-
Laser point-scanning fluorescence microscopy serves as an indispensable tool in the life science research, owing to its merits of excellent resolution, high sensitivity, remarkable specificity, three-dimensional optical-sectioning capability, and dynamic imaging. However, conventional laser point-scanning fluorescence microscopy confronts a series of challenges in the rapidly evolving field of life sciences, because of the limitations imposed by optical diffraction and point scanning detection. Over the past two decades, substantial advancements have been made in super-resolution fluorescence microscopic imaging techniques. Researchers have developed various high spatial and temporal resolution point-scanning microtechniques, which hold great significance for biological optical imaging and other relevant applications. Regrettably, there are still few review articles covering the recent progress of this field. It is essential to provide a comprehensive review of laser point-scanning fluorescence microscopic techniques for their future developments and trends. In this article, the basic principles and recent advances in different point-scanning fluorescence microscopy imaging techniques are introduced from the perspectives of temporal resolution and spatial resolution, and the progress and applications of high spatio-temporal resolution microscopic imaging techniques based on point-scanning mode are summarized. Finally, the development trends and challenges of high spatio-temporal resolution point scanning fluorescence microscopic imaging technique are discussed.
-
Keywords:
- laser-scanning confocal microscopy /
- super-resolution microscopy /
- fluorescence microscopy /
- spatio-temporal resolution
[1] Abee E 1873 Arch. f. Mikr. Anat. 9 413
[2] 赵光远, 郑程, 方月, 匡翠方, 刘旭 2017 66 148702
 Google Scholar
Google Scholar
Zhao G Y, Zheng C, Fang Y, Kuang C F, Liu X 2017 Acta Phys. Sin. 66 148702
 Google Scholar
Google Scholar
[3] Betzig E, Patterson G H, Sougrat R, Lindwasser O W, Olenych S, Bonifacino J S, Davidson M W, Lippincott-Schwartz J, Hess H F 2006 Science 313 1642
 Google Scholar
Google Scholar
[4] Rust M J, Bates M, Zhuang X W 2006 Nat. Methods 3 793
 Google Scholar
Google Scholar
[5] Gustafsson M G 2000 J. Microsc. 198 82
 Google Scholar
Google Scholar
[6] Hell S W, Wichmann J 1994 Opt. Lett. 19 780
 Google Scholar
Google Scholar
[7] Jungmann R, Steinhauer C, Scheible M, Kuzyk A, Tinnefeld P, Simmel F C 2010 Nano Lett. 10 4756
 Google Scholar
Google Scholar
[8] Jungmann R, Avendaño M S, Woehrstein J B, Dai M J, Shih W M, Yin P 2014 Nat. Methods 11 313
 Google Scholar
Google Scholar
[9] Schermelleh L, Ferrand A, Huser T, Eggeling C, Sauer M, Biehlmaier O, Drummen G P C 2019 Nat. Cell Biol. 21 72
 Google Scholar
Google Scholar
[10] Huang X S, Fan J C, Li L J, Liu H S, Wu R L, Wu Y, Wei L S, Mao H, Lal A, Xi P, Tang L Q, Zhang Y F, Liu Y M, Tan S, Chen L Y 2018 Nat. Biotechnol. 36 451
 Google Scholar
Google Scholar
[11] Shao L, Kner P, Rego E H, Gustafsson M G L 2011 Nat. Methods 8 1044
 Google Scholar
Google Scholar
[12] Nägerl U V, Willig K I, Hein B, Hell S W, Bonhoeffer T 2008 Proc. Natl. Acad. Sci. U. S. A. 105 18982
 Google Scholar
Google Scholar
[13] Wildanger D, Medda R, Kastrup L, Hell S 2009 J. Microsc. 236 35
 Google Scholar
Google Scholar
[14] Nwaneshiudu A, Kuschal C, Sakamoto F H, Anderson R R, Schwarzenberger K, Young R C 2012 J. Invest. Dermatol. 132 1
 Google Scholar
Google Scholar
[15] Wilson T 1989 J. Microsc. 154 143
 Google Scholar
Google Scholar
[16] Pawley J 2006 Handbook of Biological Confocal Microscopy (Vol. 236) (Berlin: Springer Science & Business Media
[17] Muller M 2006 Introduction to Confocal Fluorescence Microscopy (Vol. 69) (Bellingham: SPIE Press
[18] Sticker M, Elsässer R, Neumann M, Wolff H 2020 J. Microsc. 28 36
 Google Scholar
Google Scholar
[19] Sheppard C R 1988 Optik (Stuttgart) 80 53
[20] Huff J 2015 Nat. Methods 12 i
 Google Scholar
Google Scholar
[21] Müller C B, Enderlein J 2010 Phys. Rev. Lett. 104 198101
 Google Scholar
Google Scholar
[22] York A G, Parekh S H, Dalle Nogare D, Fischer R S, Temprine K, Mione M, Chitnis A B, Combs C A, Shroff H 2012 Nat. Methods 9 749
 Google Scholar
Google Scholar
[23] Ingaramo M, York A G, Wawrzusin P, Milberg O, Hong A, Weigert R, Shroff H, Patterson G H 2014 Proc. Natl. Acad. Sci. U. S. A. 111 5254
 Google Scholar
Google Scholar
[24] Wu Y C, Shroff H 2018 Nat. Methods 15 1011
 Google Scholar
Google Scholar
[25] Qin S, Isbaner S, Gregor I, Enderlein J 2021 Nat. Protoc. 16 164
 Google Scholar
Google Scholar
[26] Gräf R, Rietdorf J, Zimmermann T 2005 Microscopy Techniques (Berlin:Springer) -/- 57
[27] Toomre D, Pawley J B 2006 Handbook of Biological Confocal Microscopy (Boston: Springer US) p221
[28] Inoué S, Inoué T 2002 Cell Biological Applications of Confocal Microscopy(San Diego: Academic Press) p88
[29] Wang E, Babbey C, Dunn K W 2005 J. Microsc. 218 148
 Google Scholar
Google Scholar
[30] Enoki R, Ono D, Hasan M T, Honma S, Honma K I 2012 J. Neurosci. Methods 207 72
 Google Scholar
Google Scholar
[31] Abreu-Blanco M T, Verboon J M, Parkhurst S M 2011 J. Cell Biol. 193 455
 Google Scholar
Google Scholar
[32] York A G, Chandris P, Nogare D D, Head J, Wawrzusin P, Fischer R S, Chitnis A, Shroff H 2013 Nat. Methods 10 1122
 Google Scholar
Google Scholar
[33] Schulz O, Pieper C, Clever M, Pfaff J, Ruhlandt A, Kehlenbach R H, Wouters F S, Großhans J, Bunt G, Enderlein J 2013 Proc. Natl. Acad. Sci. U. S. A. 110 21000
 Google Scholar
Google Scholar
[34] Xu Y Z, Xu R H, Wang Z, Zhou Y, Shen Q F, Ji W C, Dang D F, Meng L J, Tang B Z 2021 Chem. Soc. Rev. 50 667
 Google Scholar
Google Scholar
[35] Vicidomini G, Bianchini P, Diaspro A 2018 Nat. Methods 15 173
 Google Scholar
Google Scholar
[36] Westphal V, Hell S W 2005 Phys. Rev. Lett. 94 143903
 Google Scholar
Google Scholar
[37] Wildanger D, Patton B R, Schill H, Marseglia L, Hadden J, Knauer S, Schönle A, Rarity J G, O'Brien J L, Hell S W 2012 Adv. Mater. 24 OP309
 Google Scholar
Google Scholar
[38] Wäldchen S, Lehmann J, Klein T, Van De Linde S, Sauer M 2015 Sci. Rep. 5 1
 Google Scholar
Google Scholar
[39] Hell S W, Kroug M 1995 Appl. Phys. B 60 495
 Google Scholar
Google Scholar
[40] Bretschneider S, Eggeling C, Hell S W 2007 Phys. Rev. Lett. 98 218103
 Google Scholar
Google Scholar
[41] Göttfert F, Pleiner T, Heine J, Westphal V, Görlich D, Sahl S J, Hell S W 2017 Proc. Natl. Acad. Sci. U. S. A. 114 2125
 Google Scholar
Google Scholar
[42] Heine J, Reuss M, Harke B, D’Este E, Sahl S J, Hell S W 2017 Proc. Natl. Acad. Sci. U. S. A. 114 9797
 Google Scholar
Google Scholar
[43] Kuang C F, Li S, Liu W, Hao X, Gu Z T, Wang Y F, Ge J H, Li H F, Liu X 2013 Sci. Rep. 3 1441
 Google Scholar
Google Scholar
[44] Huang B R, Wu Q S, Peng X Y, Yao L Q, Peng D F, Zhan Q Q 2018 Nanoscale 10 21025
 Google Scholar
Google Scholar
[45] Moeyaert B, Dedecker P 2014 Photoswitching Proteins: Methods and Protocols(New York: Humana Press) p261
[46] Grotjohann T, Testa I, Leutenegger M, Bock H, Urban N T, Lavoie-Cardinal F, Willig K I, Eggeling C, Jakobs S, Hell S W 2011 Nature 478 204
 Google Scholar
Google Scholar
[47] Wang N, Kobayashi T 2014 Opt. Express 22 28819
 Google Scholar
Google Scholar
[48] Wang N, Kobayashi T 2015 Opt. Express 23 13704
 Google Scholar
Google Scholar
[49] Zhao G Y, Kuang C F, Ding Z H, Liu X 2016 Opt. Express 24 23596
 Google Scholar
Google Scholar
[50] Hell S W, Jakobs S, Kastrup L 2003 Appl. Phys. A 77 859
 Google Scholar
Google Scholar
[51] Sharma R, Singh M, Sharma R 2020 Spectrochim. Acta Part A 231 117715
 Google Scholar
Google Scholar
[52] Grotjohann T, Testa I, Reuss M, Brakemann T, Eggeling C, Hell S W, Jakobs S 2012 Elife 1 e00248
 Google Scholar
Google Scholar
[53] Balzarotti F, Eilers Y, Gwosch K C, Gynnå A H, Westphal V, Stefani F D, Elf J, Hell S W 2017 Science 355 606
 Google Scholar
Google Scholar
[54] Gwosch K C, Pape J K, Balzarotti F, Hoess P, Ellenberg J, Ries J, Hell S W 2020 Nat. Methods 17 217
 Google Scholar
Google Scholar
[55] Weber M, von der Emde H, Leutenegger M, Gunkel P, Sambandan S, Khan T A, Keller-Findeisen J, Cordes V C, Hell S W 2023 Nat. Biotechnol. 41 569
 Google Scholar
Google Scholar
[56] Wu R T, Zhan Q Q, Liu H C, Wen X Y, Wang B J, He S L 2015 Opt. Express 23 32401
 Google Scholar
Google Scholar
[57] Zhan Q Q, Liu H C, Wang B J, Wu Q S, Pu R, Zhou C, Huang B R, Peng X Y, Ågren H, He S L 2017 Nat. Commun. 8 1058
 Google Scholar
Google Scholar
[58] Liu Y J, Lu Y Q, Yang X S, Zheng X L, Wen S H, Wang F, Vidal X, Zhao J B, Liu D M, Zhou Z G, Ma C S, Zhou J J, Piper J A, Xi P, Jin D Y 2017 Nature 543 229
 Google Scholar
Google Scholar
[59] Guo X, Pu R, Zhu Z M, Qiao S Q, Liang Y S, Huang B R, Liu H C, Labrador-Páez L, Kostiv U, Zhao P, Wu Q S, Widengren J, Zhan Q Q 2022 Nat. Commun. 13 2843
 Google Scholar
Google Scholar
[60] Pu R, Zhan Q Q, Peng X Y, Liu S Y, Guo X, Liang L L, Qin X, Zhao Z W, Liu X 2022 Nat. Commun. 13 6636
 Google Scholar
Google Scholar
[61] Liang Y S, Zhu Z M, Qiao S Q, Guo X, Pu R, Tang H, Liu H C, Dong H, Peng T T, Sun L-D, Widengren J, Zhan Q Q 2022 Nat. Nanotechnol. 17 524
 Google Scholar
Google Scholar
[62] Wu Q S, Huang B R, Peng X Y, He S L, Zhan Q Q 2017 Opt. Express 25 30885
 Google Scholar
Google Scholar
[63] Chen C C, Wang F, Wen S H, Su Q P, Wu M C, Liu Y T, Wang B M, Li D, Shan X C, Kianinia M, Aharonovich I, Toth I, Jackson M S, Xi P, Jin D Y 2018 Nat. Commun. 9 3290
 Google Scholar
Google Scholar
[64] Denkova D, Ploschner M, Das M, Parker L M, Zheng X, Lu Y, Orth A, Packer N H, Piper J A 2019 Nat. Commun. 10 3695
 Google Scholar
Google Scholar
[65] Lee C, Xu E Z, Liu Y, Teitelboim A, Yao K, Fernandez-Bravo A, Kotulska A M, Nam S H, Suh Y D, Bednarkiewicz A 2021 Nature 589 230
 Google Scholar
Google Scholar
[66] Klar T A, Jakobs S, Dyba M, Egner A, Hell S W 2000 Proc. Natl. Acad. Sci. U. S. A. 97 8206
 Google Scholar
Google Scholar
[67] Harke B, Ullal C K, Keller J, Hell S W 2008 Nano Lett. 8 1309
 Google Scholar
Google Scholar
[68] Gould T J, Burke D, Bewersdorf J, Booth M J 2012 Opt. Express 20 20998
 Google Scholar
Google Scholar
[69] Lenz M O, Sinclair H G, Savell A, Clegg J H, Brown A C, Davis D M, Dunsby C, Neil M A, French P M 2013 J. Biophotonics 1 29
 Google Scholar
Google Scholar
[70] Schmidt R, Wurm C A, Jakobs S, Engelhardt J, Egner A, Hell S W 2008 Nat. Methods 5 539
 Google Scholar
Google Scholar
[71] Chmyrov A, Keller J, Grotjohann T, Ratz M, d'Este E, Jakobs S, Eggeling C, Hell S W 2013 Nat. Methods 10 737
 Google Scholar
Google Scholar
[72] Böhm U, Hell S W, Schmidt R 2016 Nat. Commun. 7 10504
 Google Scholar
Google Scholar
[73] Hao X, Allgeyer E S, Lee D R, Antonello J, Watters K, Gerdes J A, Schroeder L K, Bottanelli F, Zhao J, Kidd P 2021 Nat. Methods 18 688
 Google Scholar
Google Scholar
[74] Staudt T, Engler A, Rittweger E, Harke B, Engelhardt J, Hell S W 2011 Opt. Express 19 5644
 Google Scholar
Google Scholar
[75] Dreier J, Castello M, Coceano G, Cáceres R, Plastino J, Vicidomini G, Testa I 2019 Nat. Commun. 10 556
 Google Scholar
Google Scholar
[76] Alvelid J, Damenti M, Sgattoni C, Testa I 2022 Nat. Methods 19 1268
 Google Scholar
Google Scholar
[77] Bingen P, Reuss M, Engelhardt J, Hell S W 2011 Opt. Express 19 23716
 Google Scholar
Google Scholar
[78] Hofmann M, Eggeling C, Jakobs S, Hell S W 2005 Proc. Natl. Acad. Sci. U. S. A. 102 17565
 Google Scholar
Google Scholar
[79] Brakemann T, Stiel A C, Weber G, Andresen M, Testa I, Grotjohann T, Leutenegger M, Plessmann U, Urlaub H, Eggeling C 2011 Nat. Biotechnol. 29 942
 Google Scholar
Google Scholar
[80] Bergermann F, Alber L, Sahl S J, Engelhardt J, Hell S W 2015 Opt. Express 23 211
 Google Scholar
Google Scholar
[81] Boden A, Pennacchietti F, Coceano G, Damenti M, Ratz M, Testa I 2021 Nat. Biotechnol. 39 609
 Google Scholar
Google Scholar
[82] Wang H D, Rivenson Y, Jin Y Y, Wei Z S, Gao R, Günaydın H, Bentolila L A, Kural C, Ozcan A 2019 Nat. Methods 16 103
 Google Scholar
Google Scholar
[83] Li M, Shan H, Pryshchep S, Lopez M M, Wang G 2020 J. Nanophotonics 14 016009
 Google Scholar
Google Scholar
[84] Chen J J, Sasaki H, Lai H Y, Su Y J, Liu J M, Wu Y C, Zhovmer A, Combs C A, Rey-Suarez I, Chang H Y, Huang C C, Li X S, Guo M, Nizambad S, Upadhyaya A, Lee S J, Lucas L A, Shroff H 2021 Nat. Methods 18 678
 Google Scholar
Google Scholar
[85] Ebrahimi V, Stephan T, Kim J, Carravilla P, Eggeling C, Jakobs S, Han K Y 2023 bioRxiv 2023.01. 26.525571
[86] Matthews T E, Piletic I R, Selim M A, Simpson M J, Warren W S 2011 Sci. Transl. Med. 3 71ra15
 Google Scholar
Google Scholar
[87] Simpson M J, Wilson J W, Robles F E, Dall C P, Glass K, Simon J D, Warren W S 2014 J. Phys. Chem. A 118 993
 Google Scholar
Google Scholar
[88] Simpson M J, Wilson J W, Phipps M A, Robles F E, Selim M A, Warren W S 2013 J. Invest. Dermatol. 133 1822
 Google Scholar
Google Scholar
[89] Massaro E S, Hill A H, Grumstrup E M 2016 ACS Photonics 3 501
 Google Scholar
Google Scholar
-
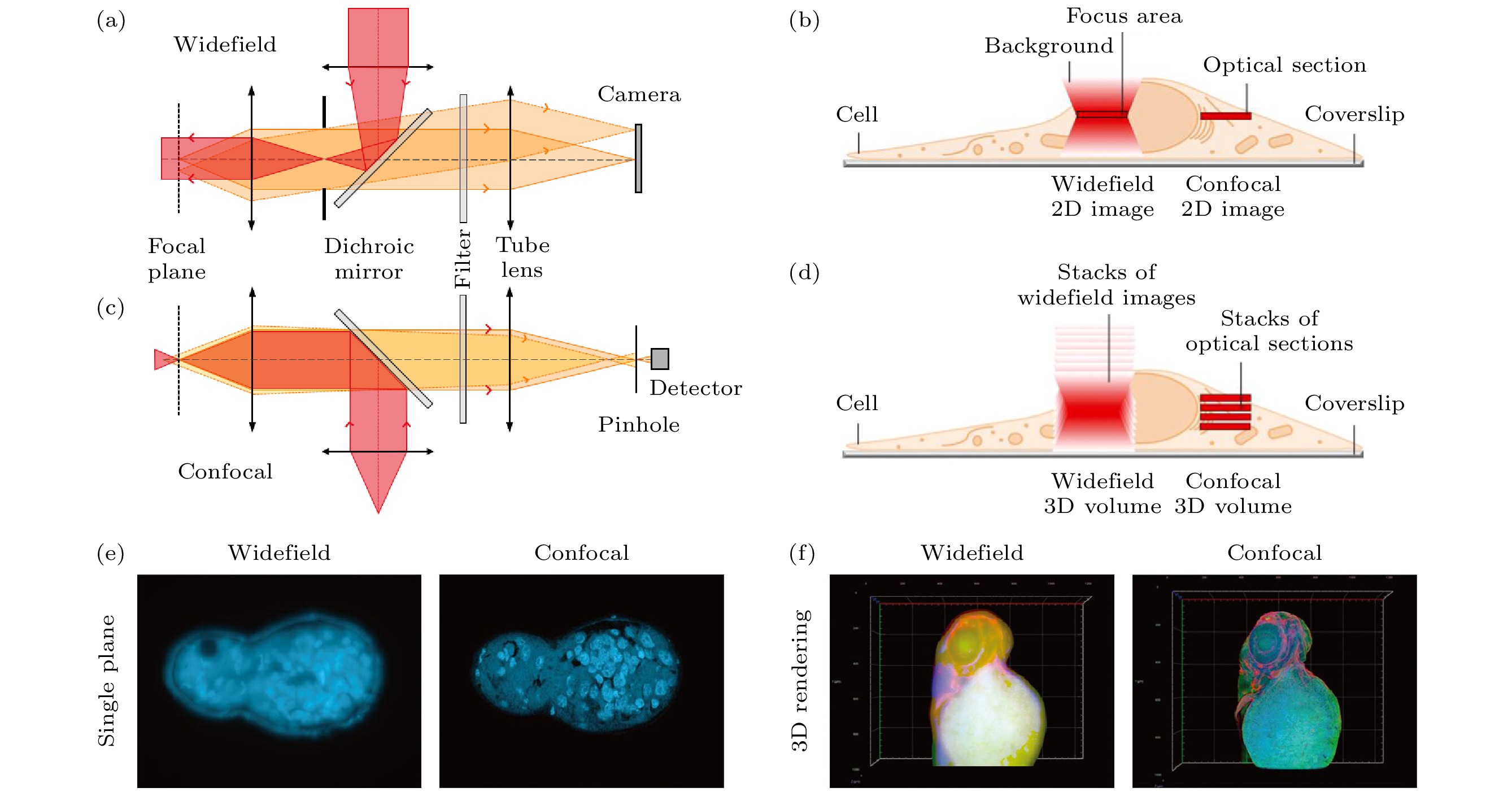
图 1 宽场和共聚焦显微成像技术对比 (a) 宽场显微镜系统简化图; (b) 宽场显微镜荧光信号采集方式[18]; (c) 共聚焦显微镜系统简化图; (d) 共聚焦显微镜荧光信号采集方式[18]; (e) 二维切面图对比[18]; (f) 三维重构图对比[18]
Figure 1. Comparison of wide-field and confocal microscopy: (a) Simplified system schematic of wide-field microscopy; (b) fluorescent signal acquisition of wide-field microscopy[18]; (c) simplified system schematic of confocal microscopy; (d) fluorescent signal acquisition of confocal microscopy[18]; (e) comparison of two-dimensional section images[18]; (f) comparison of three-dimensional reconstruction images[18].
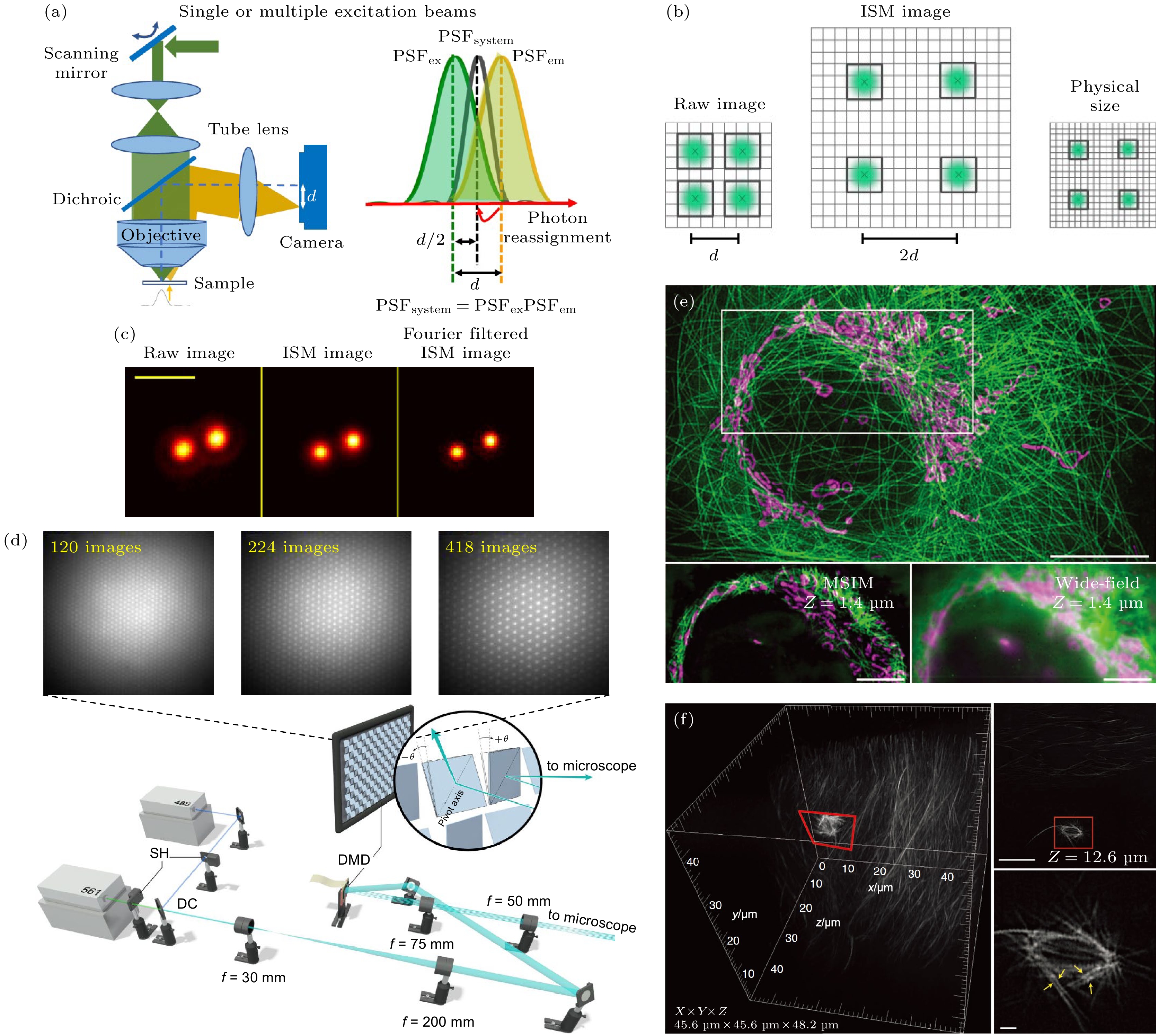
图 2 基于多焦点阵列的快速成像技术示意图 (a), (b) 像素重定位原理[24,25]; (c) 共聚焦显微镜和ISM对100 nm直径荧光球的成像对比[21]; (d) MSIM照明系统[22]; (e) MSIM和宽场显微镜的活细胞双色成像对比[22]; (f) MSIM的活细胞三维成像[22]
Figure 2. Schematic diagram of fast imaging technology based on multifocus array: (a), (b) Principle of pixel reassignment[24,25]; (c) imaging comparison of confocal microscope and ISM on 100 nm diameter fluorescent spheres[21]; (d) illumination system for MSIM[22]; (e) dual-color imaging comparison of MSIM and wide-field microscope on live-cell[22]; (f) 3D imaging of live cells by MSIM[22].
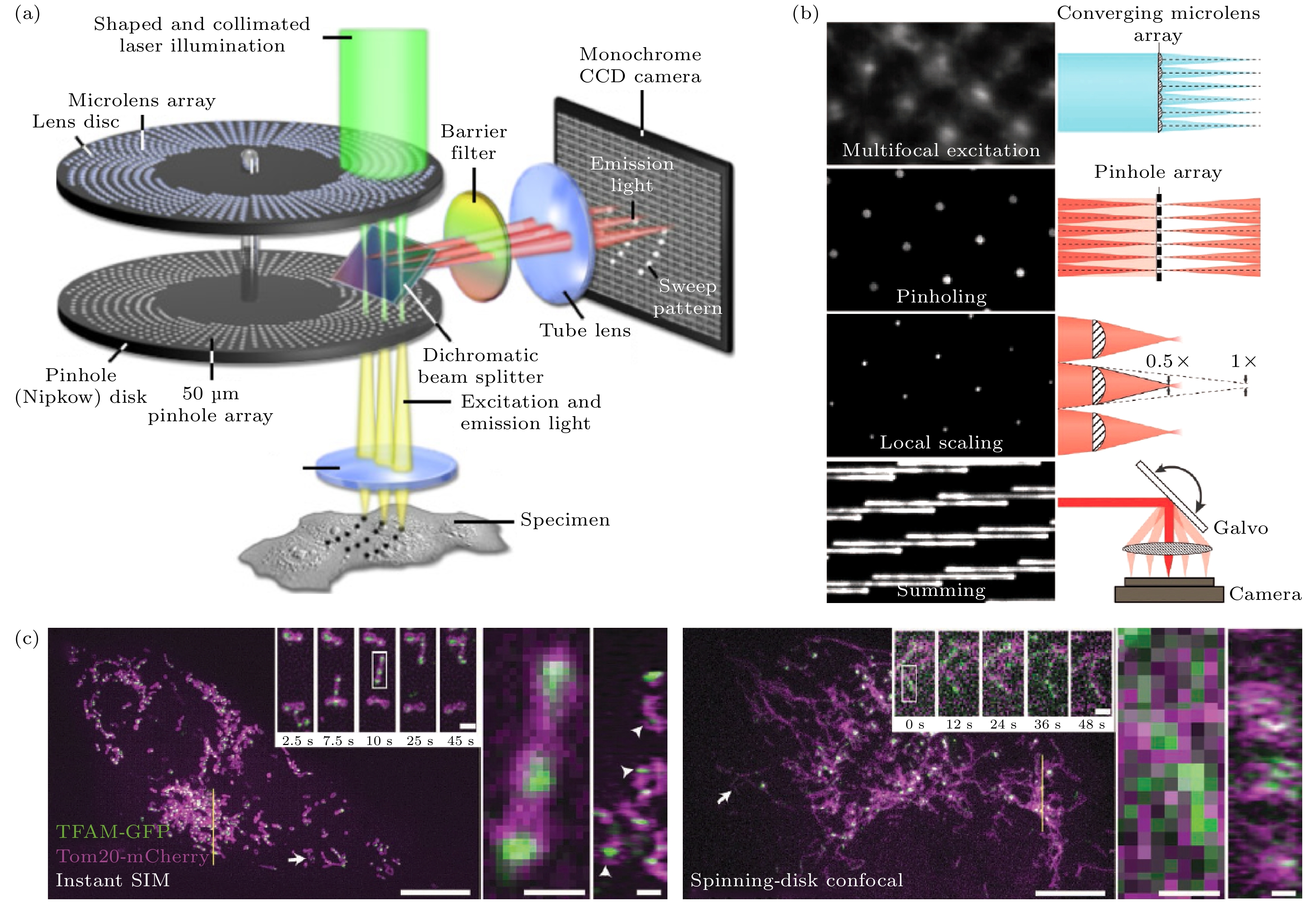
图 3 基于微透镜阵列的快速成像技术示意图 (a) 转盘共聚焦系统简化图; (b) 实现Instant-SIM的关键步骤[32]; (c) Instant-SIM和转盘共聚焦显微镜的活细胞双色成像对比[32]
Figure 3. Schematic diagram of fast imaging technology based on microlens array: (a) Simplified schematic of the spinning disk confocal system; (b) key steps in implementing Instant-SIM[32]; (c) dual-color imaging comparison of Instant-SIM and spinning disk confocal microscope on live-cell[32].
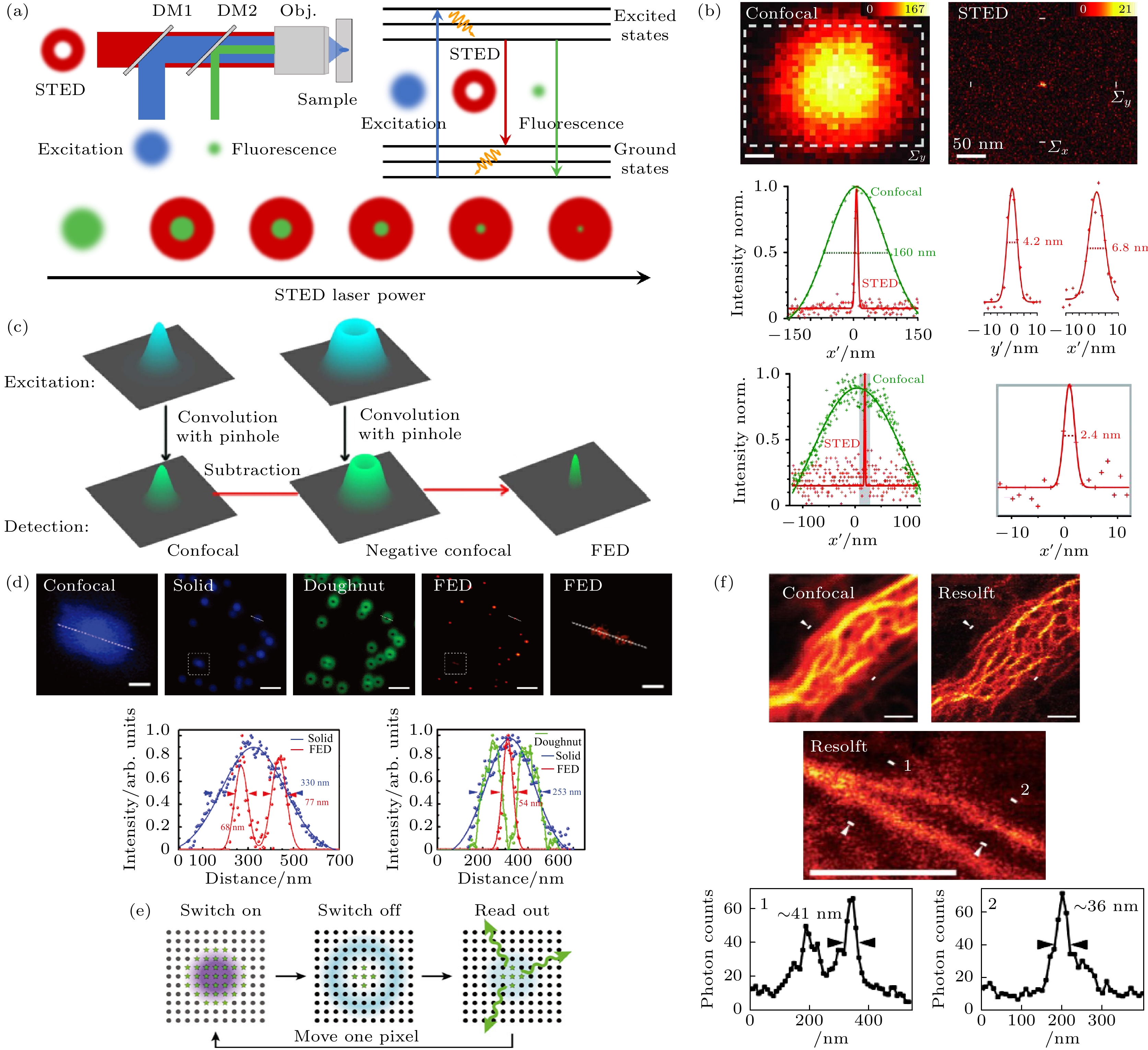
图 4 点扫描超分辨成像技术示意图 (a) STED原理图; (b) STED在NV色心上实现2.4 nm分辨率[37]; (c) FED原理图[43]; (d) 单颗粒FED成像[44]; (e) RESOLFT原理图[45]; (f)共聚焦显微镜和RESOLFT的细胞成像对比[46]
Figure 4. Schematic diagram of point scanning super-resolution imaging technology: (a) STED schematic; (b) STED achieves 2.4 nm resolution on NV point[37]; (c) FED schematic[43]; (d) UCNPs-FED single particle imaging[44]; (e) RESOLFT schematic[45]; (f) cell imaging comparison of confocal microscope and RESOLFT[46].
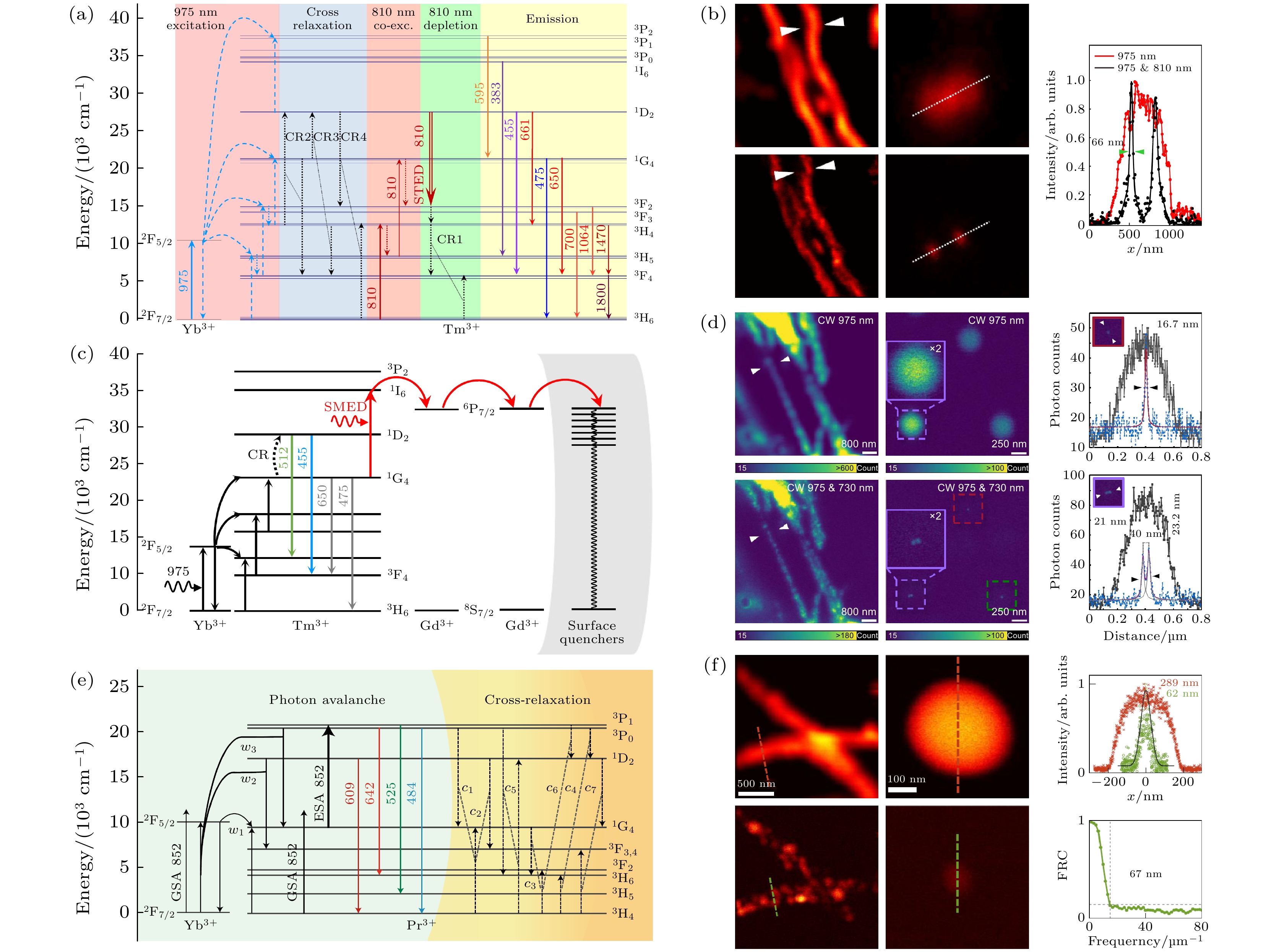
图 5 上转换超分辨成像技术示意图 (a) 交叉弛豫传能荧光损耗机制[57]; (b) 上转换STED超分辨成像结果[57]; (c) 表面迁移荧光损耗机制[60]; (d) SMED超分辨成像结果[60]; (e) Yb3+/Pr3+共掺杂纳米颗粒的光子雪崩机制[61]; (f) 光子雪崩超分辨成像结果[61]
Figure 5. Schematic diagram of up-conversion super-resolution imaging technology: (a) Cross-relaxation energy transfer fluorescence loss mechanism[57]; (b) up-conversion STED super-resolution imaging results[57]; (c) surface migration fluorescence loss mechanism[60]; (d) SMED super-resolution imaging results[60]; (e) photon avalanche mechanism of Yb3+/Pr3+ co-doped nanoparticles[61]; (f) photon avalanche super-resolution Resolution imaging results[61].
图 6 点扫描三维超分辨成像技术示意图 (a) 3D-STED PSF xz平面强度分布; (b) 3D-STED系统简化图[67]; (c) 单SLM实现AO-3DSTED 装置[69]; (d) 共聚焦显微镜和3D-STED对20 nm直径荧光球的成像对比[67]; (e) AO-isoSTED系统简化图以及PSF xz平面强度分布[73]; (f) 共聚焦显微镜和AO-isoSTED对细胞微管的成像对比[73]
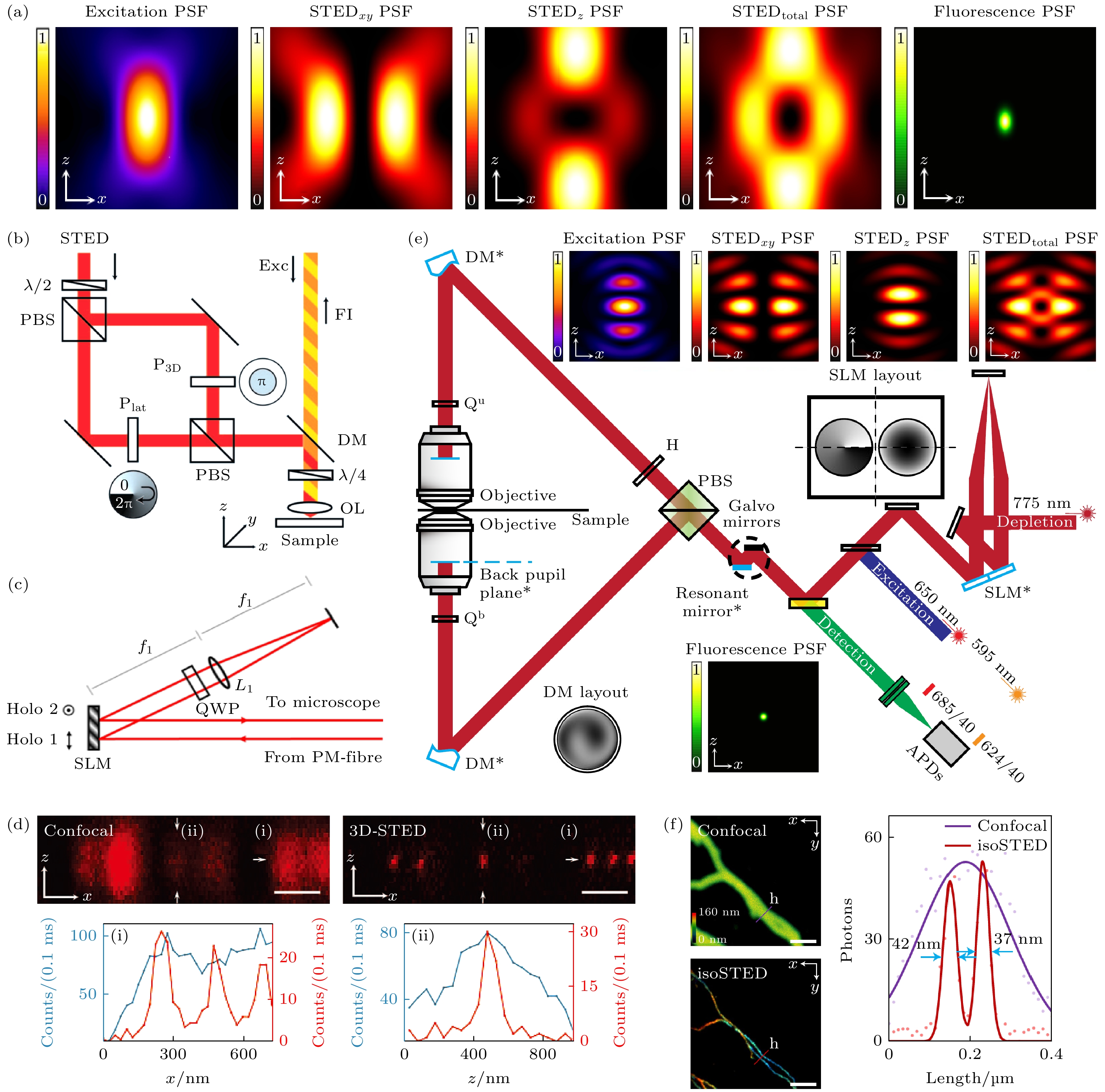
Figure 6. Schematic diagram of point scanning 3D super-resolution imaging technology: (a) 3D-STED PSF xz plane intensity distribution; (b) simplified schematic of 3D-STED system[67]; (c) AO-3DSTED with a single SLM[69]; (d) imaging comparison of confocal microscope and 3D-STED on 20 nm diameter fluorescent spheres[67]; (e) simplified schematic of AO-isoSTED system and PSF xz plane intensity distribution[73]; (f) imaging comparison of confocal microscope and AO-isoSTED on cell microtubules[73].
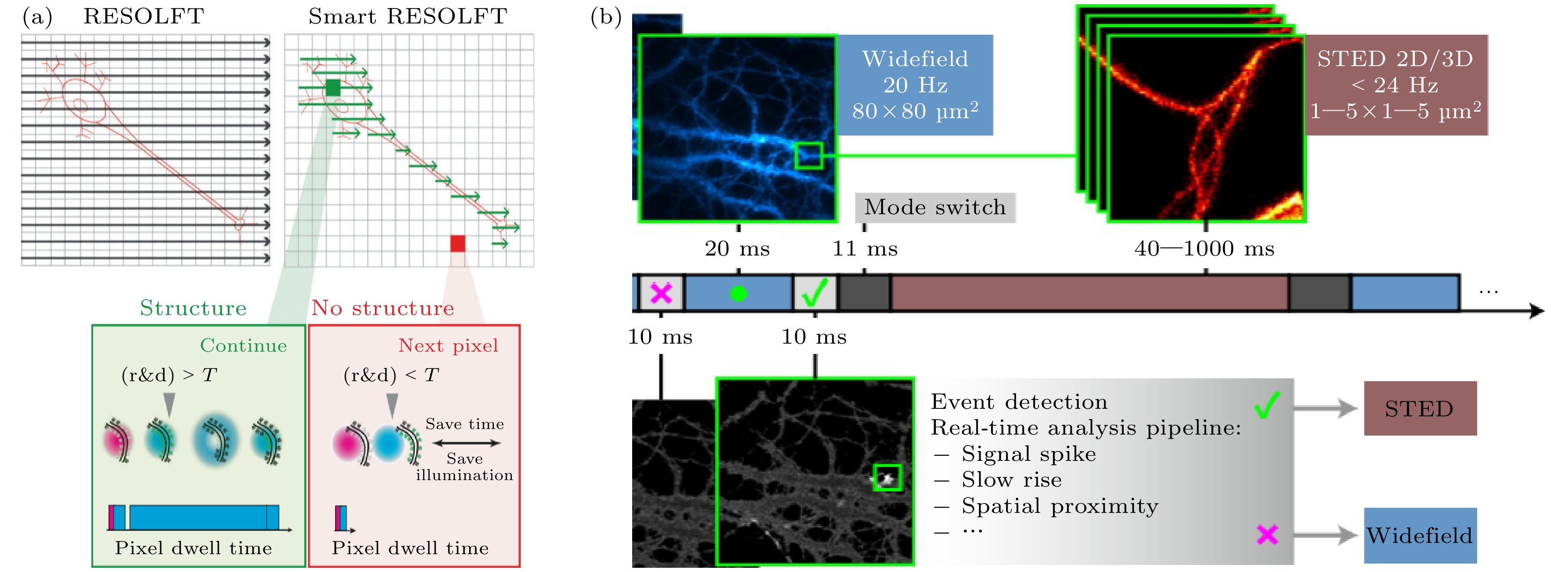
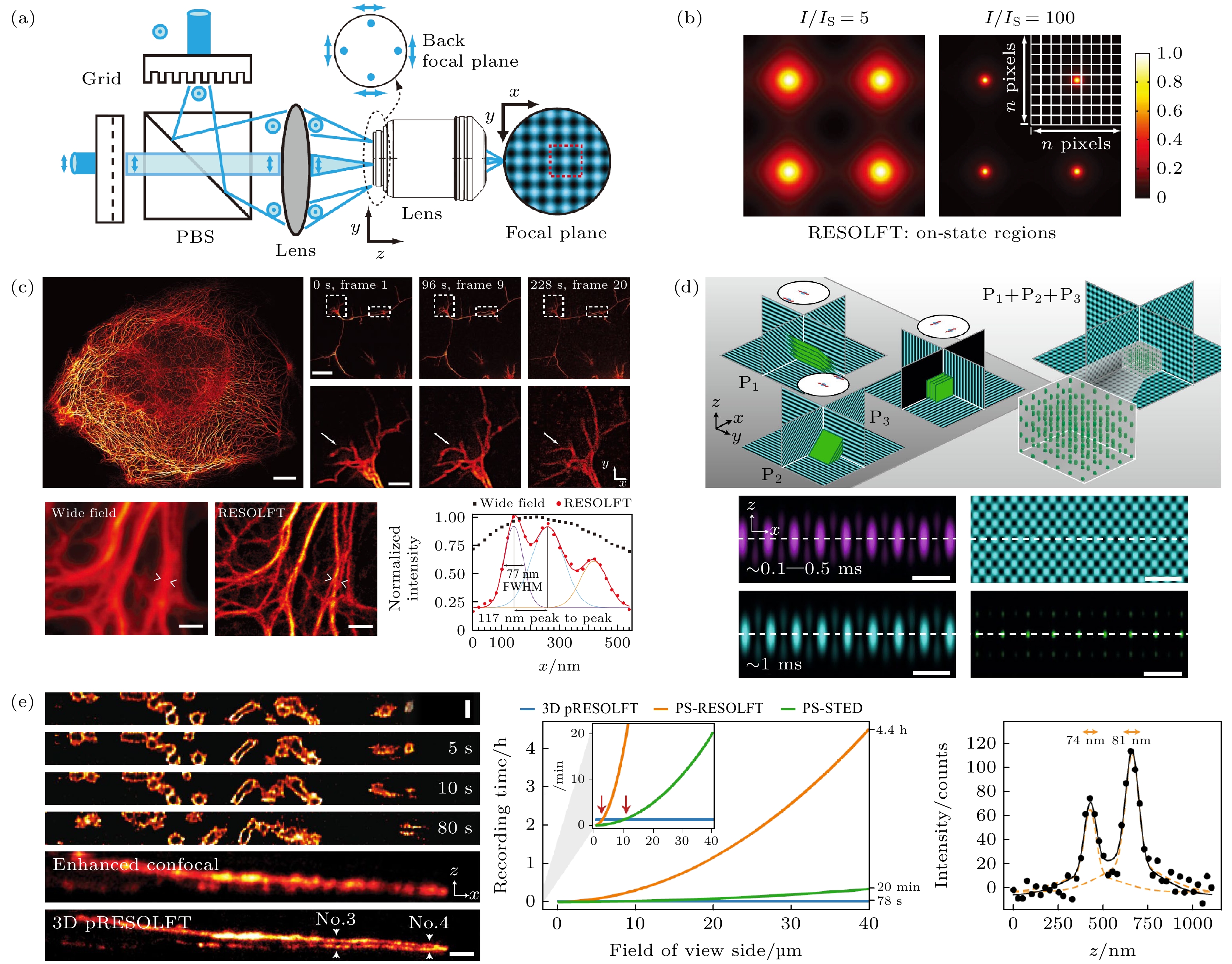
图 8 并行扫描超分辨成像技术示意图 (a) pRESOLFT 系统简化图[71]; (b) pRESOLFT “ON” 状态下不同 I/Is 的二维强度分布图[71]; (c) pRESOLFT 纳米显微镜的活细胞成像[71]; (d) 3D-pRESOLFT 三种模式的驻波叠加产生蜂窝状照明阵列[81]; (e) 3D-pRESOLFT 纳米显微镜的活细胞成像[81]
Figure 8. Schematic diagram of parallel scanning super-resolution imaging technology: (a) Simplified schematic of pRESOLFT system[71]; (b) 2D profiles of the on-state probability distribution for different I/Is [71]; (c) live-cell imaging with pRESOLFT nanoscopy[71]; (d) three patterns of standing waves are superimposed to create a honeycomb lighting array in 3D-pRESOLFT[81]; (e) live-cell imaging with 3D-pRESOLFT nanoscopy[81].
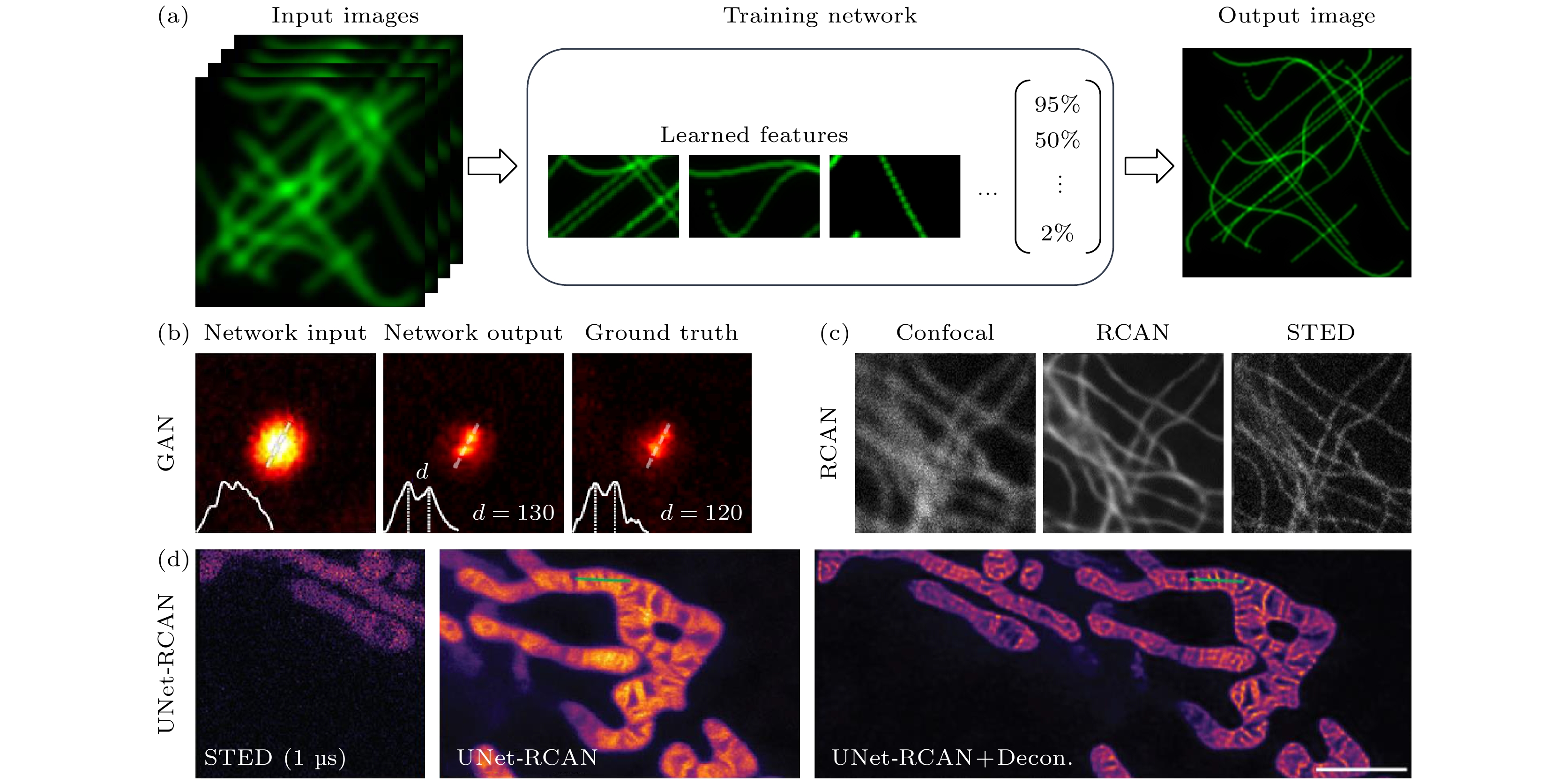
图 9 深度学习助力超高时空分辨率成像示意图 (a) 深度学习提高图像分辨率; (b) GAN网络训练结果[82]; (c) RCAN网络训练结果[84]; (d) Unet-RCAN网络训练结果[85]
Figure 9. Schematic diagram of deep learning-assisted ultra-high spatial-temporal resolution imaging: (a) Deep learning improves image resolution; (b) GAN network training results[82]; (c) RCAN network training results[84]; (d) Unet-RCAN network training results[85]
表 1 SMLM, SIM, STED超分辨技术的关键性能指标对比
Table 1. Technical comparison of SMLM, SIM, STED super-resolution microscopy.
表 2 不同点扫描超分辨成像技术的关键性能指标对比
Table 2. Overview of point-scanning super-resolution fluorescence microscopy techniques.
技术 横向分辨率
/nm轴向分辨率
/nm二维时间分辨率 激光类型 激光强度 荧光漂白
程度激光
波段组织成像
深度重构
算法Confocal[19] 200 500 0.2—2.0 s
(50 μm×50 μm)CW 40 μW—1 mW ☆☆☆ 可见光 中等 无需 Airyscan[20] 120—140 400 0.2—1.0 s
(50 μm×50 μm)CW 4—20 μW ☆☆ 可见光 低 需要 MSIM [22] 145 400 1 s
(45.6 μm×45.6 μm)CW
fs/ps1—25 μW
1.1 W☆☆ 可见光/
近红外低 需要 STED [6,12,13] 20—50 40—150 13 s
(50 μm×50 μm)fs/ps 1—10 GW/cm2 ☆☆☆☆☆ 可见光 中等 无需 UCNPs-STED
(SMED[60])17 ~45 10—50 s
(50 μm×50 μm)CW 18 kW/cm2 零漂白 近红外 大 无需 RESOFLT[50,52] ~40 ~120 100 s
(10 μm×10 μm)fs/ps 1 kW/cm2 ☆☆ 可见光 中等 无需 pRESOFLT[71,81] ~80 ~80 0.4 s
(10 μm×10 μm)fs/ps 1 kW/cm2 ☆☆ 可见光 很低 需要 isoSTED[70,73] ~40 ~40 0.5—5.0 s
(50 μm×50 μm)fs/ps 50—100 mW ☆☆☆☆☆ 可见光 中等 无需 MINFLUX[53,54] 1—3 1—3 1—2 min
(20 μm×20 μm)CW 10—50 kW/cm2 ☆ 可见光 很低 需要 MINSTED[55] 0.23 — 68 min
(1.37 μm×1.37 μm)fs/ps 1.5 μW ☆ 可见光 很低 需要 -
[1] Abee E 1873 Arch. f. Mikr. Anat. 9 413
[2] 赵光远, 郑程, 方月, 匡翠方, 刘旭 2017 66 148702
 Google Scholar
Google Scholar
Zhao G Y, Zheng C, Fang Y, Kuang C F, Liu X 2017 Acta Phys. Sin. 66 148702
 Google Scholar
Google Scholar
[3] Betzig E, Patterson G H, Sougrat R, Lindwasser O W, Olenych S, Bonifacino J S, Davidson M W, Lippincott-Schwartz J, Hess H F 2006 Science 313 1642
 Google Scholar
Google Scholar
[4] Rust M J, Bates M, Zhuang X W 2006 Nat. Methods 3 793
 Google Scholar
Google Scholar
[5] Gustafsson M G 2000 J. Microsc. 198 82
 Google Scholar
Google Scholar
[6] Hell S W, Wichmann J 1994 Opt. Lett. 19 780
 Google Scholar
Google Scholar
[7] Jungmann R, Steinhauer C, Scheible M, Kuzyk A, Tinnefeld P, Simmel F C 2010 Nano Lett. 10 4756
 Google Scholar
Google Scholar
[8] Jungmann R, Avendaño M S, Woehrstein J B, Dai M J, Shih W M, Yin P 2014 Nat. Methods 11 313
 Google Scholar
Google Scholar
[9] Schermelleh L, Ferrand A, Huser T, Eggeling C, Sauer M, Biehlmaier O, Drummen G P C 2019 Nat. Cell Biol. 21 72
 Google Scholar
Google Scholar
[10] Huang X S, Fan J C, Li L J, Liu H S, Wu R L, Wu Y, Wei L S, Mao H, Lal A, Xi P, Tang L Q, Zhang Y F, Liu Y M, Tan S, Chen L Y 2018 Nat. Biotechnol. 36 451
 Google Scholar
Google Scholar
[11] Shao L, Kner P, Rego E H, Gustafsson M G L 2011 Nat. Methods 8 1044
 Google Scholar
Google Scholar
[12] Nägerl U V, Willig K I, Hein B, Hell S W, Bonhoeffer T 2008 Proc. Natl. Acad. Sci. U. S. A. 105 18982
 Google Scholar
Google Scholar
[13] Wildanger D, Medda R, Kastrup L, Hell S 2009 J. Microsc. 236 35
 Google Scholar
Google Scholar
[14] Nwaneshiudu A, Kuschal C, Sakamoto F H, Anderson R R, Schwarzenberger K, Young R C 2012 J. Invest. Dermatol. 132 1
 Google Scholar
Google Scholar
[15] Wilson T 1989 J. Microsc. 154 143
 Google Scholar
Google Scholar
[16] Pawley J 2006 Handbook of Biological Confocal Microscopy (Vol. 236) (Berlin: Springer Science & Business Media
[17] Muller M 2006 Introduction to Confocal Fluorescence Microscopy (Vol. 69) (Bellingham: SPIE Press
[18] Sticker M, Elsässer R, Neumann M, Wolff H 2020 J. Microsc. 28 36
 Google Scholar
Google Scholar
[19] Sheppard C R 1988 Optik (Stuttgart) 80 53
[20] Huff J 2015 Nat. Methods 12 i
 Google Scholar
Google Scholar
[21] Müller C B, Enderlein J 2010 Phys. Rev. Lett. 104 198101
 Google Scholar
Google Scholar
[22] York A G, Parekh S H, Dalle Nogare D, Fischer R S, Temprine K, Mione M, Chitnis A B, Combs C A, Shroff H 2012 Nat. Methods 9 749
 Google Scholar
Google Scholar
[23] Ingaramo M, York A G, Wawrzusin P, Milberg O, Hong A, Weigert R, Shroff H, Patterson G H 2014 Proc. Natl. Acad. Sci. U. S. A. 111 5254
 Google Scholar
Google Scholar
[24] Wu Y C, Shroff H 2018 Nat. Methods 15 1011
 Google Scholar
Google Scholar
[25] Qin S, Isbaner S, Gregor I, Enderlein J 2021 Nat. Protoc. 16 164
 Google Scholar
Google Scholar
[26] Gräf R, Rietdorf J, Zimmermann T 2005 Microscopy Techniques (Berlin:Springer) -/- 57
[27] Toomre D, Pawley J B 2006 Handbook of Biological Confocal Microscopy (Boston: Springer US) p221
[28] Inoué S, Inoué T 2002 Cell Biological Applications of Confocal Microscopy(San Diego: Academic Press) p88
[29] Wang E, Babbey C, Dunn K W 2005 J. Microsc. 218 148
 Google Scholar
Google Scholar
[30] Enoki R, Ono D, Hasan M T, Honma S, Honma K I 2012 J. Neurosci. Methods 207 72
 Google Scholar
Google Scholar
[31] Abreu-Blanco M T, Verboon J M, Parkhurst S M 2011 J. Cell Biol. 193 455
 Google Scholar
Google Scholar
[32] York A G, Chandris P, Nogare D D, Head J, Wawrzusin P, Fischer R S, Chitnis A, Shroff H 2013 Nat. Methods 10 1122
 Google Scholar
Google Scholar
[33] Schulz O, Pieper C, Clever M, Pfaff J, Ruhlandt A, Kehlenbach R H, Wouters F S, Großhans J, Bunt G, Enderlein J 2013 Proc. Natl. Acad. Sci. U. S. A. 110 21000
 Google Scholar
Google Scholar
[34] Xu Y Z, Xu R H, Wang Z, Zhou Y, Shen Q F, Ji W C, Dang D F, Meng L J, Tang B Z 2021 Chem. Soc. Rev. 50 667
 Google Scholar
Google Scholar
[35] Vicidomini G, Bianchini P, Diaspro A 2018 Nat. Methods 15 173
 Google Scholar
Google Scholar
[36] Westphal V, Hell S W 2005 Phys. Rev. Lett. 94 143903
 Google Scholar
Google Scholar
[37] Wildanger D, Patton B R, Schill H, Marseglia L, Hadden J, Knauer S, Schönle A, Rarity J G, O'Brien J L, Hell S W 2012 Adv. Mater. 24 OP309
 Google Scholar
Google Scholar
[38] Wäldchen S, Lehmann J, Klein T, Van De Linde S, Sauer M 2015 Sci. Rep. 5 1
 Google Scholar
Google Scholar
[39] Hell S W, Kroug M 1995 Appl. Phys. B 60 495
 Google Scholar
Google Scholar
[40] Bretschneider S, Eggeling C, Hell S W 2007 Phys. Rev. Lett. 98 218103
 Google Scholar
Google Scholar
[41] Göttfert F, Pleiner T, Heine J, Westphal V, Görlich D, Sahl S J, Hell S W 2017 Proc. Natl. Acad. Sci. U. S. A. 114 2125
 Google Scholar
Google Scholar
[42] Heine J, Reuss M, Harke B, D’Este E, Sahl S J, Hell S W 2017 Proc. Natl. Acad. Sci. U. S. A. 114 9797
 Google Scholar
Google Scholar
[43] Kuang C F, Li S, Liu W, Hao X, Gu Z T, Wang Y F, Ge J H, Li H F, Liu X 2013 Sci. Rep. 3 1441
 Google Scholar
Google Scholar
[44] Huang B R, Wu Q S, Peng X Y, Yao L Q, Peng D F, Zhan Q Q 2018 Nanoscale 10 21025
 Google Scholar
Google Scholar
[45] Moeyaert B, Dedecker P 2014 Photoswitching Proteins: Methods and Protocols(New York: Humana Press) p261
[46] Grotjohann T, Testa I, Leutenegger M, Bock H, Urban N T, Lavoie-Cardinal F, Willig K I, Eggeling C, Jakobs S, Hell S W 2011 Nature 478 204
 Google Scholar
Google Scholar
[47] Wang N, Kobayashi T 2014 Opt. Express 22 28819
 Google Scholar
Google Scholar
[48] Wang N, Kobayashi T 2015 Opt. Express 23 13704
 Google Scholar
Google Scholar
[49] Zhao G Y, Kuang C F, Ding Z H, Liu X 2016 Opt. Express 24 23596
 Google Scholar
Google Scholar
[50] Hell S W, Jakobs S, Kastrup L 2003 Appl. Phys. A 77 859
 Google Scholar
Google Scholar
[51] Sharma R, Singh M, Sharma R 2020 Spectrochim. Acta Part A 231 117715
 Google Scholar
Google Scholar
[52] Grotjohann T, Testa I, Reuss M, Brakemann T, Eggeling C, Hell S W, Jakobs S 2012 Elife 1 e00248
 Google Scholar
Google Scholar
[53] Balzarotti F, Eilers Y, Gwosch K C, Gynnå A H, Westphal V, Stefani F D, Elf J, Hell S W 2017 Science 355 606
 Google Scholar
Google Scholar
[54] Gwosch K C, Pape J K, Balzarotti F, Hoess P, Ellenberg J, Ries J, Hell S W 2020 Nat. Methods 17 217
 Google Scholar
Google Scholar
[55] Weber M, von der Emde H, Leutenegger M, Gunkel P, Sambandan S, Khan T A, Keller-Findeisen J, Cordes V C, Hell S W 2023 Nat. Biotechnol. 41 569
 Google Scholar
Google Scholar
[56] Wu R T, Zhan Q Q, Liu H C, Wen X Y, Wang B J, He S L 2015 Opt. Express 23 32401
 Google Scholar
Google Scholar
[57] Zhan Q Q, Liu H C, Wang B J, Wu Q S, Pu R, Zhou C, Huang B R, Peng X Y, Ågren H, He S L 2017 Nat. Commun. 8 1058
 Google Scholar
Google Scholar
[58] Liu Y J, Lu Y Q, Yang X S, Zheng X L, Wen S H, Wang F, Vidal X, Zhao J B, Liu D M, Zhou Z G, Ma C S, Zhou J J, Piper J A, Xi P, Jin D Y 2017 Nature 543 229
 Google Scholar
Google Scholar
[59] Guo X, Pu R, Zhu Z M, Qiao S Q, Liang Y S, Huang B R, Liu H C, Labrador-Páez L, Kostiv U, Zhao P, Wu Q S, Widengren J, Zhan Q Q 2022 Nat. Commun. 13 2843
 Google Scholar
Google Scholar
[60] Pu R, Zhan Q Q, Peng X Y, Liu S Y, Guo X, Liang L L, Qin X, Zhao Z W, Liu X 2022 Nat. Commun. 13 6636
 Google Scholar
Google Scholar
[61] Liang Y S, Zhu Z M, Qiao S Q, Guo X, Pu R, Tang H, Liu H C, Dong H, Peng T T, Sun L-D, Widengren J, Zhan Q Q 2022 Nat. Nanotechnol. 17 524
 Google Scholar
Google Scholar
[62] Wu Q S, Huang B R, Peng X Y, He S L, Zhan Q Q 2017 Opt. Express 25 30885
 Google Scholar
Google Scholar
[63] Chen C C, Wang F, Wen S H, Su Q P, Wu M C, Liu Y T, Wang B M, Li D, Shan X C, Kianinia M, Aharonovich I, Toth I, Jackson M S, Xi P, Jin D Y 2018 Nat. Commun. 9 3290
 Google Scholar
Google Scholar
[64] Denkova D, Ploschner M, Das M, Parker L M, Zheng X, Lu Y, Orth A, Packer N H, Piper J A 2019 Nat. Commun. 10 3695
 Google Scholar
Google Scholar
[65] Lee C, Xu E Z, Liu Y, Teitelboim A, Yao K, Fernandez-Bravo A, Kotulska A M, Nam S H, Suh Y D, Bednarkiewicz A 2021 Nature 589 230
 Google Scholar
Google Scholar
[66] Klar T A, Jakobs S, Dyba M, Egner A, Hell S W 2000 Proc. Natl. Acad. Sci. U. S. A. 97 8206
 Google Scholar
Google Scholar
[67] Harke B, Ullal C K, Keller J, Hell S W 2008 Nano Lett. 8 1309
 Google Scholar
Google Scholar
[68] Gould T J, Burke D, Bewersdorf J, Booth M J 2012 Opt. Express 20 20998
 Google Scholar
Google Scholar
[69] Lenz M O, Sinclair H G, Savell A, Clegg J H, Brown A C, Davis D M, Dunsby C, Neil M A, French P M 2013 J. Biophotonics 1 29
 Google Scholar
Google Scholar
[70] Schmidt R, Wurm C A, Jakobs S, Engelhardt J, Egner A, Hell S W 2008 Nat. Methods 5 539
 Google Scholar
Google Scholar
[71] Chmyrov A, Keller J, Grotjohann T, Ratz M, d'Este E, Jakobs S, Eggeling C, Hell S W 2013 Nat. Methods 10 737
 Google Scholar
Google Scholar
[72] Böhm U, Hell S W, Schmidt R 2016 Nat. Commun. 7 10504
 Google Scholar
Google Scholar
[73] Hao X, Allgeyer E S, Lee D R, Antonello J, Watters K, Gerdes J A, Schroeder L K, Bottanelli F, Zhao J, Kidd P 2021 Nat. Methods 18 688
 Google Scholar
Google Scholar
[74] Staudt T, Engler A, Rittweger E, Harke B, Engelhardt J, Hell S W 2011 Opt. Express 19 5644
 Google Scholar
Google Scholar
[75] Dreier J, Castello M, Coceano G, Cáceres R, Plastino J, Vicidomini G, Testa I 2019 Nat. Commun. 10 556
 Google Scholar
Google Scholar
[76] Alvelid J, Damenti M, Sgattoni C, Testa I 2022 Nat. Methods 19 1268
 Google Scholar
Google Scholar
[77] Bingen P, Reuss M, Engelhardt J, Hell S W 2011 Opt. Express 19 23716
 Google Scholar
Google Scholar
[78] Hofmann M, Eggeling C, Jakobs S, Hell S W 2005 Proc. Natl. Acad. Sci. U. S. A. 102 17565
 Google Scholar
Google Scholar
[79] Brakemann T, Stiel A C, Weber G, Andresen M, Testa I, Grotjohann T, Leutenegger M, Plessmann U, Urlaub H, Eggeling C 2011 Nat. Biotechnol. 29 942
 Google Scholar
Google Scholar
[80] Bergermann F, Alber L, Sahl S J, Engelhardt J, Hell S W 2015 Opt. Express 23 211
 Google Scholar
Google Scholar
[81] Boden A, Pennacchietti F, Coceano G, Damenti M, Ratz M, Testa I 2021 Nat. Biotechnol. 39 609
 Google Scholar
Google Scholar
[82] Wang H D, Rivenson Y, Jin Y Y, Wei Z S, Gao R, Günaydın H, Bentolila L A, Kural C, Ozcan A 2019 Nat. Methods 16 103
 Google Scholar
Google Scholar
[83] Li M, Shan H, Pryshchep S, Lopez M M, Wang G 2020 J. Nanophotonics 14 016009
 Google Scholar
Google Scholar
[84] Chen J J, Sasaki H, Lai H Y, Su Y J, Liu J M, Wu Y C, Zhovmer A, Combs C A, Rey-Suarez I, Chang H Y, Huang C C, Li X S, Guo M, Nizambad S, Upadhyaya A, Lee S J, Lucas L A, Shroff H 2021 Nat. Methods 18 678
 Google Scholar
Google Scholar
[85] Ebrahimi V, Stephan T, Kim J, Carravilla P, Eggeling C, Jakobs S, Han K Y 2023 bioRxiv 2023.01. 26.525571
[86] Matthews T E, Piletic I R, Selim M A, Simpson M J, Warren W S 2011 Sci. Transl. Med. 3 71ra15
 Google Scholar
Google Scholar
[87] Simpson M J, Wilson J W, Robles F E, Dall C P, Glass K, Simon J D, Warren W S 2014 J. Phys. Chem. A 118 993
 Google Scholar
Google Scholar
[88] Simpson M J, Wilson J W, Phipps M A, Robles F E, Selim M A, Warren W S 2013 J. Invest. Dermatol. 133 1822
 Google Scholar
Google Scholar
[89] Massaro E S, Hill A H, Grumstrup E M 2016 ACS Photonics 3 501
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 11207
- PDF Downloads: 176
- Cited By: 0














 DownLoad:
DownLoad: