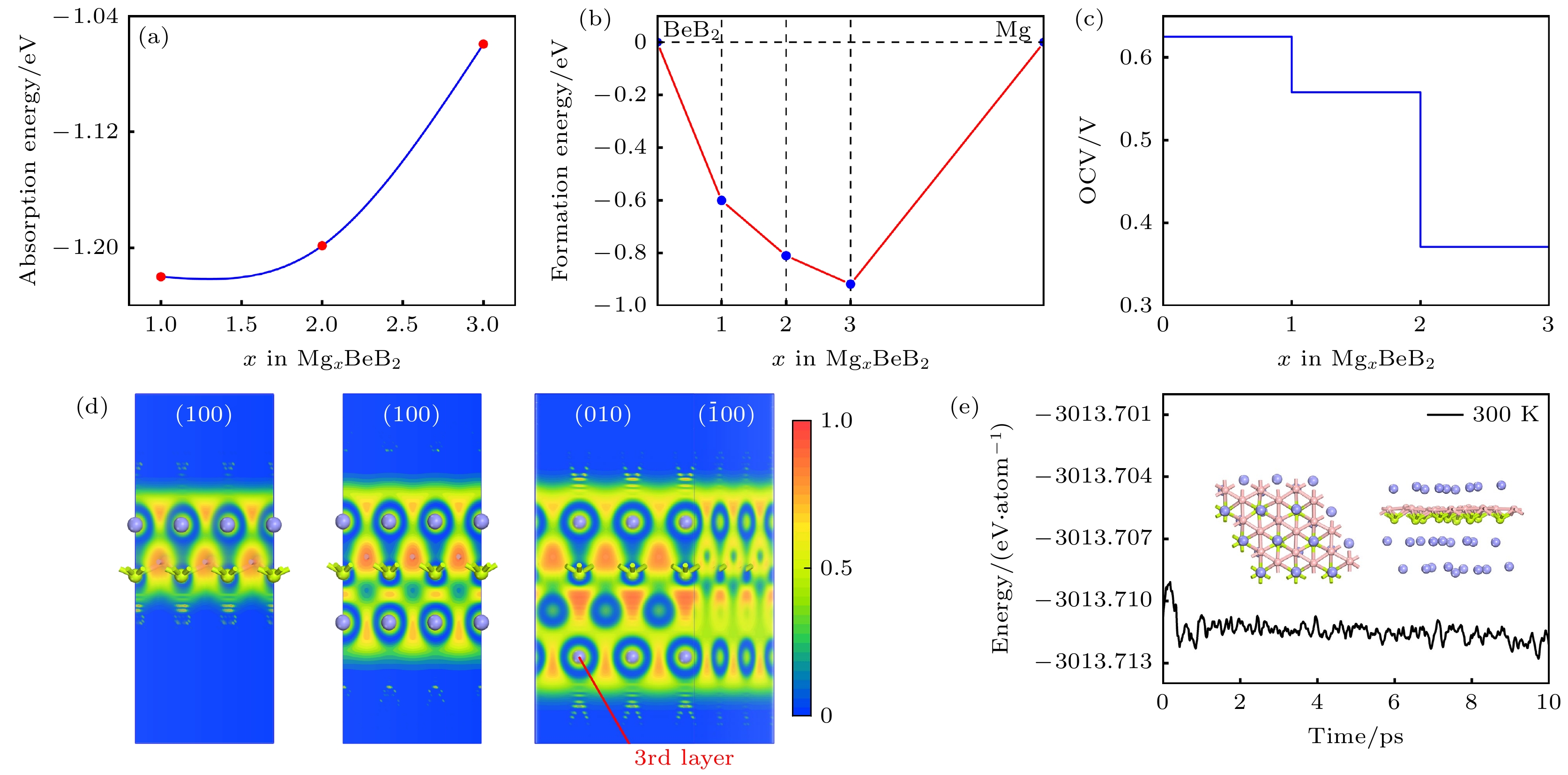
图 6 (a) 平均吸附能随吸附浓度变化的曲线; (b) MgxBeB2形成能曲线; (c) MgBeB2, Mg2BeB2和 Mg3BeB2的ELF侧视图; (d) 吸附Mg2+的BeB2单层的开路电压; (e) Mg3BeB2在 300 K条件下分子动力学模拟 10 ps 后的能量曲线, 插图为模拟结束时体系的俯视图及侧视图
Fig. 6. (a) Variation of average adsorption energy with the concentration of adsorbed Mg; (b) formation energy of MgxBeB2; (c) side views of electron function localization for MgBeB2, Mg2BeB2 and Mg3BeB2, respectively; (d) OCV of BeB2 monolayer with different Mg concentration; (e) total energy variation of Mg3BeB2 during ab initio molecular dynamics at 300 K. Inset exhibits the top and side snapshots at the end of 10 ps.
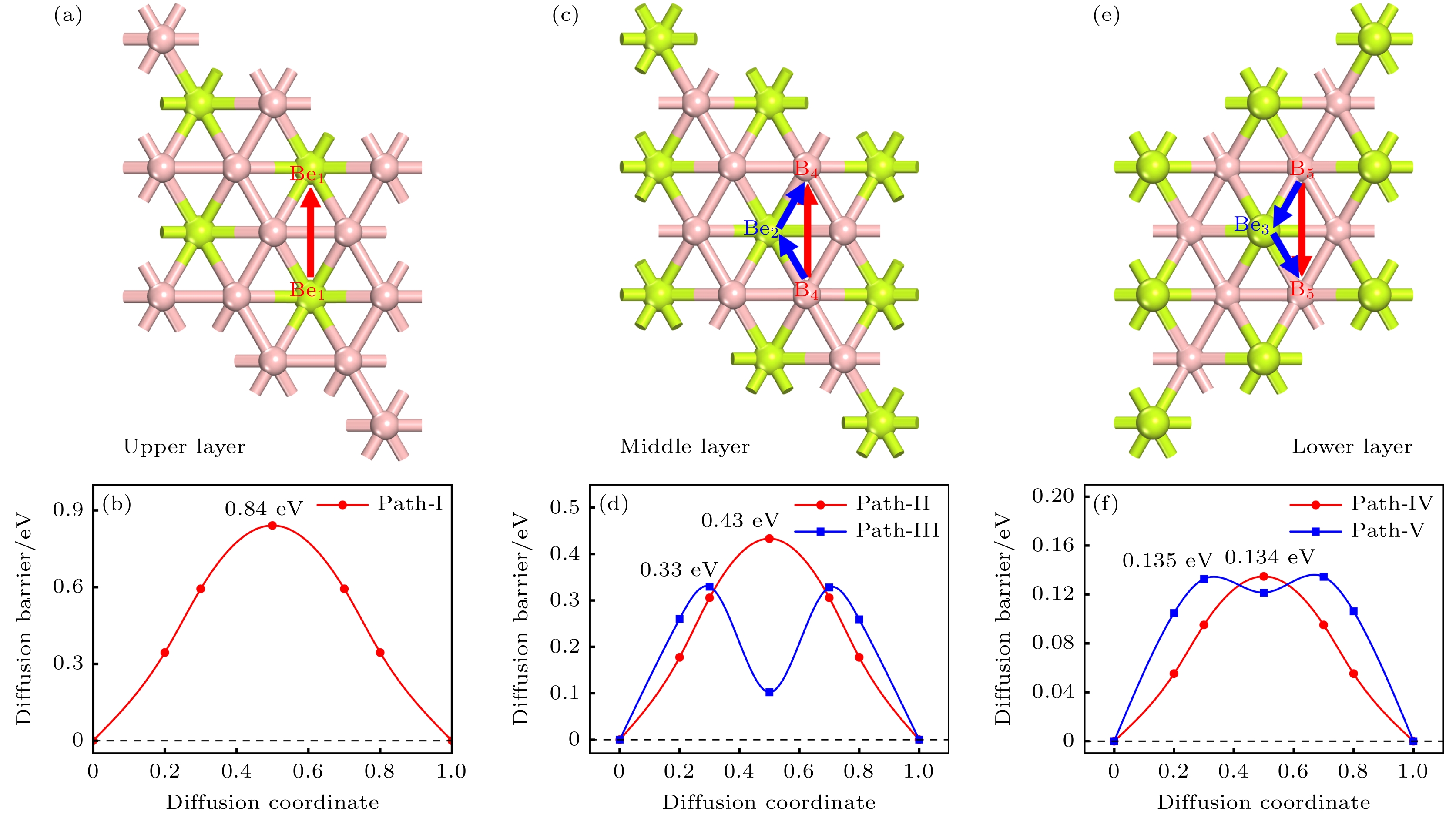
图 9 双层BeB2各层的扩散路径俯视图, 分别对应上层 (a)、中层(c)、下层(e), 以及对应路径的扩散势垒: 上层(b)、中层(d)、下层(f)
Fig. 9. Top view of diffusion paths in each region of bilayer BeB2 in AB stacking, corresponding to the upper layer (a), middle layer (c), and lower layer (e); the diffusion barriers corresponding to the paths: upper layer (b), middle layer (d), and lower layer (f).
表 1 Mg2+在 BeB2表面上不同吸附点位的吸附能及转移电荷数量
Table 1. Adsorption energy at different sites and the charge transfers of Mg2+ on BeB2 monolayer.
Adsorption site Eads/eV ΔQ/e Be1 –2.67 0.29 Be2 –2.24 0.20 B2 –2.16 0.18 表 2 几种镁离子电池阳极材料与BeB2的性能对比 (*标识为实验数据)
Table 2. Comparison of several two-dimensional materials with BeB2 as MIBs anode (*experimental results).
阳极材料 吸附能/eV 扩散势垒/eV 理论容量/(mA·h·g–1) 平均开路电压/V 体积膨胀率/% BeB2 –2.67 0.04 5250 0.33 2 β12 borophene[6] –0.696 0.97 2480 — — χ3 borophene[6] –0.199 — 2400 — — Be2B[9] –0.7 0.1 7436 0.29 0.3 α-beryllene[18] –0.24 0.099 5956 0.24 –0.18 Si2BN[19] –1.22 0.08 648 0.67 — BSi[20] –2.34 0.86 2749 0.84 — Arsenene[21] 2.48 0.21 1429 0.83 <16 B-MoS2[22] –0.024 0.6 921 0.154 2.67 B40[23] — 0.20 744 5.5 — *TiP2O7[24] — 0.62 72* 2.4* 3.2* *Ge57Bi43[25] — — 847.5* 0.32—0.35* — Ge[26] — 0.7 1476 0.241 –178 *Mg2Ga5[27] — — 290* 0.01—0.7* — *Nano-Bi[28] — — 350* 0—0.25* — *石墨[29] — — 22* 0.15* — *RGO/Bi[30] — — 372* 0.25* — -
[1] Perveen T, Siddiq M, Shahzad N, Ihsan R, Ahmad A, Shahzad M I 2020 Renewable Sustainable Energy Rev. 119 109549
 Google Scholar
Google Scholar
[2] Ma Y, Doeff M M, Visco S J, Jonghe L C D 1993 J. Electrochem. Soc. 140 2726
 Google Scholar
Google Scholar
[3] Hwang J Y, Myung S T, Sun Y K 2017 Chem. Soc. Rev. 46 3529
 Google Scholar
Google Scholar
[4] Rajagopalan R, Tang Y G, Ji X B, Jia C K, Wang H Y 2020 Adv. Funct. Mater. 30 1909486
 Google Scholar
Google Scholar
[5] Lin J Y, Yu T, Han F J J, Yang G C 2020 Wiley Interdiscip. Rev. Comput. Mol. Sci. 10 e1473
 Google Scholar
Google Scholar
[6] Mortazavi B, Rahaman O, Ahzi S, Rabczuk T 2017 Appl. Mater. Today 8 60
 Google Scholar
Google Scholar
[7] Yeoh K H, Chew K H, Chu Y Z, Yoon T L, Rusi, Ong D S 2019 J. Appl. Phys. 126 125302
 Google Scholar
Google Scholar
[8] Ullah S, Denis P A, Sato F 2017 Appl. Mater. Today 9 333
 Google Scholar
Google Scholar
[9] Ye X J, Gao Q, Cao H B, Wang X H, Liu C S 2023 Appl. Phys. Lett. 122 223902
 Google Scholar
Google Scholar
[10] Wan M Q, Zhao S Q, Zhang Z Y, Zhou N G 2022 J. Phys. Chem. C 126 9642
 Google Scholar
Google Scholar
[11] Wu Y, Hou J 2022 Phys. Chem. Chem. Phys. 24 14953
 Google Scholar
Google Scholar
[12] Segall M D, Lindan P J D, Probert M J, Pickard C J, Hasnip P J, Clark S J, Payne M C 2002 J. Phys. Condens. Matter 14 2717
 Google Scholar
Google Scholar
[13] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[14] Zhang G X, Tkatchenko A, Paier J, Appel H, Scheffler M 2011 Phys. Rev. Lett. 107 245501
 Google Scholar
Google Scholar
[15] Tkatchenko A, Scheffler M 2009 Phys. Rev. Lett. 102 073005
 Google Scholar
Google Scholar
[16] Govind N, Petersen M, Fitzgerald G, King-Simith D 2003 Comput. Mater. Sci. 28 250
 Google Scholar
Google Scholar
[17] Henkelman G, Jónsson H 2000 J. Chem. Phys. 113 9978
 Google Scholar
Google Scholar
[18] Gao Q, Ye X J, Liu C S 2023 Phys. Chem. Chem. Phys. 25 6519
 Google Scholar
Google Scholar
[19] Panigrahi P, Mishra S B, Hussain T, Nanda B. R. K, Ahuja Rajeev 2020 ACS Appl. Nano Mater. 3 9055
 Google Scholar
Google Scholar
[20] Xiao C, Tang X Q, Peng J F, Ding Y H 2021 Appl. Surf. Sci. 563 150278
 Google Scholar
Google Scholar
[21] Khan A A, Muhammad I, Ahmad R, Ahmad L 2021 Ionics 27 4819
 Google Scholar
Google Scholar
[22] Wu D H, Yang B C, Zhang S R, Ruckenstein E Chen H Y 2021 J. Colloid Interface Sci. 597 401
 Google Scholar
Google Scholar
[23] Shakerzadeh E, Kazemimoghadam F 2021 Appl. Surf. Sci. 538 148060
 Google Scholar
Google Scholar
[24] Xiong F Y, Jiang Y L, Cheng L, Yu R H, Tan S S, Tang C, Zuo C L, An Q Y, Zhao Y L, Gaumet J J, Mai L Q 2022 Interdiscip. Mater. 1 140
 Google Scholar
Google Scholar
[25] Zhang Z H, Song M J, Si C H, Cui W R, Wang Y 2023 eScience 3 100070
 Google Scholar
Google Scholar
[26] Malyi O. I, Tan T. L, Manzhos S 2013 J. Power Sources 233 341
 Google Scholar
Google Scholar
[27] Wang L, Welborn S S, Kumar H, Li M N, Wang Z Y, Shenoy V B, Detsi E 2019 Adv. Energy Mater. 9 1902086
 Google Scholar
Google Scholar
[28] Shao Y Y, Gu M, Li X Y, Nie Z M, Zuo P J, Li G S, Liu T B, Xiao J Cheng Y W, Wang C M, Zhang J G, Liu J 2014 Nano Lett. 14 255
 Google Scholar
Google Scholar
[29] God C, Bitschnau B, Kapper K, Lenardt C, Schmuck M , Mautner F, Koller S 2017 RSC Adv. 7 14168
[30] Penki T R, Valurouthu G, Shivakumara S, Sethuraman V A, Munichandraiah N 2018 New J. Chem. 42 5996
 Google Scholar
Google Scholar
计量
- 文章访问数: 4077
- PDF下载量: 307
- 被引次数: 0














 下载:
下载: