-
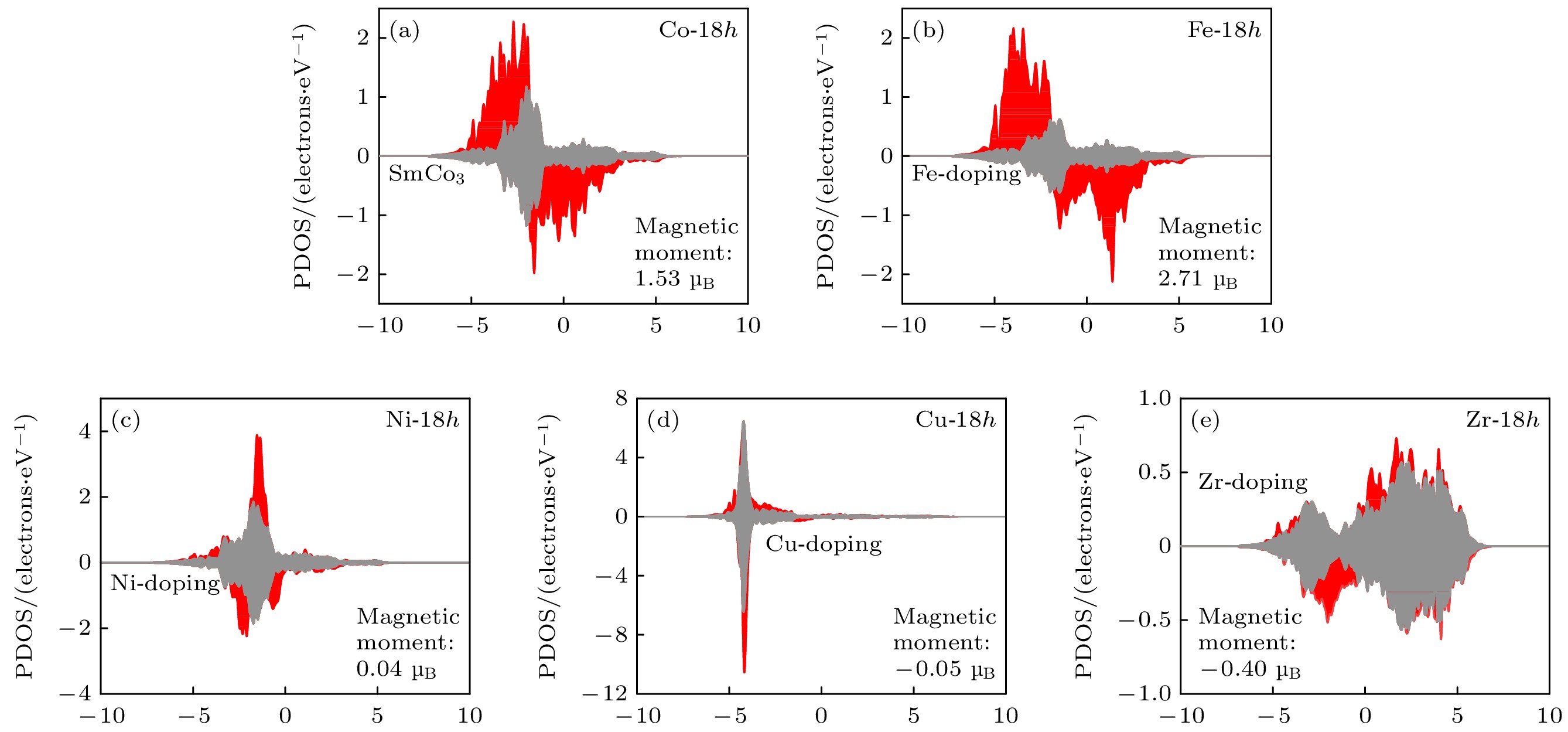
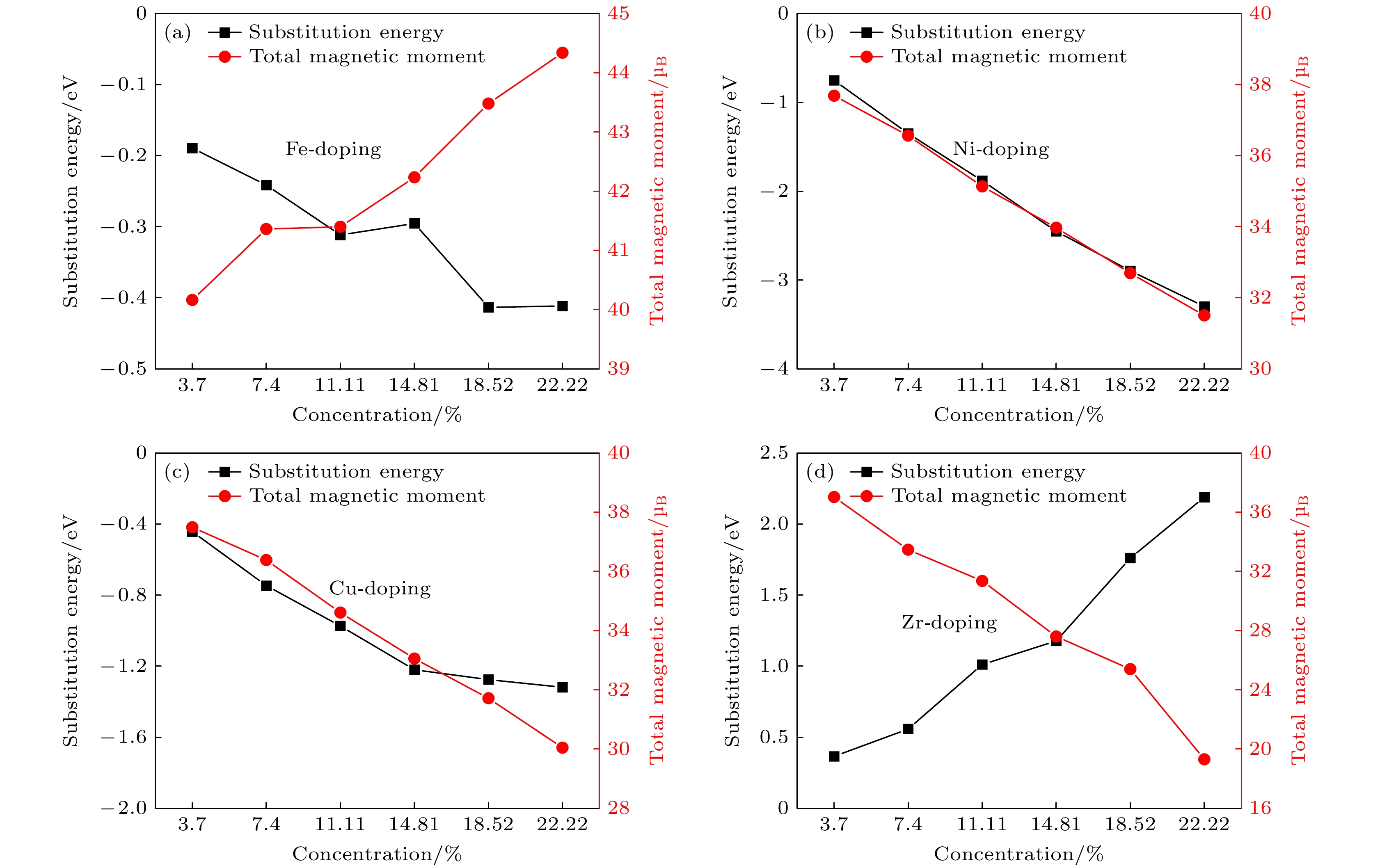
在稀土永磁材料中, Sm-Co基合金具有优异的高温磁性能, 是目前最有发展前景的永磁材料. 然而, 这些合金在高温环境的实际应用中, 由于其相对较低的饱和磁化强度和结构稳定性而受到限制. 本研究采用Fe, Ni, Cu, Zr作为代表的过渡金属元素, 通过第一性原理计算, 研究掺杂元素对SmCo3合金结构稳定性、磁性能和电子结构的影响. 计算结果表明, 元素Ni, Cu和Fe的掺杂有利于提升SmCo3体系结构稳定性, 而元素Zr的掺杂不利于体系结构稳定性. 磁性能计算表明, 掺杂非磁性元素在一定程度上会降低SmCo3体系的总磁矩, 而掺杂磁性元素可以增大SmCo3体系的总磁矩, 在SmCo3体系中并不是掺杂所有的磁性元素都可以增大体系总磁矩, 并通过电子结构的分析阐明了其微观机制. 最后筛选出了过渡元素Fe有利于提升SmCo3的磁性能和结构稳定性, 并在其原胞中掺杂原子百分比为0—22.22%范围内, 预测了其最佳掺杂原子百分比为18.52%.
-
关键词:
- SmCo3型永磁合金 /
- 第一性原理计算 /
- 过渡元素掺杂 /
- 综合磁性能
Among the spectra of rare-earth permanent magnetic materials, the Sm-Co-based alloys stand out with their excellent magnetic properties in high-temperature environments. However, the practical applications of these alloys in high-temperature settings face constraints due to their comparatively lower saturation magnetization and structural stability. In this study, Fe, Ni, Cu, and Zr are used as representative transition metal elements to investigate the effects of doping elements on the structural stability, magnetic properties, and electronic structure of SmCo3 alloy by first-principles calculations. The findings indicate that the doping of elements Ni, Cu, and Fe contributes positively to enhancing the structural stability of the SmCo3, while the introduction of Zr element has an adverse effect. Magnetic property calculations reveal that the incorporation of non-magnetic elements leads the total magnetic moment of the SmCo3 to decrease to a certain extent, whereas the introduction of magnetic elements can enhance the total magnetic moment. Notably, not all doped magnetic elements in the SmCo3 result in an increasing total magnetic moment. The underlying microscopic mechanisms are elucidated through electronic structure analysis. Finally, it is screened out that the transition element Fe is beneficial to improving the magnetic properties and structural stability of SmCo3, and the doping concentration (atomic percentage) in its unit cell ranges from 0 to 22.22%, the optimal doping concentration (atomic percentage) is predicted to be 18.52%.-
Keywords:
- SmCo3-type permanent magnet alloy /
- first-principal calculation /
- transition element doping /
- comprehensive magnetic properties
[1] Guo Z H, Pan W, Li W 2006 J. Magn. Magn. Mater. 303 396
 Google Scholar
Google Scholar
[2] 张杨, 宋晓艳, 徐文武, 张哲旭 2012 61 016102
 Google Scholar
Google Scholar
Zhang Y, Song X Y, Xu W W, Zhang Z X 2012 Acta Phys. Sin. 61 016102
 Google Scholar
Google Scholar
[3] Gutfleisch O, Willard M A, Brück E, Chen C H, Sankar S G, Liu J P 2011 Adv. Mater. 23 821
 Google Scholar
Google Scholar
[4] 周相龙, 袁涛, 宋欣, 贾文涛, 马天宇 2020 中国材料进展 39 384
 Google Scholar
Google Scholar
Zhou X L, Yuan T, Song X, Jia W T, Ma T Y 2020 Mater. China 39 384
 Google Scholar
Google Scholar
[5] 张昌文, 李华, 董建敏, 王永娟, 潘凤春, 郭永权, 李卫 2005 54 1814
 Google Scholar
Google Scholar
Zhang C W, Li H, Dong J M, Wang Y J, Pan F C, Guo Y Q, Li W 2005 Acta Phys. Sin. 54 1814
 Google Scholar
Google Scholar
[6] Xue Z Q, Liu L, Liu Z, Li M, Lee D, Chen R J, Guo Y Q, Yan A R 2016 Scr. Mater. 113 226
 Google Scholar
Google Scholar
[7] Strnat K, Hoffer G, Olson J, Ostertag W, Becker J J 1967 J. Appl. Phys. 38 1001
 Google Scholar
Google Scholar
[8] Streever R L 1979 Phys. Rev. B. 19 2704
 Google Scholar
Google Scholar
[9] Buschow K H J, Van der Goot A S 1968 J. Less Common Met. 14 323
 Google Scholar
Google Scholar
[10] Gaidukova I Y, Granovsky S A, Markosyan A S, Rodimin V E 2006 J. Magn. Magn. Mater. 301 124
 Google Scholar
Google Scholar
[11] Saito T, Nishio-Hamane D 2014 J. Alloys Compd. 585 423
 Google Scholar
Google Scholar
[12] Guo K, Lü H, Xu G J, Liu D, Wang H B, Liu X M, Song X Y 2022 Mater. Today Chem. 25 100983
 Google Scholar
Google Scholar
[13] Mao F, Lü H, Liu D, Guo K, Tang F W, Song X Y 2019 J. Alloys Compd. 810 151888
 Google Scholar
Google Scholar
[14] 毛斐, 吕皓, 唐法威, 郭凯, 刘东, 宋晓艳 2021 金属学报 57 948
 Google Scholar
Google Scholar
Mao F, Lü H, Tang F W, Guo K, Song X Y 2021 Acta. Metall. Sin. 57 948
 Google Scholar
Google Scholar
[15] Guo K, Lu H, Mao F, Liu D, Tang F W, Wang H B, Song X Y 2020 Nanoscale 12 5567
 Google Scholar
Google Scholar
[16] Wang D, Liu D, Hou C, Wang H B, Liu X, Song X Y 2017 J. Alloys Compd. 717 93
 Google Scholar
Google Scholar
[17] Das B, Choudhary R, Skomski R, Balasubramanian B, Pathak A K, Paudyal D, Sellmyer D J 2019 Phys. Rev. B 100 024419
 Google Scholar
Google Scholar
[18] Liu X B, Altounian Z 2011 Comput. Mater. Sci. 50 841
[19] Landa A, Söderlind P, Parker D, Åberg D, Lordi V, Perron A, Turchi P E A, Chouhan R K, Paudyal D, Lograsso T A 2018 J. Alloys Compd. 765 659
 Google Scholar
Google Scholar
[20] Antonioua E, Semprosa G, Gjokab M, Sarafidisa C, Polatogloua H M, Kioseogloua J 2021 J. Alloys Compd. 882 160699
 Google Scholar
Google Scholar
[21] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[22] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[23] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[24] Blöchl P E 1994 Phys. Rev. B. 50 17953
 Google Scholar
Google Scholar
[25] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[26] Perdew J P, Chevary J A, Vosko S H, Jackson K A, Pederson M R, Singh D J, Fiolhais C 1992 Phys. Rev. B 46 6671
 Google Scholar
Google Scholar
[27] Dudarev S L, Botton G A, Savrasov S Y, Humphreys C J, Sutton A P 1998 Phys. Rev. B 57 1505
 Google Scholar
Google Scholar
[28] Van der Marel D, Sawatzky G A 1988 Phys. Rev. B 37 10674
 Google Scholar
Google Scholar
[29] Zhang Z F, Guo Y Z, Robertson J 2020 Phys. Rev. Mater. 4 054603
 Google Scholar
Google Scholar
[30] Brooks M S S, Eriksson O, Wills J M, Johansson B 1997 Phys. Rev. Lett. 79 2546
[31] Georg K, Martijn M, Jurgen F 2018 User Manual for VASP Version 5.4
[32] Liu B J, Wang H, Xu C, Liu X P, Zhang Q F, Zhan T L, Stamenov P, Coey J M D, Jiang C B 2020 Phys. Rev. Mater. 4 044406
 Google Scholar
Google Scholar
[33] Söderlind P, Landa A, Locht I L, Åberg D, Kvashnin Y, Pereiro M, Däne M, Turchi P E A, Antropov V P, Eriksson O 2017 Phys. Rev. B 96 100404
 Google Scholar
Google Scholar
[34] Raja A, Adhikary T, Al-Omari I A, Das G P, Ghosh S, Satapathy D K, Oraon A, Shield J E, Aich S 2020 J. Magn. Magn. Mater. 504 166645
 Google Scholar
Google Scholar
-
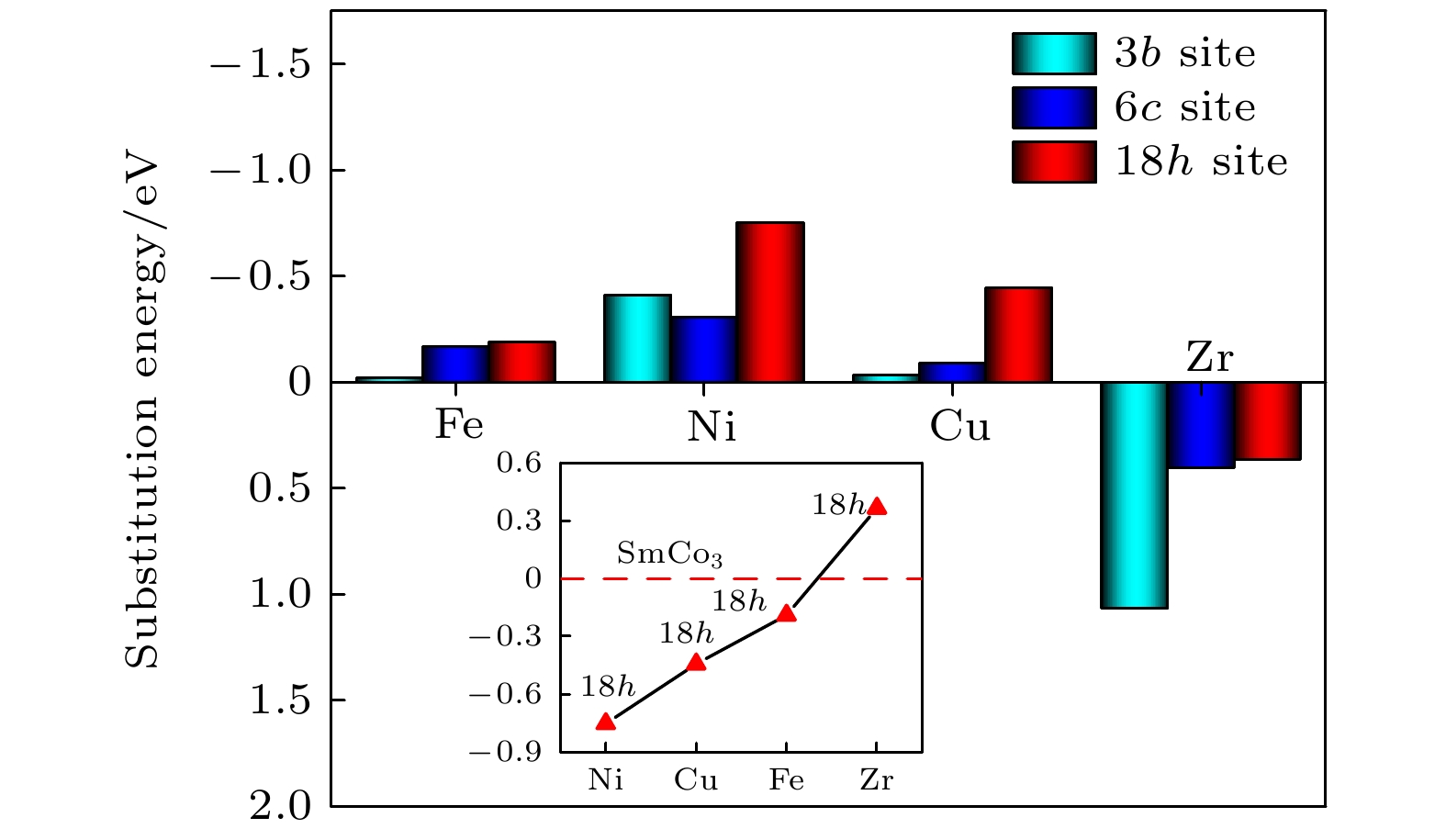
图 2 掺杂元素在3b, 6c和18h位置的替代能, 插图表示不同元素在优先占位的Sm9Co26M的替代能; 插图中红色虚线表示未掺杂SmCo3的基态能量, 其替代能为0作为参考值
Fig. 2. Substitution energy of the doping element at 3b, 6c and 18h sites, the insert shows the substitution energy of Sm9Co26M with different elements at the preferential site. The red dashed line in the illustration represents the ground state energy of undoped SmCo3, and its substitution energy is 0 eV as a reference value.
表 1 SmCo3和Sm9Co26M的晶格参数和晶胞体积
Table 1. Lattice parameters and cell volumes of SmCo3 and Sm9Co26M.
Model Lattice parameter/Å c/a V/Å3 a b c SmCo3 5.0123 5.0123 24.6424 4.9164 536.17 Experiments[10] 5.050 5.050 24.590 4.8693 543.09 Sm9Co26Ni 4.9852 4.9852 24.5962 4.9338 536.76 Sm9Co26Fe 5.0428 5.0428 24.6771 4.8935 537.62 Sm9Co26Cu 4.9793 4.9793 24.5967 4.9398 538.11 Sm9Co26Zr 5.0670 5.0670 24.8397 4.9022 548.71 -
[1] Guo Z H, Pan W, Li W 2006 J. Magn. Magn. Mater. 303 396
 Google Scholar
Google Scholar
[2] 张杨, 宋晓艳, 徐文武, 张哲旭 2012 61 016102
 Google Scholar
Google Scholar
Zhang Y, Song X Y, Xu W W, Zhang Z X 2012 Acta Phys. Sin. 61 016102
 Google Scholar
Google Scholar
[3] Gutfleisch O, Willard M A, Brück E, Chen C H, Sankar S G, Liu J P 2011 Adv. Mater. 23 821
 Google Scholar
Google Scholar
[4] 周相龙, 袁涛, 宋欣, 贾文涛, 马天宇 2020 中国材料进展 39 384
 Google Scholar
Google Scholar
Zhou X L, Yuan T, Song X, Jia W T, Ma T Y 2020 Mater. China 39 384
 Google Scholar
Google Scholar
[5] 张昌文, 李华, 董建敏, 王永娟, 潘凤春, 郭永权, 李卫 2005 54 1814
 Google Scholar
Google Scholar
Zhang C W, Li H, Dong J M, Wang Y J, Pan F C, Guo Y Q, Li W 2005 Acta Phys. Sin. 54 1814
 Google Scholar
Google Scholar
[6] Xue Z Q, Liu L, Liu Z, Li M, Lee D, Chen R J, Guo Y Q, Yan A R 2016 Scr. Mater. 113 226
 Google Scholar
Google Scholar
[7] Strnat K, Hoffer G, Olson J, Ostertag W, Becker J J 1967 J. Appl. Phys. 38 1001
 Google Scholar
Google Scholar
[8] Streever R L 1979 Phys. Rev. B. 19 2704
 Google Scholar
Google Scholar
[9] Buschow K H J, Van der Goot A S 1968 J. Less Common Met. 14 323
 Google Scholar
Google Scholar
[10] Gaidukova I Y, Granovsky S A, Markosyan A S, Rodimin V E 2006 J. Magn. Magn. Mater. 301 124
 Google Scholar
Google Scholar
[11] Saito T, Nishio-Hamane D 2014 J. Alloys Compd. 585 423
 Google Scholar
Google Scholar
[12] Guo K, Lü H, Xu G J, Liu D, Wang H B, Liu X M, Song X Y 2022 Mater. Today Chem. 25 100983
 Google Scholar
Google Scholar
[13] Mao F, Lü H, Liu D, Guo K, Tang F W, Song X Y 2019 J. Alloys Compd. 810 151888
 Google Scholar
Google Scholar
[14] 毛斐, 吕皓, 唐法威, 郭凯, 刘东, 宋晓艳 2021 金属学报 57 948
 Google Scholar
Google Scholar
Mao F, Lü H, Tang F W, Guo K, Song X Y 2021 Acta. Metall. Sin. 57 948
 Google Scholar
Google Scholar
[15] Guo K, Lu H, Mao F, Liu D, Tang F W, Wang H B, Song X Y 2020 Nanoscale 12 5567
 Google Scholar
Google Scholar
[16] Wang D, Liu D, Hou C, Wang H B, Liu X, Song X Y 2017 J. Alloys Compd. 717 93
 Google Scholar
Google Scholar
[17] Das B, Choudhary R, Skomski R, Balasubramanian B, Pathak A K, Paudyal D, Sellmyer D J 2019 Phys. Rev. B 100 024419
 Google Scholar
Google Scholar
[18] Liu X B, Altounian Z 2011 Comput. Mater. Sci. 50 841
[19] Landa A, Söderlind P, Parker D, Åberg D, Lordi V, Perron A, Turchi P E A, Chouhan R K, Paudyal D, Lograsso T A 2018 J. Alloys Compd. 765 659
 Google Scholar
Google Scholar
[20] Antonioua E, Semprosa G, Gjokab M, Sarafidisa C, Polatogloua H M, Kioseogloua J 2021 J. Alloys Compd. 882 160699
 Google Scholar
Google Scholar
[21] Kresse G, Furthmüller J 1996 Comput. Mater. Sci. 6 15
 Google Scholar
Google Scholar
[22] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[23] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[24] Blöchl P E 1994 Phys. Rev. B. 50 17953
 Google Scholar
Google Scholar
[25] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[26] Perdew J P, Chevary J A, Vosko S H, Jackson K A, Pederson M R, Singh D J, Fiolhais C 1992 Phys. Rev. B 46 6671
 Google Scholar
Google Scholar
[27] Dudarev S L, Botton G A, Savrasov S Y, Humphreys C J, Sutton A P 1998 Phys. Rev. B 57 1505
 Google Scholar
Google Scholar
[28] Van der Marel D, Sawatzky G A 1988 Phys. Rev. B 37 10674
 Google Scholar
Google Scholar
[29] Zhang Z F, Guo Y Z, Robertson J 2020 Phys. Rev. Mater. 4 054603
 Google Scholar
Google Scholar
[30] Brooks M S S, Eriksson O, Wills J M, Johansson B 1997 Phys. Rev. Lett. 79 2546
[31] Georg K, Martijn M, Jurgen F 2018 User Manual for VASP Version 5.4
[32] Liu B J, Wang H, Xu C, Liu X P, Zhang Q F, Zhan T L, Stamenov P, Coey J M D, Jiang C B 2020 Phys. Rev. Mater. 4 044406
 Google Scholar
Google Scholar
[33] Söderlind P, Landa A, Locht I L, Åberg D, Kvashnin Y, Pereiro M, Däne M, Turchi P E A, Antropov V P, Eriksson O 2017 Phys. Rev. B 96 100404
 Google Scholar
Google Scholar
[34] Raja A, Adhikary T, Al-Omari I A, Das G P, Ghosh S, Satapathy D K, Oraon A, Shield J E, Aich S 2020 J. Magn. Magn. Mater. 504 166645
 Google Scholar
Google Scholar
计量
- 文章访问数: 4541
- PDF下载量: 155
- 被引次数: 0













 下载:
下载: