-
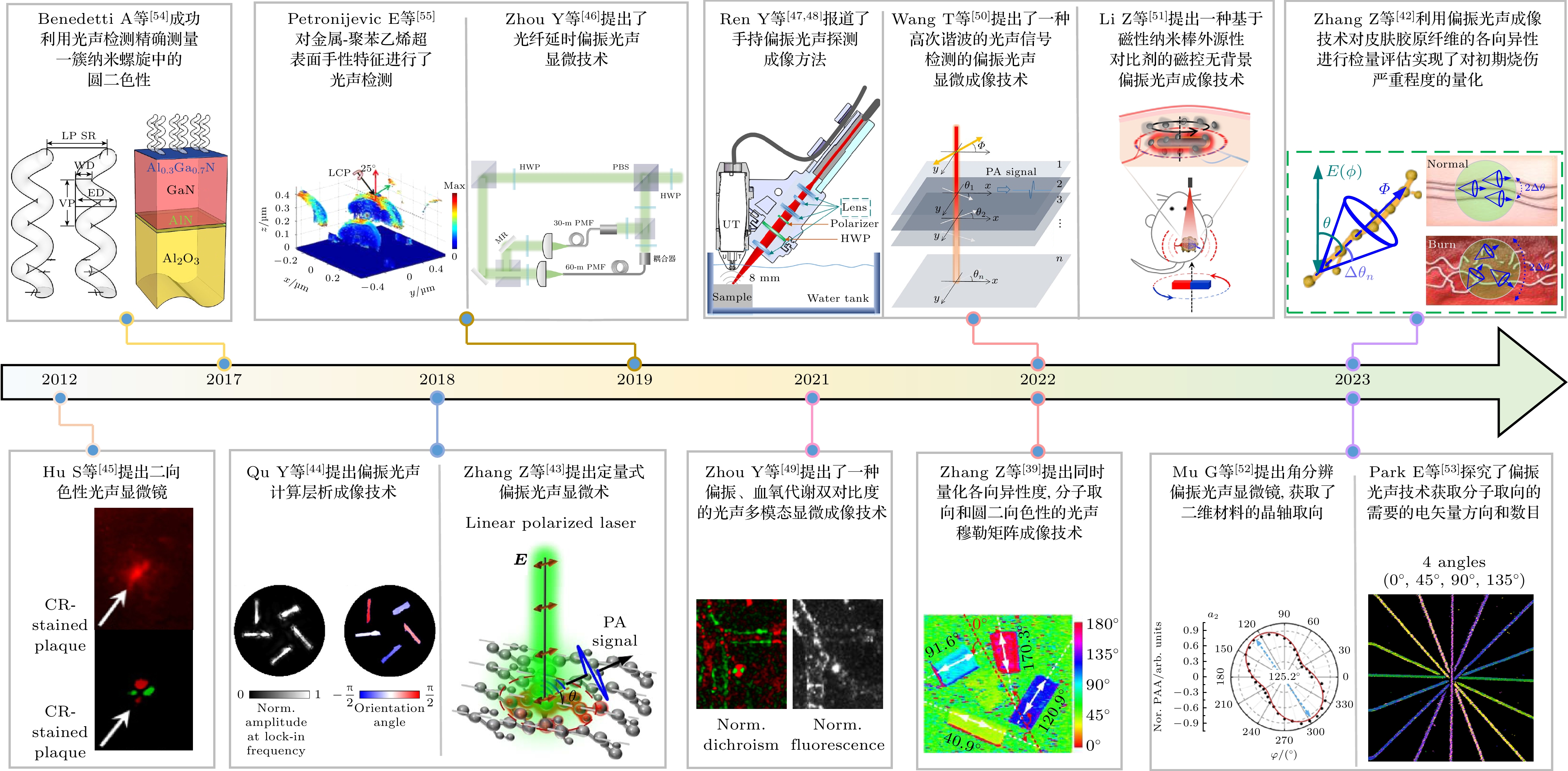
Life is a highly ordered combination, and the basic biological processes of cells and tissues are essentially controlled by the structural order of biomolecular assembly, in which the conformational characteristics of biomolecule arrangement, orientation, helix, and folding are closely related to the physiological functions of biological tissues. In the skin, muscle, and nerve tissues of living animals, for instance, fibrous proteins, collagen, nerve fibers, and DNA frequently exhibit molecular spatial conformation properties such as particular alignment or helical structure, and such tissues have distinct optical polarization responses. The fundamental structural foundation for tissues to carry out certain activities is provided by molecular conformational characteristics. Early illness diagnosis will be aided by the accurate detection and efficient revelation of molecular conformational characteristics and their changes. The microscopic organization, structure, orientation, chirality, and other structural details of living things or materials can be obtained by using polarization imaging. The analysis of the imaging depth and polarization data is challenging, despite its widespread usage in the fields of material detection and biological imaging. Photoacoustic imaging preserves both the great contrast of optical imaging and the deep penetration of ultrasonic imaging by using light as an excitation source and ultrasound as the carrier for information transmission. While keeping the benefits of non-invasiveness, it is capable of high-resolution imaging, deep penetration, and functional imaging. A polarized photoacoustic imaging technology has recently been developed to complement polarization optical imaging and allow the collection of three-dimensional polarization data from deeper layers of the medium. This provides a straightforward and efficient method of measuring the polarimetry of tissues, suggesting substantial promise for both biological imaging and substance detection. The evolution of polarized photoacoustic imaging technology is outlined in this paper. First, the technical underpinnings of polarized photoacoustic imaging are described. Then, from the two applications of biological tissue imaging and nanomaterial detection, the related research progress of polarized photoacoustic microscopic imaging, polarized photoacoustic computational tomography, and polarized photoacoustic nanoparticles' molecular imaging is presented. We briefly explain the depolarization that results from particle size, density, and organization as polarized light travels through tissue. In an anisotropic medium, the change in the mid-incident polarization state of such a sample is caused by tissue birefringence and scattering because of the inherent birefringence effect of molecules, whereas in the isotropic medium, depolarization is largely determined by the density and size of the scatter. The potential applications of polarized photoacoustic imaging are then discussed.
-
Keywords:
- anisotropy /
- molecular orientation /
- chirality /
- polarized photoacoustic imaging
[1] Röntgen W C 1896 Science 3 227
 Google Scholar
Google Scholar
[2] Castellanos F X, Giedd J N, Marsh W L, Hamburger S D, Vaituzis A C, Dickstein D P, Sarfatti S E, Vauss Y C, Snell J W, Lange N, Kaysen D, Krain A L, Ritchie G F, Rajapakse J C, Rapoport J L 1996 JAMA Psychiatry 53 607
 Google Scholar
Google Scholar
[3] Ketcham R A, Carlson W D 2001 Comp. Geos. 27 381
 Google Scholar
Google Scholar
[4] Chang J H, Kim H H, Lee J, Shung K 2010 Ultrasonics 50 453
 Google Scholar
Google Scholar
[5] Huang D, Swanson E A, Lin C P, Schuman J S, Stinson W G, Chang W, Hee M R, Flotte T, Gregory K, Puliafito C, Fujimoto J 1991 Science 254 1178
 Google Scholar
Google Scholar
[6] Hell S, Stelzer E H K 1992 JOSA A. 9 2159
 Google Scholar
Google Scholar
[7] Huang B, Bates M, Zhuang X 2009 Annu. Rev. Biochem. 78 993
 Google Scholar
Google Scholar
[8] Torrealba F, Carrasco M A 2004 Brain. Res. Rev. 47 5
 Google Scholar
Google Scholar
[9] Stuker F, Ripoll J, Rudin M 2011 Pharmaceutics 3 229
 Google Scholar
Google Scholar
[10] Cox B, Laufer J G, Arridge S R, Beard P C 2012 J. Biomed. Opt. 17 061202
 Google Scholar
Google Scholar
[11] Axer M, Grassel D, Kleiner M, Dammers J, Dickscheid T, Reckfort J, Hütz T, Eiben B, Pietrzyk U, Zilles K, Amunts K 2011 Front. Neurol. 5 34
 Google Scholar
Google Scholar
[12] Tang P, Kirby M A, Le N, Li Y, Zeinstra N, Lu G N, Murry C E, Zheng Y, Wang R 2021 Light Sci. Appl. 10 237
 Google Scholar
Google Scholar
[13] Di Martino J S, Nobre A R, Mondal C, Taha I, Farias E F, Fertig E J, Naba A, Aguirre-Ghiso J A, Bravo-Cordero J J 2022 Nature Cancer 3 90
 Google Scholar
Google Scholar
[14] Jin L W, Claborn K A, Kurimoto M, Geday M A, Maezawa I, Sohraby F, Estrada M, Kaminksy W, Kahr B 2003 Proc. Natl. Acad. Sci. 100 15294
 Google Scholar
Google Scholar
[15] Fitzpatrick A W P, Falcon B, He S, Murzin A G, Murshudov G, Garringer H J, Crowther R A, Ghetti B, Goedert M, Scheres S H W 2017 Nature 547 185
 Google Scholar
Google Scholar
[16] Zhao M, Wang L, Wang M, Zhou S, Lu Y, Cui H, Racanelli A C, Zhang L, Ye T, Ding B, Zhang B, Yang J 2022 Signal Transduct Target Ther. 7 206
 Google Scholar
Google Scholar
[17] 云天梁, 曾楠, 李伟, 马辉 2009 光学学报 29 1926
 Google Scholar
Google Scholar
Yun T L, Zeng N, Li W, Ma H 2009 Acta Optica Sinica 29 1926
 Google Scholar
Google Scholar
[18] Rodriguez L G, Lockett S J, Holtom G R 2006 Cytometry A 69 779
 Google Scholar
Google Scholar
[19] Jameson D M, Ross J A 2010 Chem. Rev. 110 2685
 Google Scholar
Google Scholar
[20] Bell A G 1880 J. Soc. Telegr. Eng. 9 34
 Google Scholar
Google Scholar
[21] Zhang Z H, Shi Y J, Yang S H, Xing D 2018 Opt. Lett. 43 2336
 Google Scholar
Google Scholar
[22] 申晓雯, 武红鹏, 董磊 2023 光子学报 52 81
 Google Scholar
Google Scholar
Shen X W, Wu H P, Dong L 2023 Acta Photonica Sinica 52 81
 Google Scholar
Google Scholar
[23] Liu Y, Nie L, Chen X 2016 Trends Biotechnol. 34 420
 Google Scholar
Google Scholar
[24] Wang L V, Hu S 2012 Science 335 1458
 Google Scholar
Google Scholar
[25] Zhao Y, Yang S H, Chen C G, Xing D 2014 Opt. Lett. 39 2565
 Google Scholar
Google Scholar
[26] Lin L, Hu P, Shi J, Appleton C M, Maslov K, Li L, Wang L V 2018 Nat. Commun. 9 2352
 Google Scholar
Google Scholar
[27] Wang Z, Yang F, Cheng Z, Zhang W, Xiong K, Yang, S 2021 Nanophotonics 10 3359
 Google Scholar
Google Scholar
[28] Wen X, Lei P, Huang S, Chen X, Yuan Y, Ke D, Yang S. 2023 Photonics Res. 1 55
 Google Scholar
Google Scholar
[29] 穆根, 张振辉, 石玉娇 2022 中国激光 49 2007208
 Google Scholar
Google Scholar
Mu G, Zhang Z H, Shi Y 2022 Chin. J. Lasers 49 2007208
 Google Scholar
Google Scholar
[30] Wang Z, Yang F, Zhang W, Xiong K, Yang S 2023 Fundamental Research online
 Google Scholar
Google Scholar
[31] Van den Berg P, Daoudi K, Steenbergen W 2015 Photoacoustics 3 89
 Google Scholar
Google Scholar
[32] Chen J, Zhang Y, Li X, Zhu J, Li D, Li S, Lee C-S, Wang L 2020 Photonics Res. 8 1875
 Google Scholar
Google Scholar
[33] 陈丛桂, 赵岳, 杨思华 2013 激光生物学报 22 486
 Google Scholar
Google Scholar
Chen C G, Zhao Y, Yang S H 2013 Acta Laser Biology Sinica 22 486
 Google Scholar
Google Scholar
[34] Yang F, Chen Z, Xing D 2019 IEEE. Trans. Med. Imaging. 39 1791
 Google Scholar
Google Scholar
[35] Wang L, Zhang C, Wang L V 2014 Phys. Rev. Lett. 113 174301
 Google Scholar
Google Scholar
[36] Wang L V, Yao J 2016 Nat. Methods. 13 627
 Google Scholar
Google Scholar
[37] 张振辉, 石玉娇 2019 激光生物学报 28 32
 Google Scholar
Google Scholar
Zhang Z H, Shi Y J 2019 Acta Laser Biology Sin. 28 32
 Google Scholar
Google Scholar
[38] Yao J, Wang L V 2013 Laser Photonics Rev. 7 758.
 Google Scholar
Google Scholar
[39] Zhang Z H, Shi Y H, Shen Q, Wang Z Y, Xing D, Yang S H 2022 arXiv: 2207. 13271v1 [physics. med-ph
[40] Rodger A 2006 Circular Dichroism and Linear Dichroism. Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation pp1–42
[41] Ma W, Xu L, de Moura A F, Wu X, Kuang H, Xu C, Kotov N A 2017 Chem. Rev. 117 8041
 Google Scholar
Google Scholar
[42] Zhang Z H, Chen W, Cui D D, Mi J, Mu G, Nie L M, Yang S H, Shi Y J 2023 Photonics Res. 11 817
 Google Scholar
Google Scholar
[43] Zhang Z H, Shi Y J, Xiang L Z, Xing D 2018 Opt. Lett. 43 5267
 Google Scholar
Google Scholar
[44] Qu Y, Li L, Shen Y, Wei X, Wong T T, Hu P, Yao J, Maslov K, Wang L V 2018 Optica 5 495
 Google Scholar
Google Scholar
[45] Hu S, Maslov K, Yan P, Lee J-M, Wang L V 2012 Biomedical. Optics Miami, USA, April 28–May 2, 2012 pBM2B-8
[46] Zhou Y, Chen J, Liu C, Liu C, Lai P, Wang L 2019 Photoacoustics 16 100148
 Google Scholar
Google Scholar
[47] Ren Y, Zhang Y, He H, Liu L, Wu X, Song L, Liu C 2022 Quant. Imaging Med. Surg. 12 2238
 Google Scholar
Google Scholar
[48] 张迎, 吴晓君, 何宏辉, 文斌华, 刘成波, 任亚光 2022 激光与光电子学报 59 0817001
 Google Scholar
Google Scholar
Zhang Y, Wu X J, He H H, Wen B H, Liu C B, Ren Y G 2022 Journal of Optoelectronics Laser 59 0817001
 Google Scholar
Google Scholar
[49] Zhou Y, Zhong F, Yan P, Lee J-M, Hu S 2021 Opt. Lett. 46 2561
 Google Scholar
Google Scholar
[50] Wang T, Yang J, Wang W S, Wang L X, Wang X C, Zhou S 2022 Ann. Transl. Med. 10 293
 Google Scholar
Google Scholar
[51] Li Z W, Meng Z Q, Tian F, Ye Z Y, Zhou X F, Zhong X J, Chen Q, Yang M, Liu Z, Yin Y D 2022 Nano Lett. 22 5158
 Google Scholar
Google Scholar
[52] Mu G, Zhang Z H, Cui D D, Chen W, Shi Y J 2023 Opt. Lett. 48 2748
 Google Scholar
Google Scholar
[53] Park E, Lee Y J, Kim C, Eom T J 2023 Photoacoustics 31 100510
 Google Scholar
Google Scholar
[54] Benedetti A, Alam B, Esposito M, Tasco V, Leahu G, Belardini A, Li Voti R, Passaseo A, Sibilia C 2017 Sci. Rep. 7 5257
 Google Scholar
Google Scholar
[55] Petronijević E, Leahu G, Li Voti R, Belardini A, Scian C, Michieli N, Cesca T, Mattei G, Sibilia C 2019 Appl. Phys. Lett. 114 053101
 Google Scholar
Google Scholar
[56] Tuchin V V 2016 J. Biomed. Opt. 21 071114
 Google Scholar
Google Scholar
[57] Schmitt J M, Gandjbakhche A H, Bonner R F 1992 Appl. Opt. 31 6535
 Google Scholar
Google Scholar
[58] Morgan S P, Khong M P, Somekh M G 1997 App. Opt. 36 1560
 Google Scholar
Google Scholar
[59] Ghosh N, Patel H S, Gupta P K 2003 Opt. Express 11 2198
 Google Scholar
Google Scholar
[60] Shen F, Zhang B, Guo K, Yin Z, Guo Z 2017 IEEE Photonics J. 10 1
 Google Scholar
Google Scholar
-
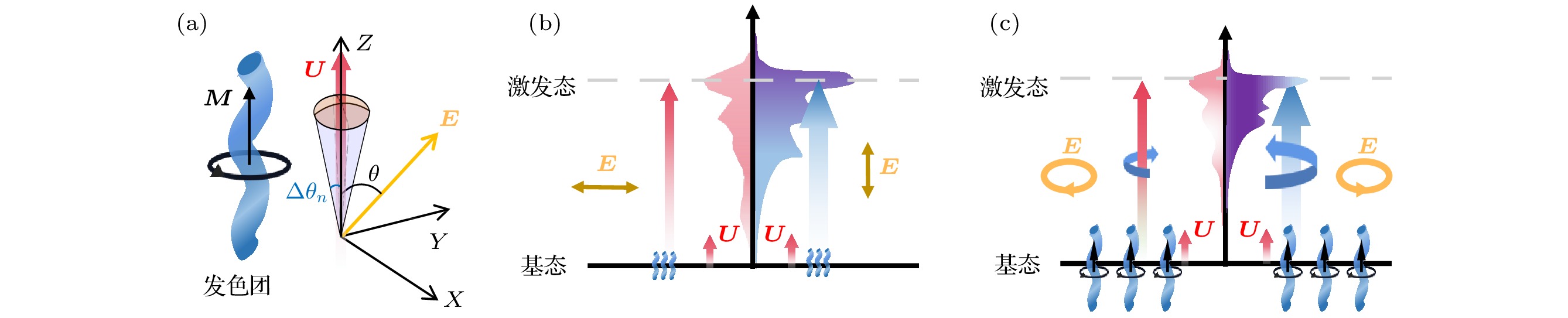
图 2 分子矢量吸收原理示意 (a) 单个发色团各向异性吸收示意图, E, U和M分别为入射光的电矢量, 发色团的电跃迁偶极矩(ETDM)和磁跃迁偶极矩(MTDM), θ为E与U之间的夹角, Δθn为单分子跃迁偶极矩取向的摆动范围; (b), (c) 分别表示线偏振光和圆偏振光激发各向异性分子的电子能级跃迁示意图
Figure 2. Schematic of molecular vector absorption principle: (a) Schematic diagram of anisotropic absorption of a single chromophore, E, U and M are the electric vector of incident light, the electric transition dipole moment (ETDM) and the magnetic transition dipole moment (MTDM) of the chromophore, respectively, and θ is the angle between E and U, Δθn is the swing range of the single molecule transition dipole moment orientation; (b), (c) schematic diagrams of electronic energy level transitions of anisotropic molecules excited by linearly polarized light and circularly polarized light, respectively.
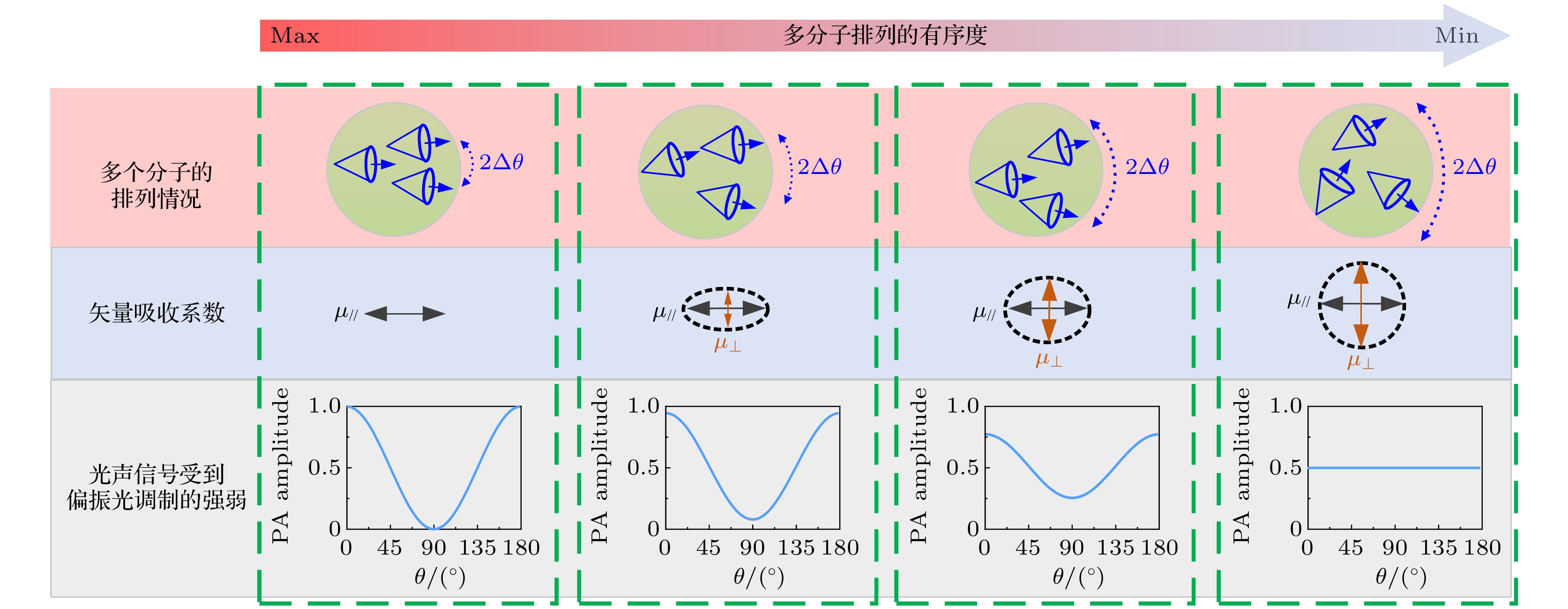
图 3 光激发范围内多个跃迁电偶极矩分布示意图, Δθ代表平均跃迁电偶极矩弥散角度, Δθ越大代表多分子排列有序度越小[42]
Figure 3. The schematic diagram of the distribution of multiple transition electric dipole moments in the photoexcitation range, where Δθ represents the average diffusion angle of the transition electric dipole moment, and the larger Δθ represents the smaller order of the multiple molecules[42].
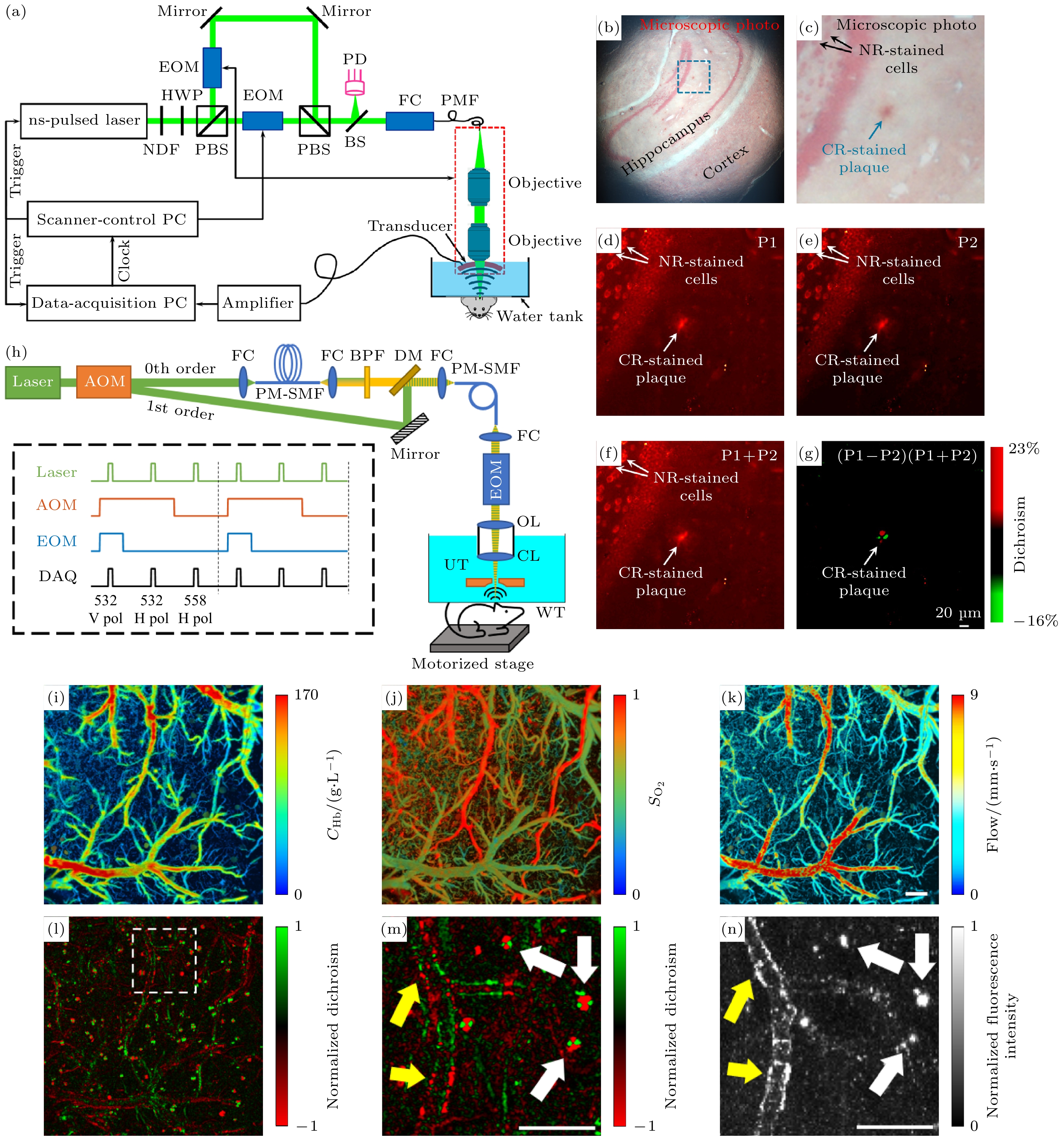
图 5 线二向色性光学分辨率光声显微镜及实验结果[45,49] (a) 线二向色性光学分辨率光声显微镜(DOR-PAM)系统原理图; (b) 脑切片的光学显微镜照片, 用刚果红(CR)和中性红(NR)双染色APP/PS1小鼠脑切片; (c) 图(b)中蓝色区域的放大图和箭头分别表示CR染色的淀粉样斑块和NR染色的背景细胞; (d), (e) 在两种正交偏振光照射下获得的DOR-PAM图像; (f), (g) 分别为图(d)和图(e)的总和差值, 图(g) 中的差异检测消除了非二向色背景, 并突出了淀粉样斑块的线二向色性对比; (h) 二向色性-血氧血流多参数双对比PAM原理图, 框形插图给出了为同时二向色性和多参数PAM成像设计的激光激励方案, 其中AOM为声光调制器, FC为光纤耦合器, PM-SMF为保偏单模光纤, BPF为带通滤波器, DM为二向镜, EOM为电光调制器, OL为物镜, CL为矫正镜, UT为超声波换能器, WT为水箱, DAQ为数据采集, 水平/垂直极化; (i)—(k) 10月龄的小鼠活体脑多参数PAM图像, 包括活体鼠脑血红蛋白的总浓度(CHb)、血红蛋白氧饱和度(sO2)和血流成像; (l) 二向色性PAM图像显示刚果红(CRD)染色的淀粉样斑块和血管壁沉积物的分布; (m) 图(l)中矩形框区域放大图; (n) 图(l)中矩形框区域共聚焦图像, 比例尺为200 μm
Figure 5. The DOR-PAM system and experimental results[45,49]: (a) Schematic of the DOR-PAM system; (b) optical microscopic photo of the brain section; (c) close-up of the boxed area in Fig.(b), the blue and black arrows indicate the CR-stained amyloid plaque and the NR-stained background cells, respectively; (d), (e) the DOR-PAM images acquired with each of the two orthogonally polarized optical irradiations, respectively; (f), (g) the summation and subtraction of Fig.(d) and Fig.(e), respectively, the differential detection in Fig.(f) eliminates the non-dichroic background and highlights the dichroic contrast of the amyloid plaque; (h) schematic of dual-contrast PAM, the boxed inset illustrates the laser excitation scheme designed for simultaneous dichroism and multi-parametric PAM, where AOM is the acousto-optic modulator; FC is the fiber coupler, PM-SMF is the polarization-maintaining single-mode optical fiber, BPF is the bandpass filter, DM is the dichroic mirror, EOM is the electro-optic modulator, OL is the objective lens, CL is the correction lens; UT is the ultrasonic transducer, WT is the water tank, DAQ is the data acquisition, H/V pol, horizontal/vertical polarization; (i)–(k) multi-parametric PAM images of a 10-month-old APP/PS1 mouse brain in vivo, including the CHb, sO2, and blood flow speed; (l) dichroism PAM image showing the distribution of CR stained amyloid plaques and deposits in the vessel wall; (m) close-up of the boxed area in Fig.(l); (n) confocal image of the boxed area in Fig.(l). Scale bars: 200 μm.
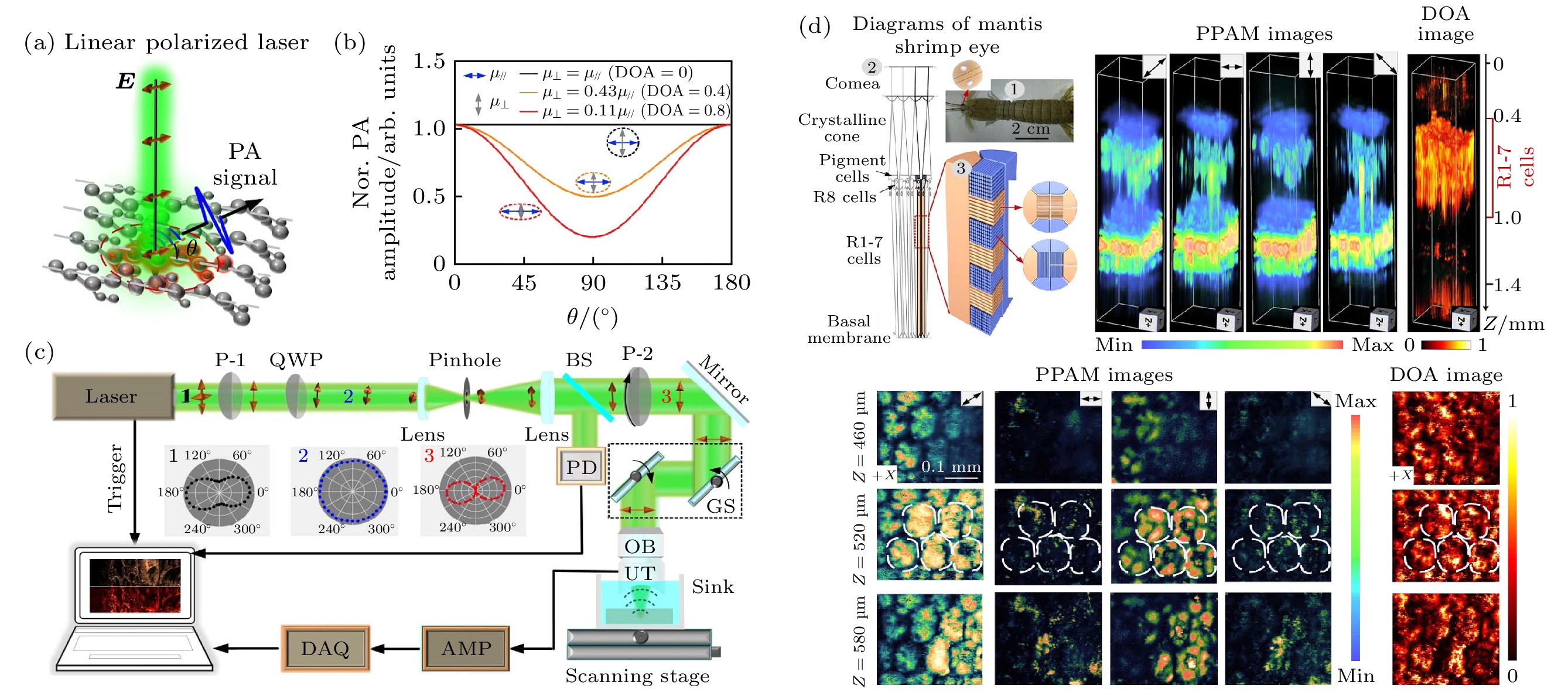
图 6 定量式偏振光声显微镜的原理装置和实验结果[43] (a) 分子矢量吸收原理示意; (b) DOA值与偏振光声信号调制强度的关联; (c) PPAM装置图; (d) PPAM三维成像螳螂虾复眼的各向异性特征
Figure 6. Theoretical setup and experimental results of a quantitative polarized photoacoustic microscopy (PPAM)[43]: (a) Schematic diagram of molecular vector absorption principle; (b) the correlation between the DOA value and the modulation intensity of polarized photoacoustic signals; (c) PPAM device diagram; (d) the anisotropic characteristics of the compound eyes of praying mantis and shrimp in PPAM three-dimensional imaging.
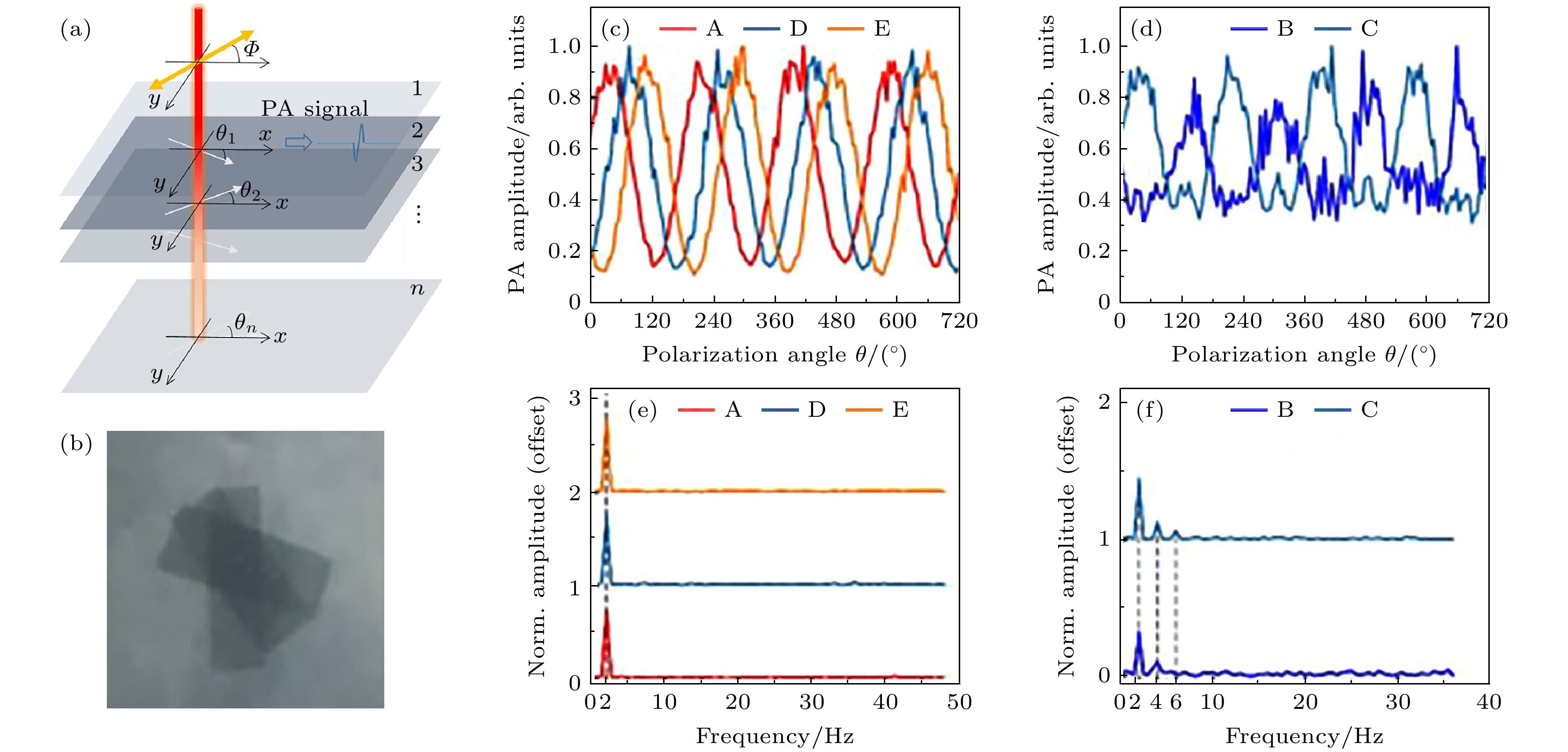
图 7 高次谐波的偏振光声信号检测技术和实验结果[50] (a) 线性偏振激光在不同层产生的光声信号, 黄色箭头表示激光的电矢量方向, 白色箭头表示光轴的方向; (b) 三维各向异性样品照片; (c) 在非重叠区域的归一化光声振幅; (d) 在重叠区域的归一化光声振幅; (e) 在非重叠区域的归一化光声信号振荡的频域分布; (f) 重叠区域的归一化光声信号振荡的频域分布
Figure 7. High order harmonic polarized photoacoustic signal detection technology and experimental results[50]: (a) Photoacoustic signal generated by the linearly polarized laser at different layers, the yellow arrow indicates the polarization angle of the laser, and the white arrow indicates the orientation of the optical axis; (b) 3D anisotropy phantom; (c) normalized photoacoustic amplitude at the non-overlapping area; (d) normalized photoacoustic amplitude at the overlapping area; (e) spectrum of the normalized photoacoustic amplitude at the non-overlapping area; (f) spectrum of the normalized photoacoustic amplitude at the overlapping area.
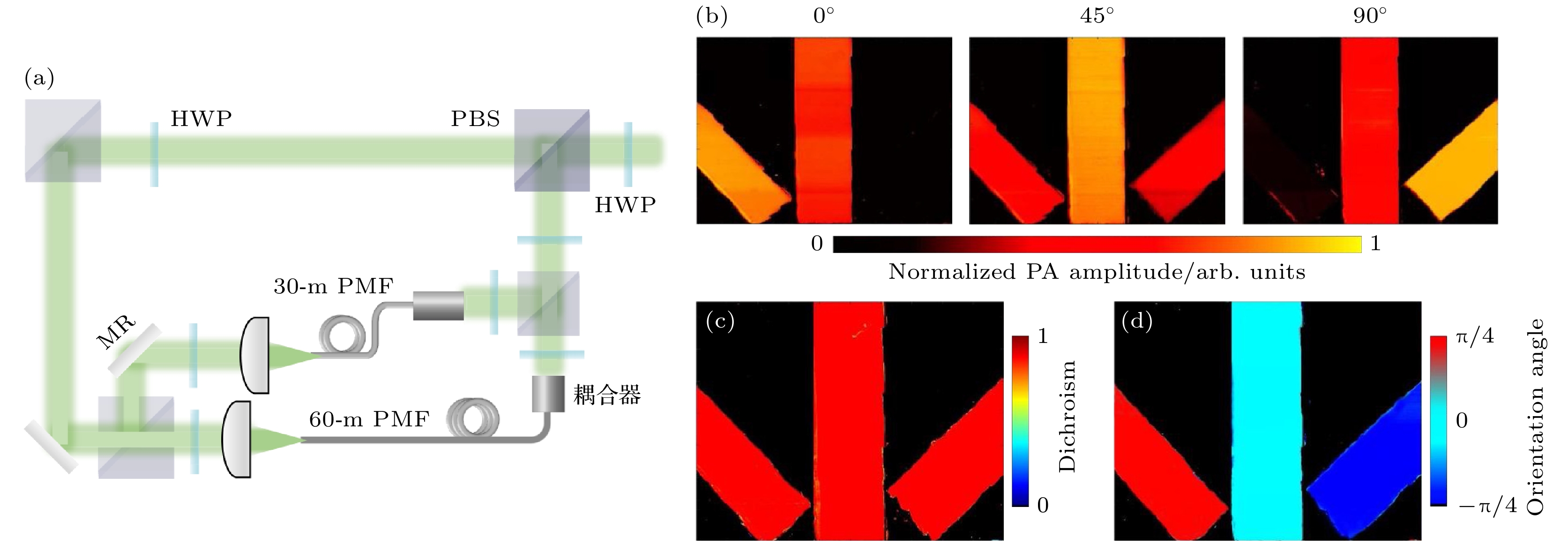
图 8 光纤延时单次扫描的偏振光声技术[46] (a) 光纤延时线二向色性系统示意图; (b) 电矢量方向为0°, 45°和90°的三束线偏振光分别激发得到的光声图像; (c), (d) 计算得到的线二向色性图像和分子取向图像
Figure 8. Polarized photoacoustic technology for fiber delay single scan[46]: (a) Schematic of the dual-fiber single-shot dichroism OR-PAM system; WT, water tank; (b) PA images of three linear polarizers excited with polarized light at 0°, 45°, and 90°; (c), (d) calculated dichroism image and polarization angle of the three linear polarizers.
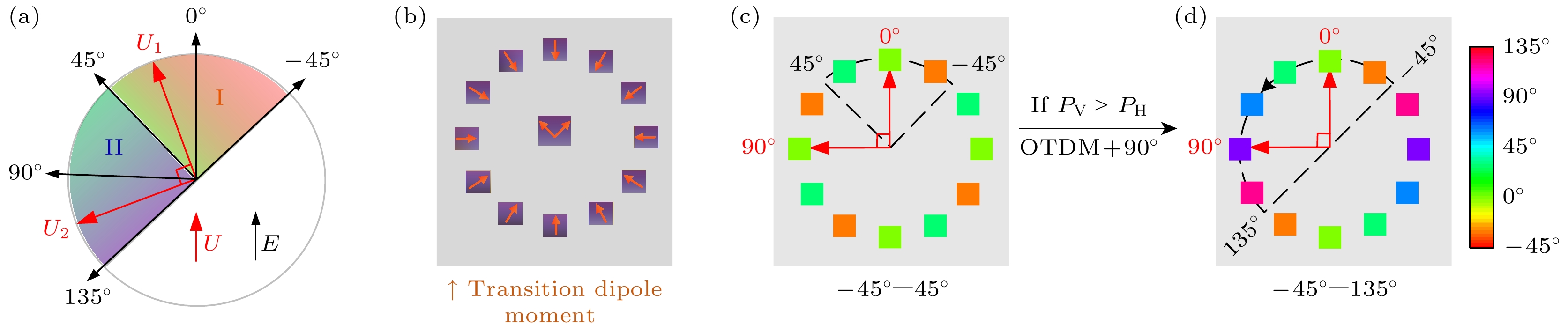
图 9 实现0—180°分子跃迁偶极矩取向[39] (a) OTDM分布示意图, E和U分别为入射光的电矢量和电跃迁偶极矩; (b) 计算机模拟的虚拟样品OTDM分布设置示意图, 这些样品的DOA值都设置为1; (c) 根据(21)式计算模拟得到的OTDM成像结果; (d) 消除简并效应后得到的OTDM成像结果
Figure 9. Measurement of 0—180° molecular transition dipole moment orientation[39]: (a) OTDM distribution diagram, E and U are the electric vector and electric transition dipole moment of incident light respectively; (b) schematic diagram of OTDM distribution settings for virtual samples simulated by computer, with DOA values set to 1 for these samples; (c) calculate the simulated OTDM imaging results according to Eq. (21); (d) the OTDM imaging results obtained after eliminating the degeneracy effect.
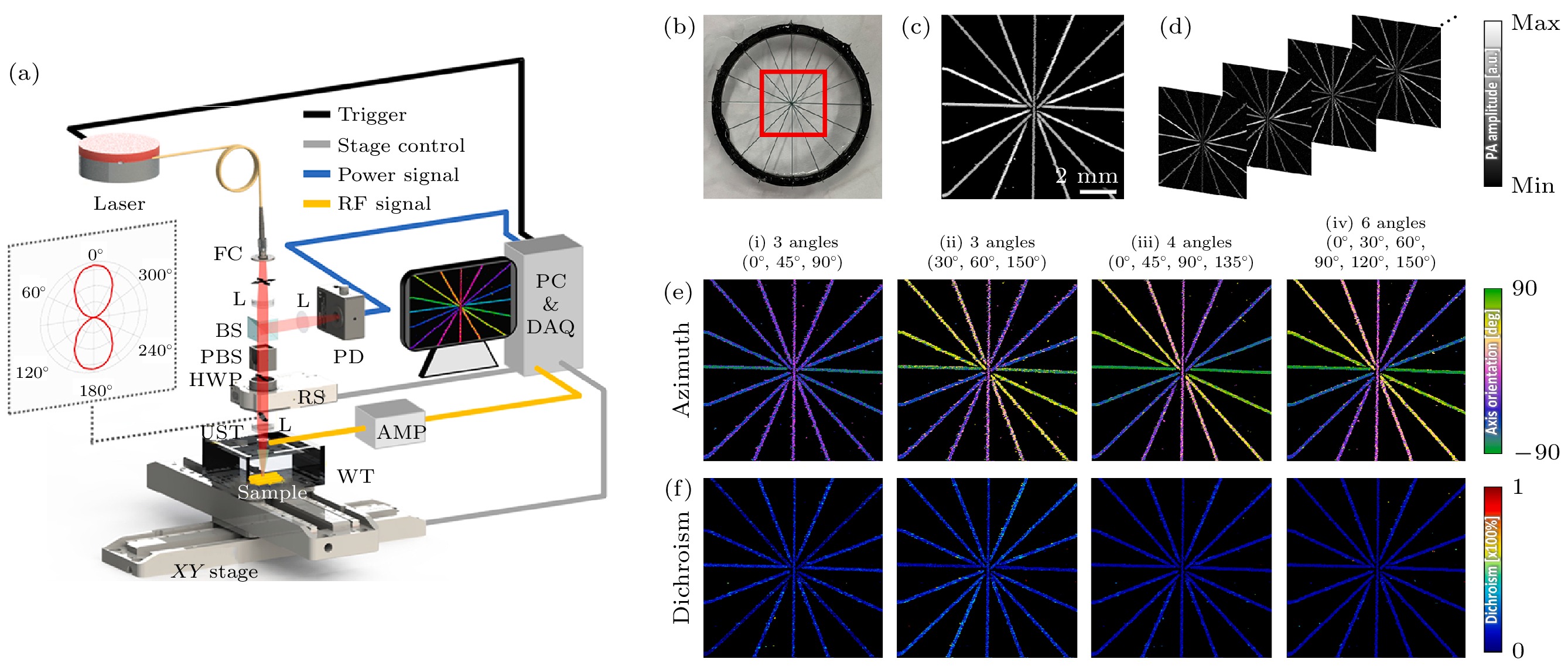
图 10 偏振光声成像分子取向的准确性与激发光电矢量数目的关联[53] (a) 线二向色性光声显微成像系统装置示意图(DS-PAM), 其中FC为光纤准直器, L为透镜, BS为分束器, PBS为偏振分束器, RS为旋转台, UST为超声波换能器, WT为水箱, AMP为放大器, PD为光电探测器, PC为电脑, DAQ为数据采集; (b) 交叉导线的照片; (c) 常规光声图像; (d) 根据激发光偏振状不同得到的一系列图像; (e) 分子取向方位角; (f) 线二向色性图
Figure 10. The correlation between the accuracy of molecular orientation in polarized photoacoustic imaging and the number of excited photoelectric vectors[53]: (a) The DS-PAM system configuration, FC is fiber collimator, L is lens, BS is beam splitter, PBS is polarizing beam splitter, RS is rotary stage, UST is ultrasonic transducer, WT is water tank, AMP is amplifier, PD is photodetector, PC is personal computer, DAQ is data acquisition; (b) photograph of crossed wires, the red box indicates the FOV for DS-PAM; (c) conventional PA MAP image; (d) series of images according to the polarization states; (e) azimuth and (f) dichroism mapping according to (i)–(iv) the number of angles applied.
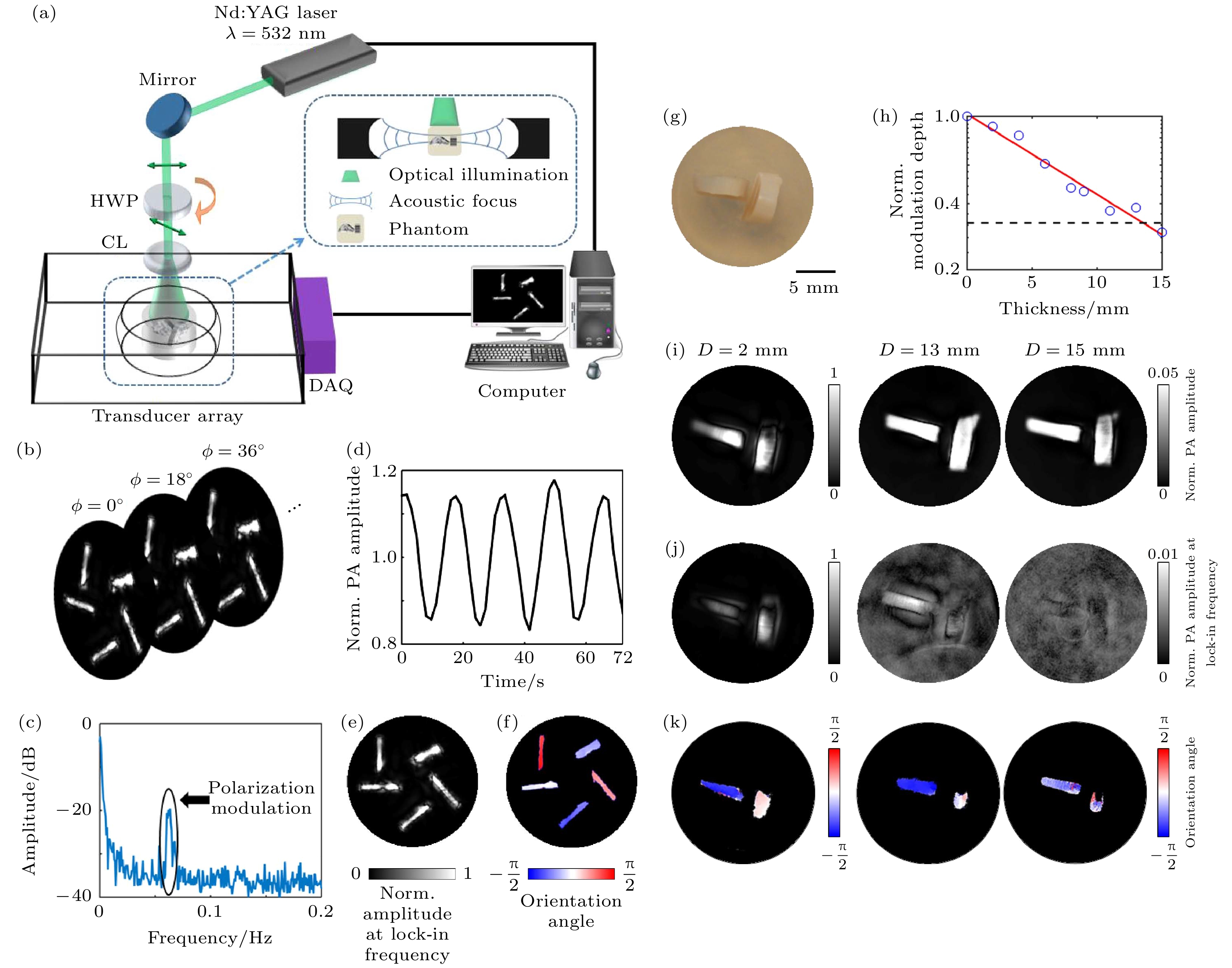
图 11 线二向色性光声计算层析成像系统与实验结果[44] (a) 线二向色性光声计算层析成像(DS-PACT)装置图; (b) 以0.625 Hz的帧率获得常规PACT图像序列, 使入射光的偏振率旋转为11.25°/s; (c) 归一化PA振幅的时间的函数; (d) 傅里叶光谱位于0.0625 Hz的峰值对应于由调制极化引起的交流光声信号; (e) 重建的DS-PACT图像; (f) 颜色编码的分子取向方向角度图; (g) 无散射介质的样品照片, 样品包含两块牛腱, 它们相互垂直放置; (h)PA振幅的调制深度随着散射介质厚度D的变化曲线, 高达15 mm, 红色实线表示与测量数据的指数拟合, 黑色虚线表示噪声水平; (i) 常规PACT图像; (j) 使用DSPACT的振幅图像; (k) 两块牛肌腱的方位角图
Figure 11. Linear dichroic photoacoustic computed tomography system and experimental results[44]: (a) Experimental setup of linear dichroism-sensitive photoacoustic computed tomography (DS-PACT); (b) a sequence of conventional PACT images is acquired at a frame rate of 0.625 Hz, rotating the polarization of the incident light at 11.25°/s; (c)normalized PA amplitude as a function of time at one representative spatial point. PA(t) is normalized with respect to its average; (d) Fourier spectrum of PA(t). The peak located at 0.0625 Hz corresponds to the alternating PA signals due to the modulated polarization; (e) DS-PACT image reconstructed by the amplitude of the lock-in term; (f) color-coded orientation angle map of the sample, which is reconstructed from the phase of the lock-in term; (g) photograph of the sample without any scattering medium. The sample contains two pieces of bovine tendon, which are placed perpendicular to each other; (h) modulation depth of the PA amplitude acquired with increasing thickness D of the scattering medium, up to 15 mm, the red solid line indicates the exponential fit to the measured data. The black dashed line represents the noise level; (i) conventional PACT images; (j) amplitude images using DSPACT; (k) orientation angle maps of the two pieces of bovine tendon.
图 12 手持式偏振光声计算机断层扫描系统与结果[47] (a)手持式偏振光声探头装置示意, UT为超声换能器, HWP为半波片; (b) 校准装置的照片; (c) 不同偏振方向的PVA和PVC的光声成像; (d) PVA和PVC的偏振光声信号受激发光电矢量方向的调制曲线; (e) 小鼠腿(上)和牛肌腱(下)的照片; (f) 小鼠腿(上)和牛肌腱(下)的HP-PACT图像, 激发光电矢量方向为0°(水平)和90°(垂直); (g) 小鼠腿(上)和牛肌腱(下)线二向色性结果图
Figure 12. Handheld polarized photoacoustic computed tomography system and results[47]: (a) Handheld polarized photoacoustic probe, UT is ultrasound transducer, HWP is half wave plate; (b) photograph of the calibration setup; (c) PAI of the PVA and PVC with different polarizations; (d) modulation curve of polarized photoacoustic signals in PVA and PVC excited by photoelectric vector direction; (e) photograph of the mouse leg (upper) and bovine tendon (lower); (f) HP-PACT images of mouse leg (upper) and bovine tendon (lower) with the polarization of the excitation light being 0° (horizontal) and 90° (vertical); (g) dichroic imaging of mouse leg (upper) and bovine tendon (lower) lines.
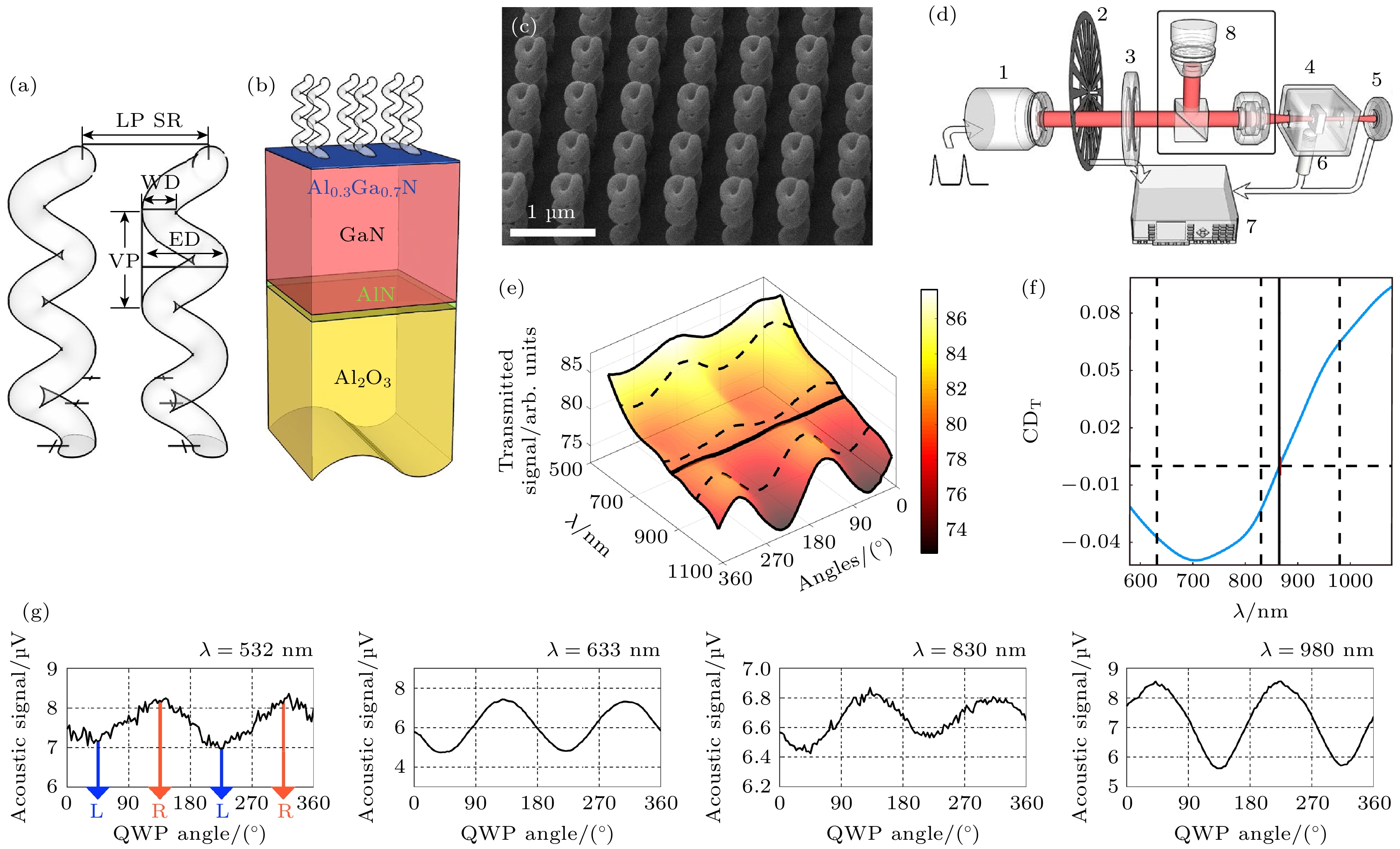
图 13 偏振光声检测纳米螺旋材料中的圆二色性[54] (a) 单个螺旋纳米材料的结构示意图, 线径(WD)为110 nm, 外径(ED)为300 nm, 垂直间距(VP)为300 nm; (b) 位于螺旋层下的基底的示意图, 它由一个GaN/AlGaN异质结构组成; (c) 扫描电子显微镜(SEM)样品视图; (d) 为PA测量的实验装置的示意图, 1为激光源, 2为斩波器, 3为四分之一波片(QWP), 4为包含所研究样品的PA室, 5为光电探测器用于检测激光功率, 6为麦克风部分安装在PA室内, 此处还有一个锁定装置7连接到主装置; (e) 光学技术测量得到的光强(归一化)作为波长(λ)和QWP方向角的函数, 被组装在图所示的三维函数中; (f) 从图(e)中在相同波长范围内提取的CD数据; (g) 不同波长辐射下产生的光声信号振幅和QWP角度之间的关系
Figure 13. Polarized photoacoustic detection of circular dichroism in nano spiral materials[54]: (a) Schematic diagram of a single helix, with a wire diameter (WD) of 110 nm, an outer diameter (ED) of 300 nm, and a vertical spacing (VP) of 300 nm; (b) schematic diagram of the base located below the spiral layer, it consists of a GaN/AlGaN heterostructure; (c) scanning electron microscopy (SEM) sample view; (d) schematic view of the experimental setup for PA measurements, we show the laser source (1), the chopper (2), the QWP (3), the PA chamber (4) containing the studied sample and the PD (5); the microphone (6) is partially installed inside the PA chamber; here again a lock-in (7) is connected to the main set-up; (e) the intensity (normalized) measured by optical technology is used as a function of wavelength and QWP direction angle; (f) extract CD data from (e) within the same wavelength range; (g) the relationship between the amplitude of photoacoustic signals generated under different wavelengths of radiation and the angle of QWP.
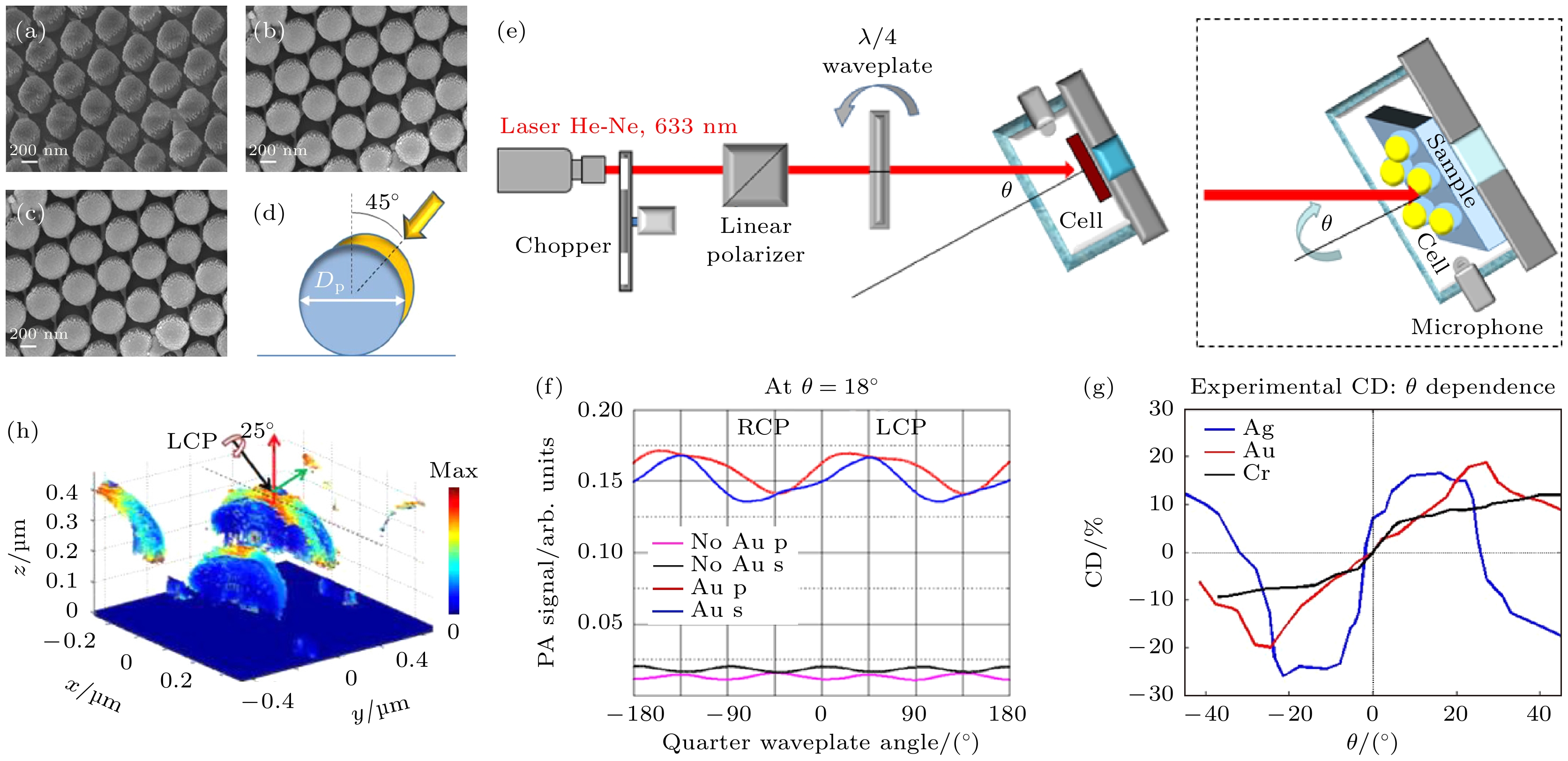
图 14 偏振光声检测金属-聚苯乙烯(SNSA)超表面的手性特性[55] (a)—(c) 分别为Au-半纳米壳阵列、Cr-半纳米壳阵列、Ag-半纳米壳阵列的场发射扫描电子显微成像(FESEM); (d) 纳米壳几何参数和蒸发角度草图; (e) 手性-PA设置装置示意, 插图为入射模式; (f) Au沉积前后旋转四分之一波片得到的光声信号幅值变化趋势; (g) 不同入射角下3个样本CD值的变化; (h) Au-SNSA在最大CD条件下吸收密度的分布, 最大吸收密度在金纳米的边界上
Figure 14. Chiral properties of metal polystyrene (SNSA) metasurfaces detected by polarized photoacoustic detection[55]: (a) Au-SNSA, (b) Cr-SNSA, and (c) Ag-SNSA and (d) sketch of the geometric parameters and the evaporation angle; (e) schematic diagram of chiral PA setting device, illustration: incidence mode; (f) the amplitude variation trend of photoacoustic signals obtained by rotating a quarter wave plate before and after Au deposition; (g) changes in CD values of three samples under different incident angles; (h) the distribution of absorption density of Au SNSA under maximum CD conditions is shown, with the maximum absorption density at the boundary of gold nanoparticles.
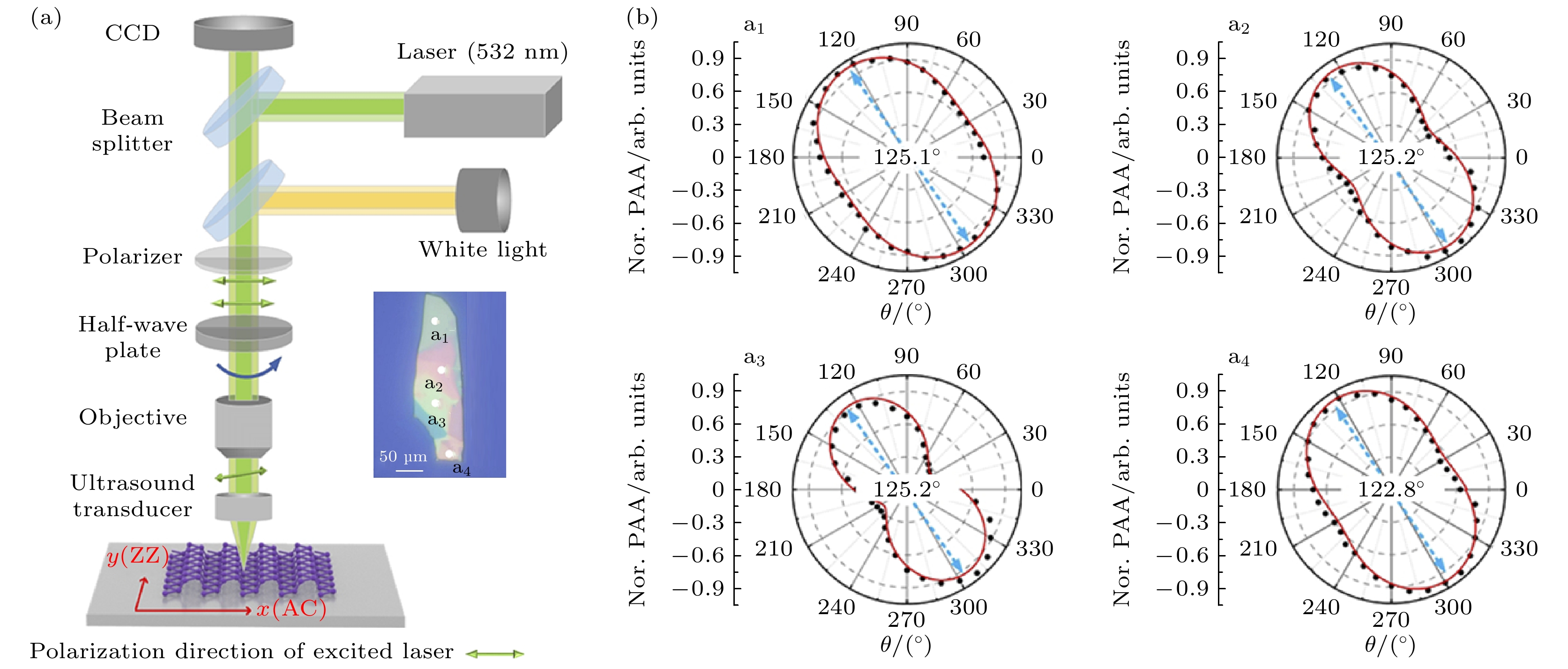
图 15 角分辨偏振光声技术检测二维材料的晶轴取向[52] (a) 角分辨偏振光声显微镜系统示意图, 插图为黑磷样品的光学照片; (b) a1, a2, a3和a4为4个采样点的晶轴(扶手椅型)取向检测结果
Figure 15. Angle resolved polarized photoacoustic technology for detecting the crystal axis orientation of two-dimensional materials[52]: (a) The diagram of the angle-resolved polarized photoacoustic microscopy (AnR-PPAM) system and the illustration is the optical photo of black phosphorus; (b) a1, a2, a3, and a4 are the crystal axis (armchair type) orientation detection results of the four sampling points.
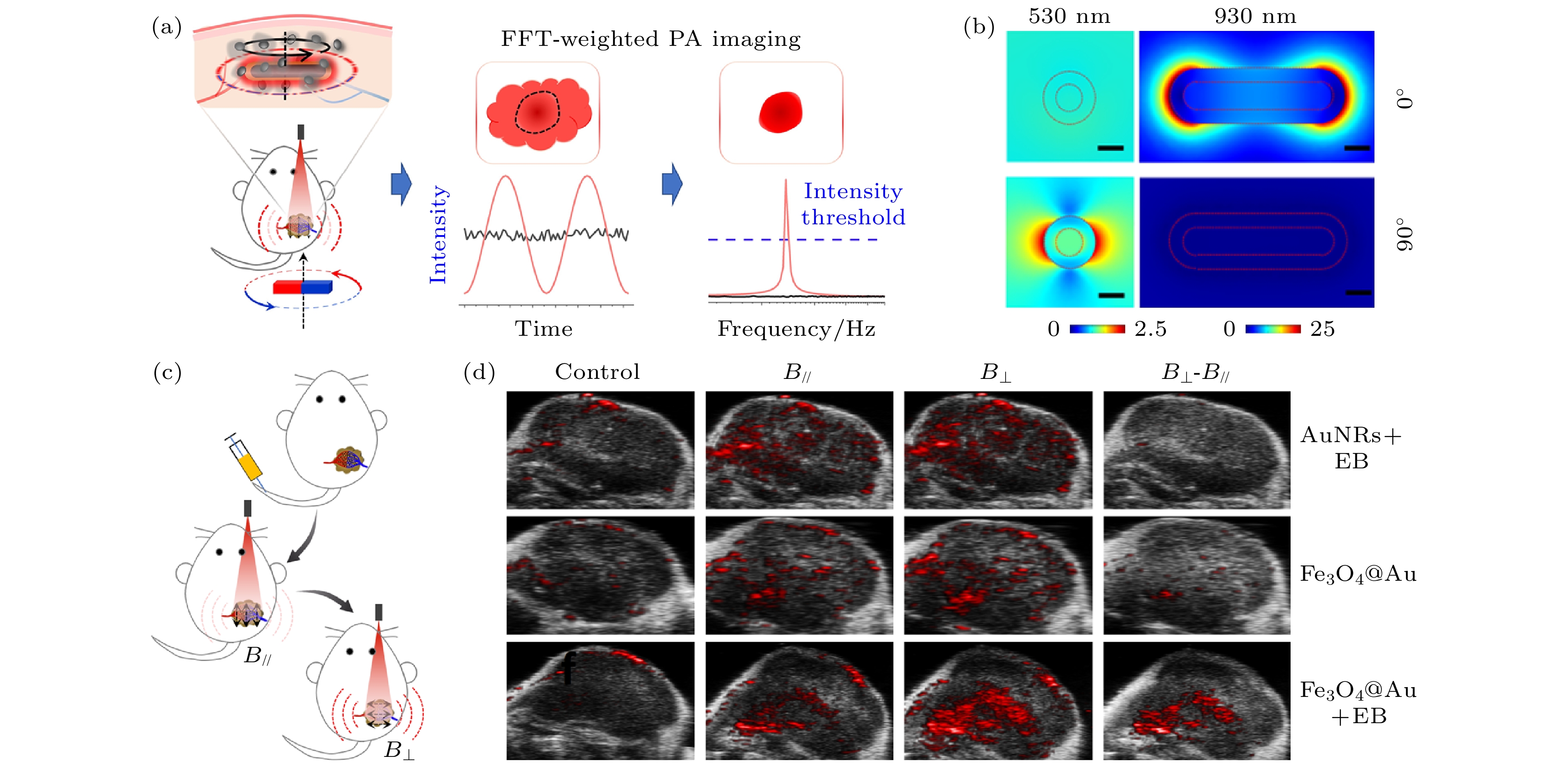
图 16 基于磁响应纳米探针的偏振光声无背景成像[51] (a) 磁控偏振光声无背景成像的原理示意图; (b) 光激发下不同共振波长纳米棒的电场分布, 比例尺为20 nm; (c) 静脉肿瘤无背景偏振成像示意图, 注入Fe3O4@Au NRs; (d) 传统光声成像和偏振光声无背景成像结果
Figure 16. Polarized photoacoustic background free imaging based on magnetic responsive nanoprobes[51]: (a) Schematic diagram of the principle of magnetic controlled polarized light acoustic background free imaging; (b) electric field distribution of orientated nanorods under light excitation at resonant wavelength. Scale bars: 20 nm; (c) background free polarization imaging schematic of venous tumors, injection Fe3O4@Au NRs; (d) control photoacoustic imaging and polarized photoacoustic imaging have no background imaging results.
-
[1] Röntgen W C 1896 Science 3 227
 Google Scholar
Google Scholar
[2] Castellanos F X, Giedd J N, Marsh W L, Hamburger S D, Vaituzis A C, Dickstein D P, Sarfatti S E, Vauss Y C, Snell J W, Lange N, Kaysen D, Krain A L, Ritchie G F, Rajapakse J C, Rapoport J L 1996 JAMA Psychiatry 53 607
 Google Scholar
Google Scholar
[3] Ketcham R A, Carlson W D 2001 Comp. Geos. 27 381
 Google Scholar
Google Scholar
[4] Chang J H, Kim H H, Lee J, Shung K 2010 Ultrasonics 50 453
 Google Scholar
Google Scholar
[5] Huang D, Swanson E A, Lin C P, Schuman J S, Stinson W G, Chang W, Hee M R, Flotte T, Gregory K, Puliafito C, Fujimoto J 1991 Science 254 1178
 Google Scholar
Google Scholar
[6] Hell S, Stelzer E H K 1992 JOSA A. 9 2159
 Google Scholar
Google Scholar
[7] Huang B, Bates M, Zhuang X 2009 Annu. Rev. Biochem. 78 993
 Google Scholar
Google Scholar
[8] Torrealba F, Carrasco M A 2004 Brain. Res. Rev. 47 5
 Google Scholar
Google Scholar
[9] Stuker F, Ripoll J, Rudin M 2011 Pharmaceutics 3 229
 Google Scholar
Google Scholar
[10] Cox B, Laufer J G, Arridge S R, Beard P C 2012 J. Biomed. Opt. 17 061202
 Google Scholar
Google Scholar
[11] Axer M, Grassel D, Kleiner M, Dammers J, Dickscheid T, Reckfort J, Hütz T, Eiben B, Pietrzyk U, Zilles K, Amunts K 2011 Front. Neurol. 5 34
 Google Scholar
Google Scholar
[12] Tang P, Kirby M A, Le N, Li Y, Zeinstra N, Lu G N, Murry C E, Zheng Y, Wang R 2021 Light Sci. Appl. 10 237
 Google Scholar
Google Scholar
[13] Di Martino J S, Nobre A R, Mondal C, Taha I, Farias E F, Fertig E J, Naba A, Aguirre-Ghiso J A, Bravo-Cordero J J 2022 Nature Cancer 3 90
 Google Scholar
Google Scholar
[14] Jin L W, Claborn K A, Kurimoto M, Geday M A, Maezawa I, Sohraby F, Estrada M, Kaminksy W, Kahr B 2003 Proc. Natl. Acad. Sci. 100 15294
 Google Scholar
Google Scholar
[15] Fitzpatrick A W P, Falcon B, He S, Murzin A G, Murshudov G, Garringer H J, Crowther R A, Ghetti B, Goedert M, Scheres S H W 2017 Nature 547 185
 Google Scholar
Google Scholar
[16] Zhao M, Wang L, Wang M, Zhou S, Lu Y, Cui H, Racanelli A C, Zhang L, Ye T, Ding B, Zhang B, Yang J 2022 Signal Transduct Target Ther. 7 206
 Google Scholar
Google Scholar
[17] 云天梁, 曾楠, 李伟, 马辉 2009 光学学报 29 1926
 Google Scholar
Google Scholar
Yun T L, Zeng N, Li W, Ma H 2009 Acta Optica Sinica 29 1926
 Google Scholar
Google Scholar
[18] Rodriguez L G, Lockett S J, Holtom G R 2006 Cytometry A 69 779
 Google Scholar
Google Scholar
[19] Jameson D M, Ross J A 2010 Chem. Rev. 110 2685
 Google Scholar
Google Scholar
[20] Bell A G 1880 J. Soc. Telegr. Eng. 9 34
 Google Scholar
Google Scholar
[21] Zhang Z H, Shi Y J, Yang S H, Xing D 2018 Opt. Lett. 43 2336
 Google Scholar
Google Scholar
[22] 申晓雯, 武红鹏, 董磊 2023 光子学报 52 81
 Google Scholar
Google Scholar
Shen X W, Wu H P, Dong L 2023 Acta Photonica Sinica 52 81
 Google Scholar
Google Scholar
[23] Liu Y, Nie L, Chen X 2016 Trends Biotechnol. 34 420
 Google Scholar
Google Scholar
[24] Wang L V, Hu S 2012 Science 335 1458
 Google Scholar
Google Scholar
[25] Zhao Y, Yang S H, Chen C G, Xing D 2014 Opt. Lett. 39 2565
 Google Scholar
Google Scholar
[26] Lin L, Hu P, Shi J, Appleton C M, Maslov K, Li L, Wang L V 2018 Nat. Commun. 9 2352
 Google Scholar
Google Scholar
[27] Wang Z, Yang F, Cheng Z, Zhang W, Xiong K, Yang, S 2021 Nanophotonics 10 3359
 Google Scholar
Google Scholar
[28] Wen X, Lei P, Huang S, Chen X, Yuan Y, Ke D, Yang S. 2023 Photonics Res. 1 55
 Google Scholar
Google Scholar
[29] 穆根, 张振辉, 石玉娇 2022 中国激光 49 2007208
 Google Scholar
Google Scholar
Mu G, Zhang Z H, Shi Y 2022 Chin. J. Lasers 49 2007208
 Google Scholar
Google Scholar
[30] Wang Z, Yang F, Zhang W, Xiong K, Yang S 2023 Fundamental Research online
 Google Scholar
Google Scholar
[31] Van den Berg P, Daoudi K, Steenbergen W 2015 Photoacoustics 3 89
 Google Scholar
Google Scholar
[32] Chen J, Zhang Y, Li X, Zhu J, Li D, Li S, Lee C-S, Wang L 2020 Photonics Res. 8 1875
 Google Scholar
Google Scholar
[33] 陈丛桂, 赵岳, 杨思华 2013 激光生物学报 22 486
 Google Scholar
Google Scholar
Chen C G, Zhao Y, Yang S H 2013 Acta Laser Biology Sinica 22 486
 Google Scholar
Google Scholar
[34] Yang F, Chen Z, Xing D 2019 IEEE. Trans. Med. Imaging. 39 1791
 Google Scholar
Google Scholar
[35] Wang L, Zhang C, Wang L V 2014 Phys. Rev. Lett. 113 174301
 Google Scholar
Google Scholar
[36] Wang L V, Yao J 2016 Nat. Methods. 13 627
 Google Scholar
Google Scholar
[37] 张振辉, 石玉娇 2019 激光生物学报 28 32
 Google Scholar
Google Scholar
Zhang Z H, Shi Y J 2019 Acta Laser Biology Sin. 28 32
 Google Scholar
Google Scholar
[38] Yao J, Wang L V 2013 Laser Photonics Rev. 7 758.
 Google Scholar
Google Scholar
[39] Zhang Z H, Shi Y H, Shen Q, Wang Z Y, Xing D, Yang S H 2022 arXiv: 2207. 13271v1 [physics. med-ph
[40] Rodger A 2006 Circular Dichroism and Linear Dichroism. Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation pp1–42
[41] Ma W, Xu L, de Moura A F, Wu X, Kuang H, Xu C, Kotov N A 2017 Chem. Rev. 117 8041
 Google Scholar
Google Scholar
[42] Zhang Z H, Chen W, Cui D D, Mi J, Mu G, Nie L M, Yang S H, Shi Y J 2023 Photonics Res. 11 817
 Google Scholar
Google Scholar
[43] Zhang Z H, Shi Y J, Xiang L Z, Xing D 2018 Opt. Lett. 43 5267
 Google Scholar
Google Scholar
[44] Qu Y, Li L, Shen Y, Wei X, Wong T T, Hu P, Yao J, Maslov K, Wang L V 2018 Optica 5 495
 Google Scholar
Google Scholar
[45] Hu S, Maslov K, Yan P, Lee J-M, Wang L V 2012 Biomedical. Optics Miami, USA, April 28–May 2, 2012 pBM2B-8
[46] Zhou Y, Chen J, Liu C, Liu C, Lai P, Wang L 2019 Photoacoustics 16 100148
 Google Scholar
Google Scholar
[47] Ren Y, Zhang Y, He H, Liu L, Wu X, Song L, Liu C 2022 Quant. Imaging Med. Surg. 12 2238
 Google Scholar
Google Scholar
[48] 张迎, 吴晓君, 何宏辉, 文斌华, 刘成波, 任亚光 2022 激光与光电子学报 59 0817001
 Google Scholar
Google Scholar
Zhang Y, Wu X J, He H H, Wen B H, Liu C B, Ren Y G 2022 Journal of Optoelectronics Laser 59 0817001
 Google Scholar
Google Scholar
[49] Zhou Y, Zhong F, Yan P, Lee J-M, Hu S 2021 Opt. Lett. 46 2561
 Google Scholar
Google Scholar
[50] Wang T, Yang J, Wang W S, Wang L X, Wang X C, Zhou S 2022 Ann. Transl. Med. 10 293
 Google Scholar
Google Scholar
[51] Li Z W, Meng Z Q, Tian F, Ye Z Y, Zhou X F, Zhong X J, Chen Q, Yang M, Liu Z, Yin Y D 2022 Nano Lett. 22 5158
 Google Scholar
Google Scholar
[52] Mu G, Zhang Z H, Cui D D, Chen W, Shi Y J 2023 Opt. Lett. 48 2748
 Google Scholar
Google Scholar
[53] Park E, Lee Y J, Kim C, Eom T J 2023 Photoacoustics 31 100510
 Google Scholar
Google Scholar
[54] Benedetti A, Alam B, Esposito M, Tasco V, Leahu G, Belardini A, Li Voti R, Passaseo A, Sibilia C 2017 Sci. Rep. 7 5257
 Google Scholar
Google Scholar
[55] Petronijević E, Leahu G, Li Voti R, Belardini A, Scian C, Michieli N, Cesca T, Mattei G, Sibilia C 2019 Appl. Phys. Lett. 114 053101
 Google Scholar
Google Scholar
[56] Tuchin V V 2016 J. Biomed. Opt. 21 071114
 Google Scholar
Google Scholar
[57] Schmitt J M, Gandjbakhche A H, Bonner R F 1992 Appl. Opt. 31 6535
 Google Scholar
Google Scholar
[58] Morgan S P, Khong M P, Somekh M G 1997 App. Opt. 36 1560
 Google Scholar
Google Scholar
[59] Ghosh N, Patel H S, Gupta P K 2003 Opt. Express 11 2198
 Google Scholar
Google Scholar
[60] Shen F, Zhang B, Guo K, Yin Z, Guo Z 2017 IEEE Photonics J. 10 1
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 13414
- PDF Downloads: 159
- Cited By: 0














 DownLoad:
DownLoad: