-
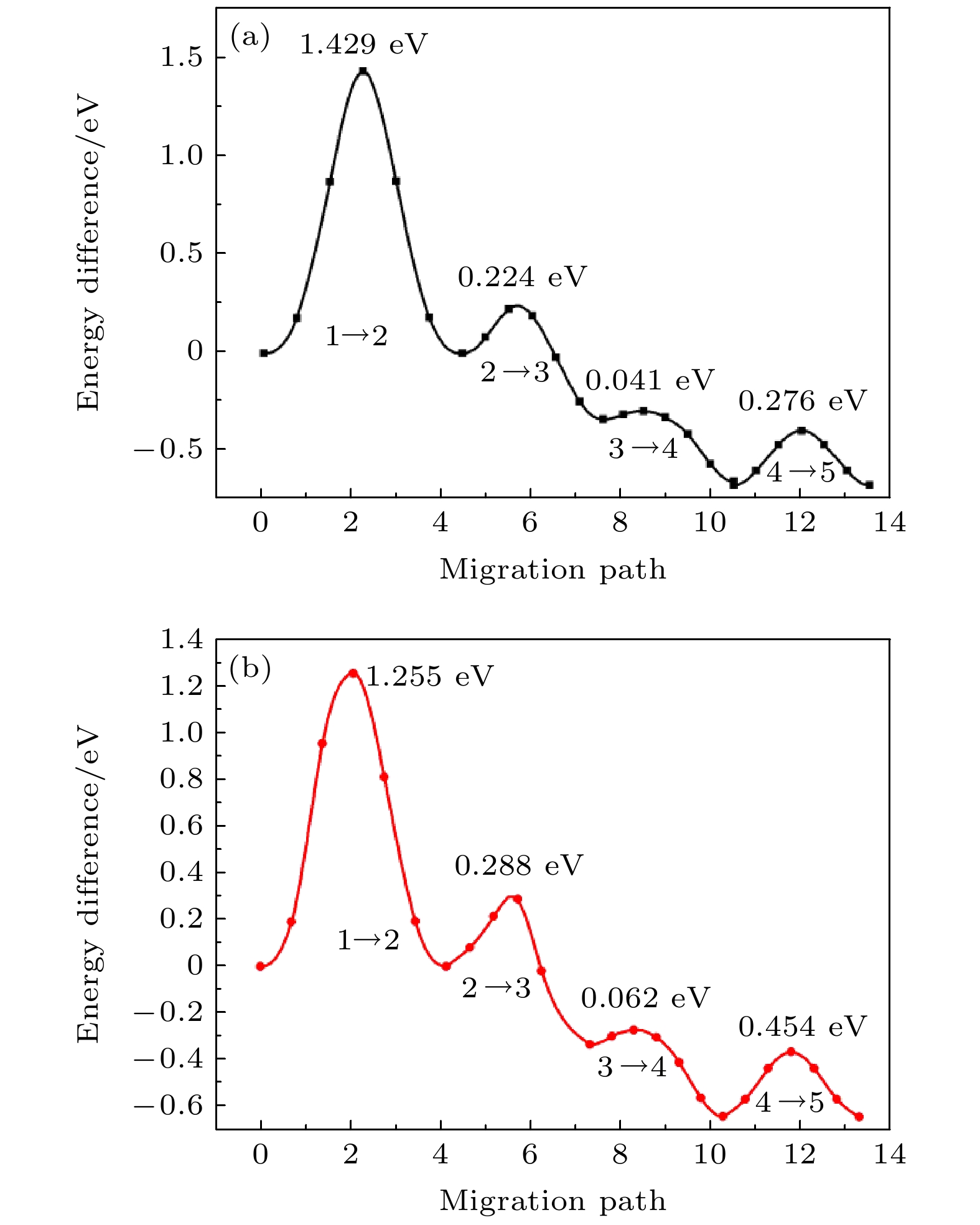
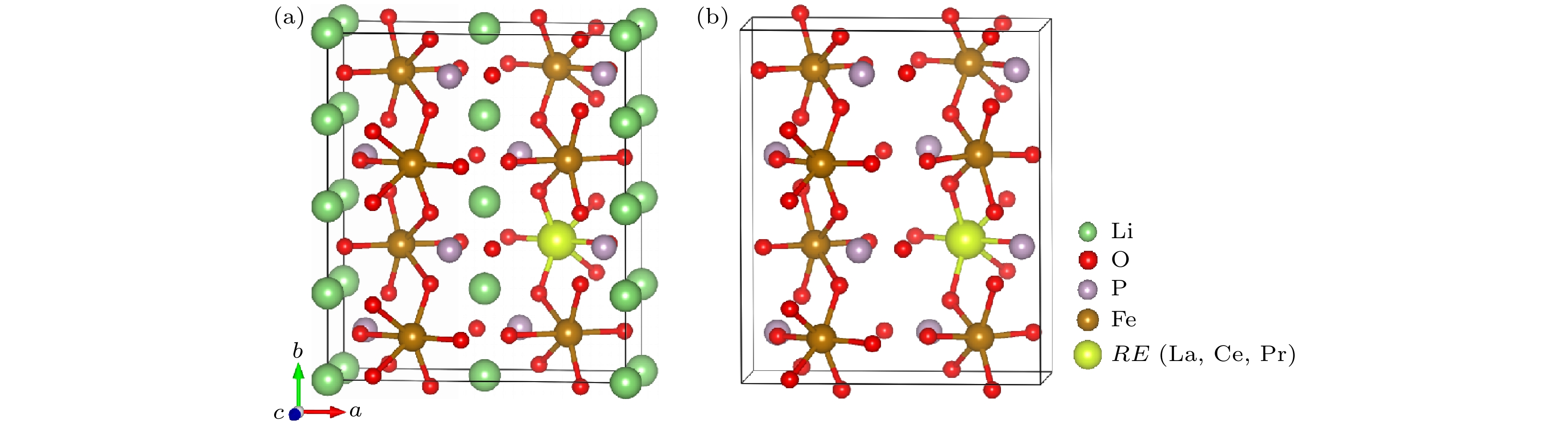
掺杂是提高LiFePO4体相电子电导率, 优化其电化学性能的重要方法之一. 稀土元素因具有高的电子电荷、大的离子半径以及强的自极化能力, 成为掺杂改性的重要选择. 本文利用基于密度泛函理论的第一性原理方法研究了稀土元素 (La, Ce, Pr) 掺杂的锂离子电池正极材料LiFePO4的性质. 计算结果表明, 稀土元素掺杂均不同程度地增加了LiFePO4的晶格常数和晶胞体积. 在脱锂过程中, 稀土掺杂后材料体积变化率明显减小, 材料的循环性能提升, 但电池能量密度下降. 稀土掺杂使LiFePO4由原来的半导体特性转变为金属特性, 增加了材料的电子电导率. 力学特性的计算表明稀土显著增加了LiFePO4材料的延展性. 另外, La和Ce掺杂后的LiFePO4在Li离子迁移过程中表现出复杂的能垒变化, 在远离稀土离子处迁移势垒呈现出不同程度的减小, 而在靠近稀土离子处迁移势垒起伏较大. 与Ce掺杂相比, La掺杂造成的离子迁移势垒的变化程度更大, 表明稀土离子掺杂对体系局域结构产生较大的影响.Doping is one of the most important methods to improve the electronic conductivity and modify its electrochemical performance of LiFePO4. Rare earth elements have become an effective selection for doping modification due to their high electronic charges, large ion radii and strong self-polarization ability. In this work, we study the structural, electronic and ionic diffusion properties of LiFePO4 with rare earth (RE) doping (La, Ce, Pr) by using first-principles calculation based on density functional theory. The calculated results show that the lattice constant and cell volume of LiFePO4 increase to a different degree after RE doping. In the delithiation process, the volume change rate of the material after RE doping is significantly reduced, indicating the cycle performance of the material is improved, on the other hand, the energy density is reduced. The calculated density of states suggests that RE-doped LiFePO4 exhibits metallic characteristics, which is different from the undoped one with semiconductor characteristics. As a result, the RE-doping can increase the electronic conductivity of the material. The calculation of elastic modulus demonstrates the increase of ductility for RE-doped LiFePO4, and it can be predicted that the cycle performance and the rate performance of the RE-doped battery have great improvement. In addition, La and Ce doped LiFePO4 materials exhibit that the complex energy barrier can change during the Li ion migration, and the migration barriers vary considerably, depending on different paths, which is related to the variation of potential energy surface caused by the doping of rare-earth elements. The Li-ions are far from the RE ions, the migration barriers are obviously lower than the undoped one, while the Li-ions are closest to RE ions, the migration barriers increase essentially. Compared with Ce doping, the change of the Li-ion migration barrier caused by La doping is great, indicating that RE ion doping has a greater influence on the local structure of the system.
-
Keywords:
- first-principles calculations /
- rare-earth doped /
- lithium-ion battery /
- LiFePO4
[1] Tarascon J M, Armand M 2001 Nature 414 359
 Google Scholar
Google Scholar
[2] Padhi A K, Nanjundawamy K S, Goodenough J B 1997 J. Electrochem. Soc. 144 1188
 Google Scholar
Google Scholar
[3] Scrosati B, Garche J 2010 J. Power Sources 195 2419
 Google Scholar
Google Scholar
[4] Yamada A, Chung S C 2001 J. Electrochem. Soc. 148 A960
 Google Scholar
Google Scholar
[5] Takahashi M, Tobishima S I, Takei K, Sakurai Y 2002 Solid State Ionics 148 283
 Google Scholar
Google Scholar
[6] Yamada A, Chung S C, Hinokuma K 2001 J. Electrochem. Soc. 148 A224
 Google Scholar
Google Scholar
[7] Huang H, Yin S C, Nazar L F 2001 Electrochem. Solid-State Lett. 4 A170
 Google Scholar
Google Scholar
[8] Chung S Y, Bloking J T, Chiang Y M 2002 Nat. Mater. 1 123
 Google Scholar
Google Scholar
[9] Chung S Y, Chiang Y M 2003 Electrochem. Solid-State Lett. 6 A278
 Google Scholar
Google Scholar
[10] Shi S Q, Liu L J, Ouyang C Y, Wang D S, Wang Z X, Chen L Q, Huang X J 2003 Phys. Rev. B 68 195108
 Google Scholar
Google Scholar
[11] Wang Z, Sun S, Xia D, Chu W, Zhang S, Wu Z 2008 J. Phys. Chem. C 112 17450
 Google Scholar
Google Scholar
[12] Thackeray M 2002 Nat. Mater. 1 81
 Google Scholar
Google Scholar
[13] Jiang B Q, Hu S F, Wang M W, Ouyang X P, Gong Z Y 2011 Rare Metals 30 115
 Google Scholar
Google Scholar
[14] Ghosh P, Mahanty S, Basu R N 2009 Electrochim. Acta 54 1654
 Google Scholar
Google Scholar
[15] Sun H B, Chen Y G, Xu C H, Zhu D, Huang L H 2012 J. Solid State Electrochem. 16 1247
 Google Scholar
Google Scholar
[16] Ding Y H, Zhang P, Jiang Y, Gao D H 2007 Solid State Ionics 178 967
 Google Scholar
Google Scholar
[17] 郑路敏, 钟淑英, 徐波, 欧阳楚英 2019 68 138201
 Google Scholar
Google Scholar
Zheng L M, Zhong S Y, Xu B, Ouyang C Y 2019 Acta Phys. Sin. 68 138201
 Google Scholar
Google Scholar
[18] Tian Y W, Kang X X, Liu L Y, Xu C Q, Qu T 2008 J. Rare Earths 26 279
 Google Scholar
Google Scholar
[19] Luo S H, Tian Y, Li H, Shi K J, Tang Z L, Zhang Z T 2010 J. Rare Earths 28 439
 Google Scholar
Google Scholar
[20] Needham S A, Wang G X, Liu H K, Drozd V A, Liu R S 2007 J. Power Sources 174 828
 Google Scholar
Google Scholar
[21] Shi S, Gao J, Liu Y, Zhao Y, Wu Q, Ju W, Ouyang C, Xiao R 2016 Chin. Phys. B 25 018212
 Google Scholar
Google Scholar
[22] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[23] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[24] Perdew J P, Chevary J A, Vosko S H, Jackson K A, Pederson M R, Singh D J, Fiolhais C 1992 Phys. Rev. B 46 6671
 Google Scholar
Google Scholar
[25] Anisimov V I, Zaanen J, Andersen O K 1991 Phys. Rev. B 44 943
 Google Scholar
Google Scholar
[26] Zhou F, Kang K, Maxisch T, Ceder G, Morgan D 2004 Solid State Commun. 132 181
 Google Scholar
Google Scholar
[27] Li B, Metiu H 2010 J. Phys. Chem. C 114 12234
 Google Scholar
Google Scholar
[28] Shi S, Tang Y, Ouyang C, Cui L, Xin X, Li P, Zhou W, Zhang H., Lei M, Chen L 2010 J. Phys. Chem. Solids 71 788
 Google Scholar
Google Scholar
[29] Ning F H, Xu B, Shi J, Wu M S, Hu Y Q, Ouyang C Y 2016 J. Phys. Chem. C 120 18428
 Google Scholar
Google Scholar
[30] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[31] Henkelman G, Jónsson H 2000 J. Chem. Phys. 113 9978
 Google Scholar
Google Scholar
[32] He B, Chi S T, Ye A J, Mi P H, Zhang L W, Pu B W, Zou Z Y, Ran Y B, Zhao Q, Wang D, Zhang W Q, Zhao J T, Adams S, Avdeev M, Shi S Q 2020 Sci. Data 7 151
 Google Scholar
Google Scholar
[33] Streltsov V A, Belokoneva E L, Tsirelson V G, Hansen N K 1993 Acta Crystallogr., Sect. B 49 147
 Google Scholar
Google Scholar
[34] Xie Y, Yu H T, Yi T F, Zhu Y R 2014 ACS Appl. Mater. Interfaces 6 4033
 Google Scholar
Google Scholar
[35] Andersson A S, Kalska B, Hggstrm L, Thomas J O 2000 Solid State Ionics 130 41
 Google Scholar
Google Scholar
[36] Wang Y, Wang Y, Hosono, E, Wang K Zhou H 2008 Angew. Chem. 47 7461
 Google Scholar
Google Scholar
[37] 张华, 唐元昊, 周薇薇, 李沛娟, 施思齐 2010 59 5135
 Google Scholar
Google Scholar
Zhang H, Tang Y H, Zhou W W, Li P J, Shi S Q 2010 Acta Phys. Sin. 59 5135
 Google Scholar
Google Scholar
[38] Maxisch T, Ceder G 2006 Phys. Rev. B 73 174112
 Google Scholar
Google Scholar
[39] Wu Z, Hao X, Liu X, Meng J 2007 Phys. Rev. B 75 054115
 Google Scholar
Google Scholar
[40] Hill R 1952 Proc. Phys. Soc. 65 349
 Google Scholar
Google Scholar
[41] Caravaca M A, Mino J C, Perez V J, Casali R A, Ponce C A 2009 J. Phys. Condens. Matter 21 015501
 Google Scholar
Google Scholar
[42] Pugh S F 2009 Philos. Mag. 45 823
 Google Scholar
Google Scholar
[43] Zhuang Y, Zou Z Y, Lu B, Li Y J, Wang D, Avdeev M, Shi S Q 2020 Chin. Phys. B 29 068202
 Google Scholar
Google Scholar
[44] Morgan D, Van der Ven A, Ceder G 2004 Electrochem. Solid-State Lett. 7 A30
 Google Scholar
Google Scholar
[45] Ouyang C Y, Shi S Q, Wang Z X, Huang X J, Chen L Q 2004 Phys. Rev. B 69 104303
 Google Scholar
Google Scholar
-
表 1 未掺杂与稀土掺杂的LiFePO4的晶格常数和超胞体积
Table 1. Optimized lattice contests and cell volume of LiFePO4 without doping and with rare-earth doping.
表 2 未掺杂LiFePO4中的Fe—O键长和稀土掺杂结构中RE—O键长
Table 2. Bond lengths between Fe atoms and O atoms, rare-earth atoms and O atoms in LiFePO4 without doping and with rare-earth doping structure, respectively.
键型 dM–O (M = Fe, La, Ce)/Å Fe—O 2.225 2.080 2.132 2.268 La—O 2.524 2.401 2.419 2.523 Ce—O 2.455 2.334 2.343 2.471 Pr—O 2.442 2.319 2.331 2.454 表 3 LiFePO4未掺杂及稀土掺杂(RE = La, Ce, Pr)的弹性常数(单位: GPa)
Table 3. Elastic constants (in GPa) of LiFePO4 without doping and with rare earth (RE = La, Ce, Pr) doping.
C11 C22 C33 C44 C55 C66 C12 C23 C13 LiFePO4 138 182 174 41 49 43 67 46 54 Ref. [38] 139 198 173 37 51 48 73 46 53 LiFe0.875La0.125PO4 113 153 142 27 35 35 84 61 65 LiFe0.875Ce0.125PO4 106 169 158 31 33 35 76 19 47 LiFe0.875Pr0.125PO4 119 108 172 35 31 28 66 95 40 表 4 未掺杂与稀土掺杂(RE = La, Ce, Pr)的LiFePO4的体模量(B)、剪切模量(G)、B/G、杨氏模量(E)、泊松比(v)
Table 4. Bulk modulus (B), shear modulus (G), B/G, Young’s modulus (E), Poisson’s ratio (v) for LiFePO4 without doping and with rare earth (RE = La, Ce, Pr) doping.
B/GPa G/GPa B/G E/GPa v LiFePO4 92 48 1.92 122 0.2782 Ref. [38] 94 48 1.92 124 0.2800 LiFe0.875La0.125PO4 91 32 2.87 85 0.3440 LiFe0.875Ce0.125PO4 79 37 2.15 95 0.2987 LiFe0.875Pr0.125PO4 87 29 3.00 78 0.3502 -
[1] Tarascon J M, Armand M 2001 Nature 414 359
 Google Scholar
Google Scholar
[2] Padhi A K, Nanjundawamy K S, Goodenough J B 1997 J. Electrochem. Soc. 144 1188
 Google Scholar
Google Scholar
[3] Scrosati B, Garche J 2010 J. Power Sources 195 2419
 Google Scholar
Google Scholar
[4] Yamada A, Chung S C 2001 J. Electrochem. Soc. 148 A960
 Google Scholar
Google Scholar
[5] Takahashi M, Tobishima S I, Takei K, Sakurai Y 2002 Solid State Ionics 148 283
 Google Scholar
Google Scholar
[6] Yamada A, Chung S C, Hinokuma K 2001 J. Electrochem. Soc. 148 A224
 Google Scholar
Google Scholar
[7] Huang H, Yin S C, Nazar L F 2001 Electrochem. Solid-State Lett. 4 A170
 Google Scholar
Google Scholar
[8] Chung S Y, Bloking J T, Chiang Y M 2002 Nat. Mater. 1 123
 Google Scholar
Google Scholar
[9] Chung S Y, Chiang Y M 2003 Electrochem. Solid-State Lett. 6 A278
 Google Scholar
Google Scholar
[10] Shi S Q, Liu L J, Ouyang C Y, Wang D S, Wang Z X, Chen L Q, Huang X J 2003 Phys. Rev. B 68 195108
 Google Scholar
Google Scholar
[11] Wang Z, Sun S, Xia D, Chu W, Zhang S, Wu Z 2008 J. Phys. Chem. C 112 17450
 Google Scholar
Google Scholar
[12] Thackeray M 2002 Nat. Mater. 1 81
 Google Scholar
Google Scholar
[13] Jiang B Q, Hu S F, Wang M W, Ouyang X P, Gong Z Y 2011 Rare Metals 30 115
 Google Scholar
Google Scholar
[14] Ghosh P, Mahanty S, Basu R N 2009 Electrochim. Acta 54 1654
 Google Scholar
Google Scholar
[15] Sun H B, Chen Y G, Xu C H, Zhu D, Huang L H 2012 J. Solid State Electrochem. 16 1247
 Google Scholar
Google Scholar
[16] Ding Y H, Zhang P, Jiang Y, Gao D H 2007 Solid State Ionics 178 967
 Google Scholar
Google Scholar
[17] 郑路敏, 钟淑英, 徐波, 欧阳楚英 2019 68 138201
 Google Scholar
Google Scholar
Zheng L M, Zhong S Y, Xu B, Ouyang C Y 2019 Acta Phys. Sin. 68 138201
 Google Scholar
Google Scholar
[18] Tian Y W, Kang X X, Liu L Y, Xu C Q, Qu T 2008 J. Rare Earths 26 279
 Google Scholar
Google Scholar
[19] Luo S H, Tian Y, Li H, Shi K J, Tang Z L, Zhang Z T 2010 J. Rare Earths 28 439
 Google Scholar
Google Scholar
[20] Needham S A, Wang G X, Liu H K, Drozd V A, Liu R S 2007 J. Power Sources 174 828
 Google Scholar
Google Scholar
[21] Shi S, Gao J, Liu Y, Zhao Y, Wu Q, Ju W, Ouyang C, Xiao R 2016 Chin. Phys. B 25 018212
 Google Scholar
Google Scholar
[22] Kresse G, Furthmüller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[23] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[24] Perdew J P, Chevary J A, Vosko S H, Jackson K A, Pederson M R, Singh D J, Fiolhais C 1992 Phys. Rev. B 46 6671
 Google Scholar
Google Scholar
[25] Anisimov V I, Zaanen J, Andersen O K 1991 Phys. Rev. B 44 943
 Google Scholar
Google Scholar
[26] Zhou F, Kang K, Maxisch T, Ceder G, Morgan D 2004 Solid State Commun. 132 181
 Google Scholar
Google Scholar
[27] Li B, Metiu H 2010 J. Phys. Chem. C 114 12234
 Google Scholar
Google Scholar
[28] Shi S, Tang Y, Ouyang C, Cui L, Xin X, Li P, Zhou W, Zhang H., Lei M, Chen L 2010 J. Phys. Chem. Solids 71 788
 Google Scholar
Google Scholar
[29] Ning F H, Xu B, Shi J, Wu M S, Hu Y Q, Ouyang C Y 2016 J. Phys. Chem. C 120 18428
 Google Scholar
Google Scholar
[30] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[31] Henkelman G, Jónsson H 2000 J. Chem. Phys. 113 9978
 Google Scholar
Google Scholar
[32] He B, Chi S T, Ye A J, Mi P H, Zhang L W, Pu B W, Zou Z Y, Ran Y B, Zhao Q, Wang D, Zhang W Q, Zhao J T, Adams S, Avdeev M, Shi S Q 2020 Sci. Data 7 151
 Google Scholar
Google Scholar
[33] Streltsov V A, Belokoneva E L, Tsirelson V G, Hansen N K 1993 Acta Crystallogr., Sect. B 49 147
 Google Scholar
Google Scholar
[34] Xie Y, Yu H T, Yi T F, Zhu Y R 2014 ACS Appl. Mater. Interfaces 6 4033
 Google Scholar
Google Scholar
[35] Andersson A S, Kalska B, Hggstrm L, Thomas J O 2000 Solid State Ionics 130 41
 Google Scholar
Google Scholar
[36] Wang Y, Wang Y, Hosono, E, Wang K Zhou H 2008 Angew. Chem. 47 7461
 Google Scholar
Google Scholar
[37] 张华, 唐元昊, 周薇薇, 李沛娟, 施思齐 2010 59 5135
 Google Scholar
Google Scholar
Zhang H, Tang Y H, Zhou W W, Li P J, Shi S Q 2010 Acta Phys. Sin. 59 5135
 Google Scholar
Google Scholar
[38] Maxisch T, Ceder G 2006 Phys. Rev. B 73 174112
 Google Scholar
Google Scholar
[39] Wu Z, Hao X, Liu X, Meng J 2007 Phys. Rev. B 75 054115
 Google Scholar
Google Scholar
[40] Hill R 1952 Proc. Phys. Soc. 65 349
 Google Scholar
Google Scholar
[41] Caravaca M A, Mino J C, Perez V J, Casali R A, Ponce C A 2009 J. Phys. Condens. Matter 21 015501
 Google Scholar
Google Scholar
[42] Pugh S F 2009 Philos. Mag. 45 823
 Google Scholar
Google Scholar
[43] Zhuang Y, Zou Z Y, Lu B, Li Y J, Wang D, Avdeev M, Shi S Q 2020 Chin. Phys. B 29 068202
 Google Scholar
Google Scholar
[44] Morgan D, Van der Ven A, Ceder G 2004 Electrochem. Solid-State Lett. 7 A30
 Google Scholar
Google Scholar
[45] Ouyang C Y, Shi S Q, Wang Z X, Huang X J, Chen L Q 2004 Phys. Rev. B 69 104303
 Google Scholar
Google Scholar
计量
- 文章访问数: 12170
- PDF下载量: 376
- 被引次数: 0













 下载:
下载: