-
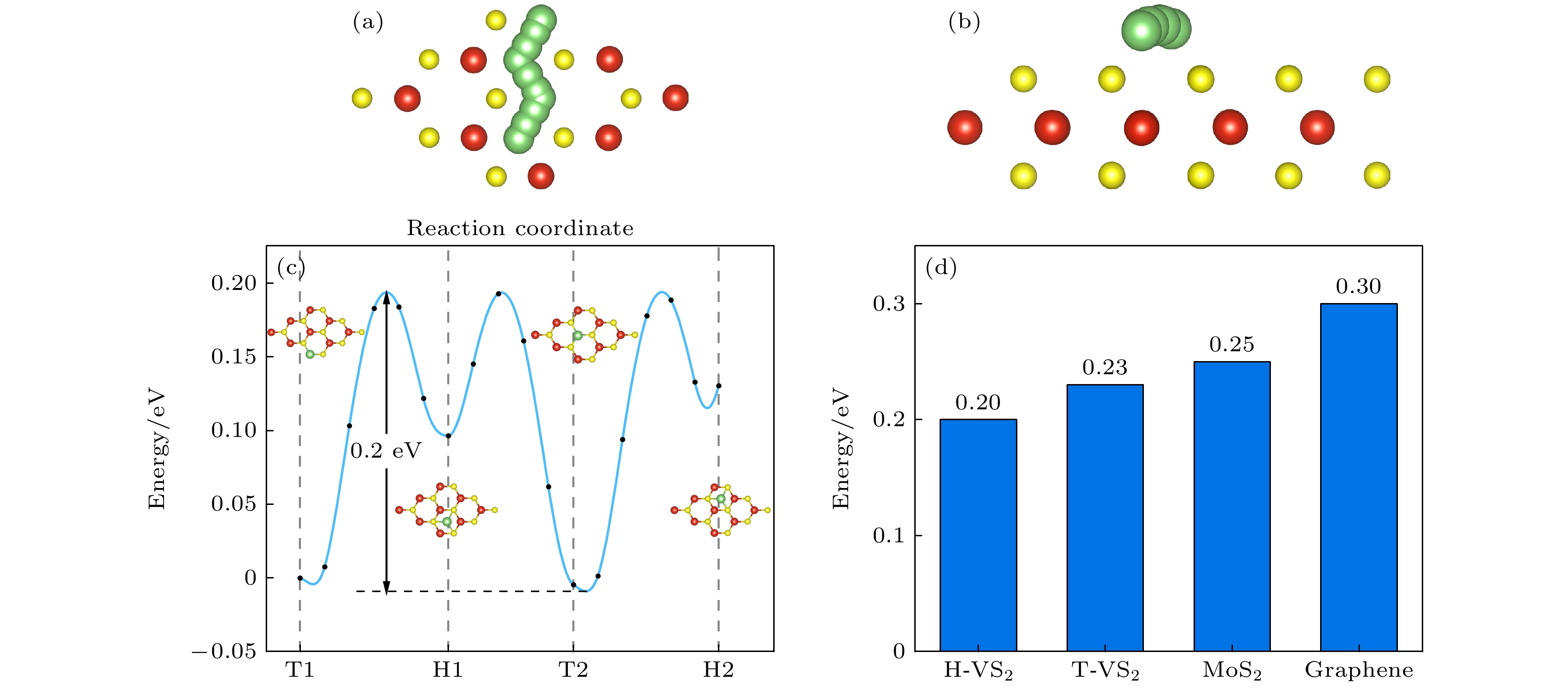
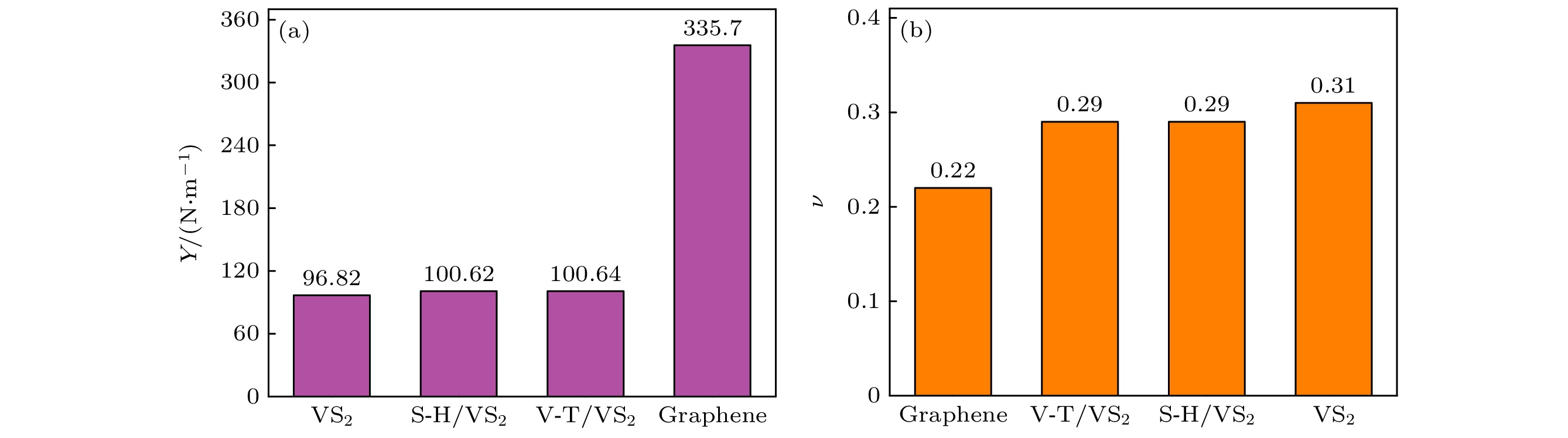
With the increase of performance requirements for lithium-ion batteries (LIBs), it is particularly important to study and develop new electrodes for lithium-ion batteries. In this work, a 3×3×1 supercell of VS2 is constructed, and the possibility of using it as an anode material for lithium-ion batteries is study by the first-principles method based on density functional theory. Through the analysis of the energy band diagram, it is found that VS2 has metallic properties. Combining the density of states diagram, the analysis shows that the energy band near the Fermi level of VS2 is contributed by the 3d state of V and the 3p state electrons of S, which means that the conductive properties of VS2 are largely affected by the 3d state of V and the 3p state electrons of S. Of the vacancies, bridge sites, and top sites of lithium adsorbing vanadium (V), the top site has the lowest adsorption energy, indicating that lithium will preferentially adsorb the top site of vanadium (V). Through first-principles molecular dynamics simulations of the top position of adsorbed vanadium (V), it is found that at a temperature of 300 K, the total energy of the system and the magnitude of the total temperature fluctuation can reach a steady state, indicating that lithium can exist at the top position of stably adsorbed vanadium (V). Moreover, the interlayer spacing of the double-layer VS2 reaches 3.67 Å, which is larger than the interlayer spacing of graphene. From the top position to the vacancy, its diffusion barrier is only 0.20 eV. Its interlayer spacing is larger than the double-layer graphene’s, and its diffusion barrier is lower than graphene’s, indicating that lithium has very good diffusivity on the VS2 surface, and lithium can migrate quickly on the VS2 surface, which is conducive to the rapid charge-discharge process of LIB. In addition to excellent electrical conductivity, VS2 has good mechanical properties. The calculated Young's modulus is 96.82 N/m, and the Young's modulus and Poisson’s ratio do not decrease after adsorbing lithium, indicating that the rigidity of VS2 will not be reduced in the diffusion and migration process of lithium. On the other hand, it has excellent deformation resistance. In order to be more accurate and closer to the actual situation, a double-layer VS2 model is constructed, with a maximum number of lithium atoms adsorbed between layers being 18. The calculated theoretical capacity of VS2 (466 mAh/g) is higher than the theoretical capacity of graphene (372 mAh/g). Our results indicate that VS2 has excellent electrical conductivity and mechanical stiffness, making it a promising cathode material for lithium-ion batteries.
-
Keywords:
- lithium-ion battery /
- energy of adsorption /
- first-principle
[1] Tarascon J M, Armand M 2001 Nature 414 359
 Google Scholar
Google Scholar
[2] Xu M S, Liang T, Shi M M, Chen H Z 2013 Chem. Rev. 113 3766
 Google Scholar
Google Scholar
[3] Liu H, Su D, Zhou R, Sun B, Wang G, Qiao S Z 2012 Adv. Energy Mater. 2 970.
 Google Scholar
Google Scholar
[4] Li Y F, Wu D H, Zhou Z, Cabrera C R, Chen Z F 2012 J. Phys. Chem. Lett. 3 2221
 Google Scholar
Google Scholar
[5] Mak K F, Lee C, Hone J, Shan J, Heinz T F 2010 Phys. Rev. Lett. 105 136805
 Google Scholar
Google Scholar
[6] Wang Z Y, Li H, Liu Z, Shi Z J, Lu J, Suenaga K, Joung S K, Okazaki T, Gu Z N, Zhou J, Gao Z X, Li G P, Sanvito S, Wang E G, Iijima S 2010 J. Am. Chem. Soc. 132 13840
 Google Scholar
Google Scholar
[7] 贺巧巧 2019 硕士学位论文(广州: 暨南大学)
He Q Q 2019 M. S. Thesis (Guangzhou: Jinan University
[8] 蒋晗涛 2022 硕士学位论文(长春: 东北师范大学)
Jiang H T 2022 M. S. Thesis (Changchun: Northeast Normal University
[9] 李传斌 2022 硕士学位论文(郑州: 华北水利水电大学)
Li C B 2022 M. S. Thesis (Zhengzhou: North China University of Water Resources and Electric Power
[10] 王羽偲嘉 2022 硕士学位论文(西安: 陕西科技大学)
Wang Y C J 2022 M. S. Thesis (Xi'an: Shaanxi University of Science & Technology
[11] 王振国 2021 博士学位论文 (上海: 华东师范大学)
Wang Z G 2021 Ph. D. Dissertation (Shanghai: East China Normal University
[12] Feng J, Peng Wu L C , Sun X , Hu S , Lin C, Dai J, Yang J L, Xie Y 2012 Adv. Mater. 15 1969
[13] Putungan D B, Lin S H, Kuo J L 2016 ACS Appl. Mater. Inter. 8 18754
 Google Scholar
Google Scholar
[14] Zhang H, Liu L M, Lau W M 2013 J. Mater. Chem. A 1 10821
 Google Scholar
Google Scholar
[15] Yu J, Zhou Z, Cabrera C R, Chen Z F 2013 J. Phys. Chem. C 117 25409
 Google Scholar
Google Scholar
[16] Kresse G, Hafner J 1993 Phys. Rev. B 47 558
 Google Scholar
Google Scholar
[17] Kresse G, Hafner J 1994 Phys-Condens Mat. 6 8245
 Google Scholar
Google Scholar
[18] Feng J, Sun X, Wu C Z, Peng L L, Lin C W, Hu S L, Yang J L, Xie Y 2011 J. Am. Chem. Soc. 133 17832
 Google Scholar
Google Scholar
[19] Ma Y D, Dai Y, Guo M, Niu C W, Zhu Y T, Huang B B 2012 ACS Nano 6 1695
 Google Scholar
Google Scholar
[20] Tang C M, Zhang M Z, Zhang K X, Gong J F 2021 Appl. Surf. Sci. 564 150468
 Google Scholar
Google Scholar
[21] Wang D S, Liu Y H, Meng X, Wei Y J, Zhao Y Y, Pang Q, Chen G 2017 J. Mater. Chem. A 5 21370
 Google Scholar
Google Scholar
[22] Mikhaleva N S, Visotin M A, Kuzubov A A, Popov Z I 2017 J. Phys. Chem. C 121 24179
 Google Scholar
Google Scholar
[23] Liu B, Gao T, Liao P, Wen Y F, Yao M J, Shi S Q, Zhang W Q 2021 Phys. Chem. Chem. Phys. 23 18784
 Google Scholar
Google Scholar
[24] Liu Y, Artyukhov V I, Liu M, Harutyunyan A R, Yakobson B I 2013 J. Phys. Chem. Lett. 4 1737
 Google Scholar
Google Scholar
[25] Liu J Z, Guo P F 2015 J. Inorg. Mater. 30 1339
 Google Scholar
Google Scholar
-
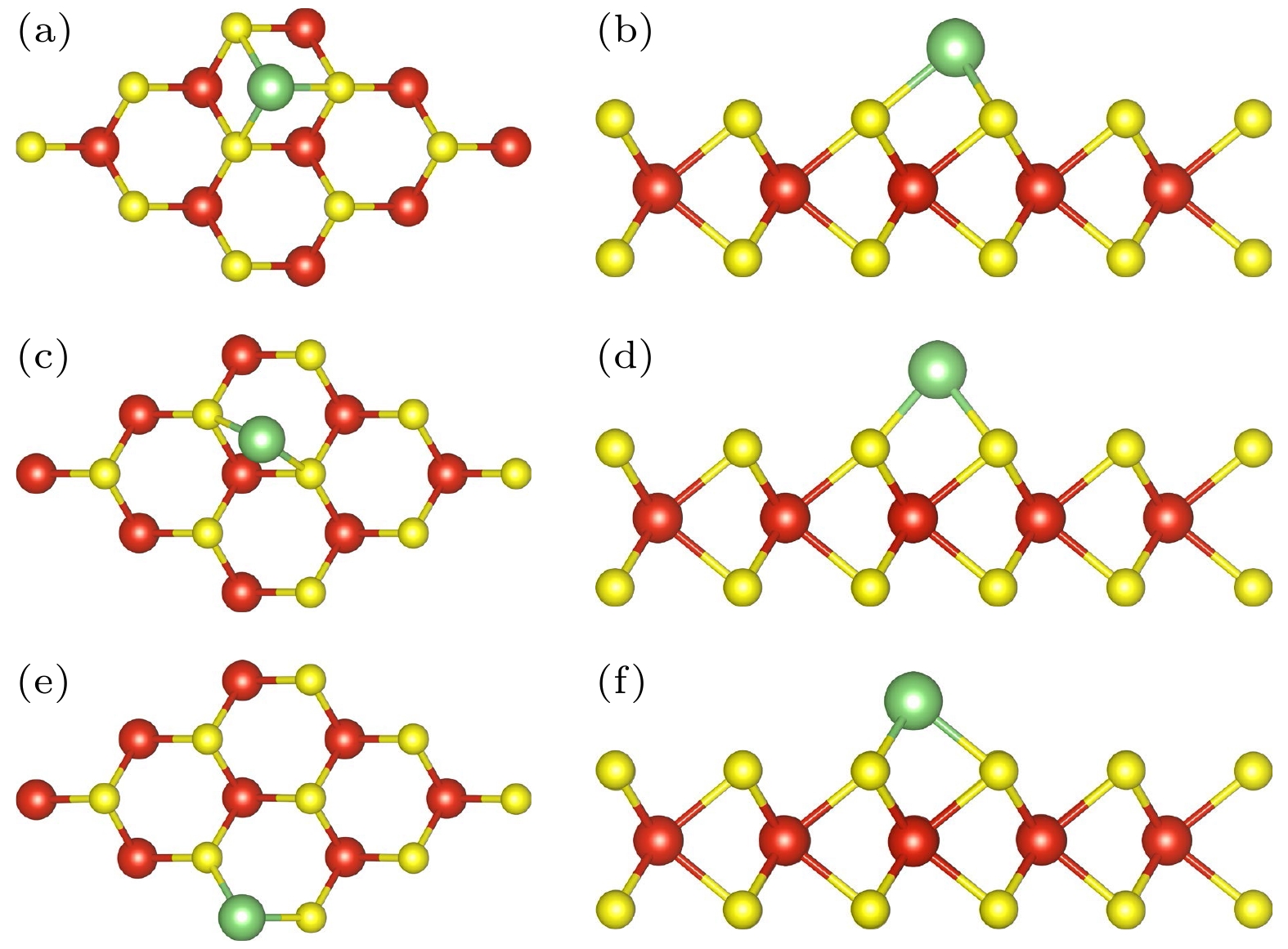
图 2 (a)吸附H-VS2中S-H位俯视图; (b)吸附H-VS2中S-H位侧视图; (c)吸附H-VS2中S-B位俯视图; (d)吸附H-VS2中S-B位侧视图; (e)吸附H-VS2中V-T位俯视图; (f)吸附H-VS2中V-T位侧视图
Figure 2. (a) Top view of S-H sites in adsorbed H-VS2; (b) side view of S-H sites in adsorbed H-VS2; (c) top view of the S-B site in the adsorbed H-VS2; (d) side view of the S-B site in the adsorbed H-VS2; (e) top view of V-T site in adsorbed H-VS2; (f) side view of V-T site in adsorbed H-VS2.
图 3 分子动力学模拟后 (a) H-VS2俯视图; (b)吸附V-T俯视图; (c), (d) 300 K, 步长1 fs下总时间5 ps内H-VS2模型FPMD期间的能量与温度波动; (e), (f) 300 K, 步长1 fs下总时间5 ps内吸附V-T模型FPMD期间的能量与温度波动
Figure 3. After molecular dynamics simulation: (a) H-VS2 top view; (b) adsorbed V-T top view; (c), (d) energy and temperature fluctuations during FPMD of H-VS2 model within a total time of 5 ps at 300 K, step size 1 fs; (e), (f) energy and temperature fluctuations during FPMD of adsorbed V-T model within a total time of 5 ps at 300 K and step size 1 fs.
图 4 (a) VS2能带图; (b)吸附Li原子后VS2能带图; (c) VS2总态密度图; (d) VS2的p和d电子态密度图; (e) VS2分态密度图; (f)吸附Li原子后分态密度图
Figure 4. (a) VS2 energy band diagram; (b) VS2 energy band diagram after adsorption of Li atoms; (c) VS2 total state density diagram; (d) VS2-p and VS2-d electronic state density diagram; (e) VS2 partial density of state diagram; (f) partial density of state diagram after adsorption of Li atoms.
表 1 VS2的总能量$ {E}_{{\mathrm{V}}{{\mathrm{S}}}_{2}} $、Li在其表面吸附后总能量$ {E}_{{\mathrm{V}}{{\mathrm{S}}}_{2}+{\mathrm{L}}{\mathrm{i}}} $、吸附能Ead、Li与VS2成键后的平均键长d
Table 1. The total energy $ {E}_{{\mathrm{V}}{{\mathrm{S}}}_{2}} $ of VS2, the total energy $ {E}_{{\mathrm{V}}{{\mathrm{S}}}_{2}+{\mathrm{L}}{\mathrm{i}}} $ after Li is adsorbed on its surface, the adsorption energy Ead, and the average bond length d of the bond between Li and VS2.
吸附位置 $ {E}_{{\mathrm{V}}{{\mathrm{S}}}_{2}} $/eV $ {E}_{{\mathrm{V}}{{\mathrm{S}}}_{2}+{\mathrm{L}}{\mathrm{i}}} $/eV Ead/eV d/Å S-H –23500.985 –23698.790 –2.303 2.38 S-B –23500.985 –23698.681 –2.194 2.31 V-T –23500.985 –23698.892 –2.405 2.37 表 2 VS2吸附V-T和S-H后VS2及石墨烯的弹性常数和杨氏模量
Table 2. Elastic constants and Young’s modulus of VS2, VS2 and graphene after adsorption of V-T and S-H.
System C11 C22 C12 Y/$ ({\mathrm{N}}\cdot {{\mathrm{m}}}^{-1}) $ v VS2 53.49 53.49 16.48 96.82 0.31 VS2吸附V-T 55.05 55.05 16.13 100.64 0.29 VS2吸附S-H 55.03 55.03 16.12 100.62 0.29 石墨烯 176.08 176.08 38.06 335.70 0.22 -
[1] Tarascon J M, Armand M 2001 Nature 414 359
 Google Scholar
Google Scholar
[2] Xu M S, Liang T, Shi M M, Chen H Z 2013 Chem. Rev. 113 3766
 Google Scholar
Google Scholar
[3] Liu H, Su D, Zhou R, Sun B, Wang G, Qiao S Z 2012 Adv. Energy Mater. 2 970.
 Google Scholar
Google Scholar
[4] Li Y F, Wu D H, Zhou Z, Cabrera C R, Chen Z F 2012 J. Phys. Chem. Lett. 3 2221
 Google Scholar
Google Scholar
[5] Mak K F, Lee C, Hone J, Shan J, Heinz T F 2010 Phys. Rev. Lett. 105 136805
 Google Scholar
Google Scholar
[6] Wang Z Y, Li H, Liu Z, Shi Z J, Lu J, Suenaga K, Joung S K, Okazaki T, Gu Z N, Zhou J, Gao Z X, Li G P, Sanvito S, Wang E G, Iijima S 2010 J. Am. Chem. Soc. 132 13840
 Google Scholar
Google Scholar
[7] 贺巧巧 2019 硕士学位论文(广州: 暨南大学)
He Q Q 2019 M. S. Thesis (Guangzhou: Jinan University
[8] 蒋晗涛 2022 硕士学位论文(长春: 东北师范大学)
Jiang H T 2022 M. S. Thesis (Changchun: Northeast Normal University
[9] 李传斌 2022 硕士学位论文(郑州: 华北水利水电大学)
Li C B 2022 M. S. Thesis (Zhengzhou: North China University of Water Resources and Electric Power
[10] 王羽偲嘉 2022 硕士学位论文(西安: 陕西科技大学)
Wang Y C J 2022 M. S. Thesis (Xi'an: Shaanxi University of Science & Technology
[11] 王振国 2021 博士学位论文 (上海: 华东师范大学)
Wang Z G 2021 Ph. D. Dissertation (Shanghai: East China Normal University
[12] Feng J, Peng Wu L C , Sun X , Hu S , Lin C, Dai J, Yang J L, Xie Y 2012 Adv. Mater. 15 1969
[13] Putungan D B, Lin S H, Kuo J L 2016 ACS Appl. Mater. Inter. 8 18754
 Google Scholar
Google Scholar
[14] Zhang H, Liu L M, Lau W M 2013 J. Mater. Chem. A 1 10821
 Google Scholar
Google Scholar
[15] Yu J, Zhou Z, Cabrera C R, Chen Z F 2013 J. Phys. Chem. C 117 25409
 Google Scholar
Google Scholar
[16] Kresse G, Hafner J 1993 Phys. Rev. B 47 558
 Google Scholar
Google Scholar
[17] Kresse G, Hafner J 1994 Phys-Condens Mat. 6 8245
 Google Scholar
Google Scholar
[18] Feng J, Sun X, Wu C Z, Peng L L, Lin C W, Hu S L, Yang J L, Xie Y 2011 J. Am. Chem. Soc. 133 17832
 Google Scholar
Google Scholar
[19] Ma Y D, Dai Y, Guo M, Niu C W, Zhu Y T, Huang B B 2012 ACS Nano 6 1695
 Google Scholar
Google Scholar
[20] Tang C M, Zhang M Z, Zhang K X, Gong J F 2021 Appl. Surf. Sci. 564 150468
 Google Scholar
Google Scholar
[21] Wang D S, Liu Y H, Meng X, Wei Y J, Zhao Y Y, Pang Q, Chen G 2017 J. Mater. Chem. A 5 21370
 Google Scholar
Google Scholar
[22] Mikhaleva N S, Visotin M A, Kuzubov A A, Popov Z I 2017 J. Phys. Chem. C 121 24179
 Google Scholar
Google Scholar
[23] Liu B, Gao T, Liao P, Wen Y F, Yao M J, Shi S Q, Zhang W Q 2021 Phys. Chem. Chem. Phys. 23 18784
 Google Scholar
Google Scholar
[24] Liu Y, Artyukhov V I, Liu M, Harutyunyan A R, Yakobson B I 2013 J. Phys. Chem. Lett. 4 1737
 Google Scholar
Google Scholar
[25] Liu J Z, Guo P F 2015 J. Inorg. Mater. 30 1339
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 3899
- PDF Downloads: 167
- Cited By: 0














 DownLoad:
DownLoad: