-
Recent experimental discovery of intrinsic ferromagnetism (FM) in chromium triiodide (CrI3) monolayer opens a new way to low-dimensional spintronics. Two-dimensional (2D) CrI3 monolayer is of great significance for its magnetic and electronic properties. Generally, surface atomic adsorption is an effective way to modify the physical properties of layered magnetic materials. Here in this work, we use the first-principles method based on density functional theory (DFT) to systematically study the electronic structure and magnetic properties of 2D CrI3 monolayers that have adsorbed other metal atoms (specifically, alkali (alkaline earth) metal (Li, K and Mg), transition metal (Ti, V, Mn, Fe, Co and Ni) and non-metal (N, P, O and S) atoms). Our results show that the metal atoms tend to be adsorbed in the center of the ring formed by the six I atoms and stay at the same height as Cr atoms, while the positions of the optimized non-metal atoms are in the ring formed by the six I atoms and depend on the type of the atoms. The adsorption of atoms (except for Ti and Mn atoms) does not change the intrinsic ferromagnetic semiconducting properties of CrI3 monolayer. The CrI3 monolayers with Ti or Mn adsorption are antiferromagnetic semiconductors. Moreover, we find that the adsorption of different atoms regulates the local magnetic moments of Cr atoms. The adsorption of metal atoms increases the local magnetic moments of Cr atoms, but not exceeding 4μB. However, the adsorption of non-metallic atoms makes the local magnetic moments of Cr atoms diversified. The adsorption of O and N atoms retain the local magnetic moment of Cr atoms, while the adsorption of P and S atoms increase the local magnetic moment. By combining the projected density of states, we analyze in detail the local magnetic moments of Cr atoms. The increase of the local magnetic moments of Cr atoms is directly related to the charges transferring. Our results provide new ideas for regulating the performance of the magnetism of 2D intrinsic ferromagnetic semiconductor CrI3, which will have potential applications in the spintronics in the future.
-
Keywords:
- atomic adsorption /
- first principles /
- two-dimensional CrI3 monolayer /
- electronic structures and magnetic properties
[1] Geim A K, Novoselov K S 2007 Nat. Mater. 6 183
 Google Scholar
Google Scholar
[2] Ferrari A C, Meyer J C, Scardaci V, Casiraghi C, Lazzeri M, Mauri F, Piscanec S, Jiang D, Novoselov K S, Roth S, Geim A K 2006 Phys. Rev. Lett. 97 187401
 Google Scholar
Google Scholar
[3] Castro Neto A H, Guinea F, Peres N M R, Novoselov K S, Geim A K 2009 Rev. Mod. Phys. 81 109
 Google Scholar
Google Scholar
[4] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[5] Chen Q, Ouyang Y, Yuan S, Li R, Wang J 2014 ACS Appl. Mater. Inter. 6 16835
 Google Scholar
Google Scholar
[6] Dong L, Kumar H, Anasori B, Gogotsi Y, Shenoy V B 2017 J. Phys. Chem. Lett. 8 422
 Google Scholar
Google Scholar
[7] Ma X C, Wu X, Wang H D, Wang Y C 2018 J. Mater. Chem. A 6 2295
 Google Scholar
Google Scholar
[8] Kadantsev E S, Hawrylak P 2012 Solid State Commun. 152 909
 Google Scholar
Google Scholar
[9] Li F, Wei W, Zhao P, Huang B, Dai Y 2017 J. Phys. Chem. Lett. 8 5959
 Google Scholar
Google Scholar
[10] Mak K F, Lee C, Hone J, Shan J, Heinz T F 2010 Phys. Rev. Lett. 105 136805
 Google Scholar
Google Scholar
[11] Dean C R, Young A F, Meric I, Lee C, Wang L, Sorgenfrei S, Watanabe K, Taniguchi T, Kim P, Shepard K L, Hone J 2010 Nat. Nanotechnol. 5 722
 Google Scholar
Google Scholar
[12] Qiao J, Kong X, Hu Z X, Yang F, Ji W 2014 Nat. Commun. 5 4475
 Google Scholar
Google Scholar
[13] Li L, Yu Y, Ye G J, Ge Q, Ou X, Wu H, Feng D, Chen X H, Zhang Y 2014 Nat. Nanotechnol. 9 372
 Google Scholar
Google Scholar
[14] Wang Q H, Kalantar-Zadeh K, Kis A, Coleman J N, Strano M S 2012 Nat. Nanotechnol. 7 699
 Google Scholar
Google Scholar
[15] Ghorbani-Asl M, Kuc A, Miro P, Heine T 2016 Adv. Mater. 28 853
 Google Scholar
Google Scholar
[16] Radisavljevic B, Radenovic A, Brivio J, Giacometti V, Kis A 2011 Nat. Nanotechnol. 6 147
 Google Scholar
Google Scholar
[17] Tao L, Cinquanta E, Chiappe D, Grazianetti C, Fanciulli M, Dubey M, Molle A, Akinwande D 2015 Nat. Nanotechnol. 10 227
 Google Scholar
Google Scholar
[18] Ma L, Dai J, Zeng X C 2017 Adv. Energy Mater. 7
 Google Scholar
Google Scholar
[19] Jing Y, Zhou Z, Cabrera C R, Chen Z 2014 J. Mater. Chem. A 2
 Google Scholar
Google Scholar
[20] Bonaccorso F, Colombo L, Yu G, Stoller M, Tozzini V, Ferrari A C, Ruoff R S, Pellegrini V 2015 Science 347 1246501
 Google Scholar
Google Scholar
[21] Huang B, Clark G, Navarro-Moratalla E, Klein D R, Cheng R, Seyler K L, Zhong D, Schmidgall E, McGuire M A, Cobden D H, Yao W, Xiao D, Jarillo-Herrero P, Xu X 2017 Nature 546 270
 Google Scholar
Google Scholar
[22] Zhang W B, Qu Q, Zhu P, Lam C H 2015 J. Mater. Chem. C 3 12457
 Google Scholar
Google Scholar
[23] Fu Y K, Sun Y, Luo X 2019 J. Appl. Phys. 125 053901
 Google Scholar
Google Scholar
[24] Wang H B, Fan F R, Zhu S S, Wu H 2016 Europhys. Lett. 114 47001
 Google Scholar
Google Scholar
[25] McGuire M A, Dixit H, Cooper V R, Sales B C 2015 Chem. Mater. 27 612
 Google Scholar
Google Scholar
[26] Webster L, Liang L, Yan J A 2018 Phys. Chem. Chem. Phys. 20 23546
 Google Scholar
Google Scholar
[27] Larson D T, Kaxiras E 2018 Phys. Rev. B 98 085406
 Google Scholar
Google Scholar
[28] Shcherbakov D, Stepanov P, Weber D, Wang Y, Hu J, Zhu Y, Watanabe K, Taniguchi T, Mao Z, Windl W, Goldberger J, Bockrath M, Lau C N 2018 Nano Lett. 18 4214
 Google Scholar
Google Scholar
[29] Chen L B, Chung J H, Gao B, Chen T, Stone M B, Kolesnikov A I, Huang Q Z, Dai P C 2018 Phys. Rev. X 8 041028
 Google Scholar
Google Scholar
[30] Zeng Y, Wang L, Li S, He C, Zhong D, Yao D X 2019 J. Phys. Codens. Mat. 31 395502
 Google Scholar
Google Scholar
[31] Zhou Y G, Wang Z G, Yang P, Zu X T, Yang L, Sun X, Gao F 2012 ACS Nano 6 9727
 Google Scholar
Google Scholar
[32] Zhu S Z, Li T 2016 Phys. Rev. B 93 115401
 Google Scholar
Google Scholar
[33] 吴木生, 徐波, 刘刚, 欧阳楚英 2012 61 227102
 Google Scholar
Google Scholar
Wu M S, Xu B, Liu G, Ouyang C Y 2012 Acta Phys. Sin. 61 227102
 Google Scholar
Google Scholar
[34] Rai H M, Saxena S K, Mishra V, Late R, Kumar R, Sagdeo P R, Jaiswal N K, Srivastava P 2016 RSC Adv. 6 11014
 Google Scholar
Google Scholar
[35] Osada M, Yoguchi S, Itose M, Li B W, Ebina Y, Fukuda K, Kotani Y, Ono K, Ueda S, Sasaki T 2014 Nanoscale 6 14227
 Google Scholar
Google Scholar
[36] Guan J, Yu G, Ding X, Chen W, Shi Z, Huang X, Sun C 2013 Chemphyschem. 14 2841
 Google Scholar
Google Scholar
[37] Du A J, Chen Y, Zhu Z H, Amal R, Lu G Q, Smith S C 2009 J. Am. Chem. Soc. 131 17354
 Google Scholar
Google Scholar
[38] 黄炳铨, 周铁戈, 吴道雄, 张召富, 李百奎 2019 68 246301
 Google Scholar
Google Scholar
Huang B Q, Zhou T G, Wu D X, Zhang Z F, Li B K 2019 Acta Phys. Sin. 68 246301
 Google Scholar
Google Scholar
[39] Kan E, Li M, Hu S, Xiao C, Xiang H, Deng K 2013 J. Phys. Chem. Lett. 4 1120
 Google Scholar
Google Scholar
[40] Barone V, Peralta J E 2008 Nano Lett. 8 2210
 Google Scholar
Google Scholar
[41] Allen M J, Tung V C, Kaner R B 2010 J. Am. Chem. Soc. 110 132
 Google Scholar
Google Scholar
[42] Lee K W, Lee C E 2012 Adv. Mater. 24 2019
 Google Scholar
Google Scholar
[43] 栾晓玮, 孙建平, 王凡嵩, 韦慧兰, 胡艺凡 2019 68 026802
 Google Scholar
Google Scholar
Luan X W, Sun J P, Wang F S, Wei H L, Hu Y F 2019 Acta Phys. Sin. 68 026802
 Google Scholar
Google Scholar
[44] 杨光敏, 梁志聪, 黄海华 2017 66 057301
 Google Scholar
Google Scholar
Yang G M, Liang Z C, Huang H H 2017 Acta Phys. Sin. 66 057301
 Google Scholar
Google Scholar
[45] Zheng F W, Zhao J Z, Liu Z, Li M L, Zhou M, Zhang S B, Zhang P 2018 Nanoscale 10 14298
 Google Scholar
Google Scholar
[46] Gao Y, Wang J, Li Z P, Yang J J, Xia M R, Hao X F, Xu Y H, Gao F M 2019 Phys. Status. Solidi-R. 13 1800410
 Google Scholar
Google Scholar
[47] Qin W J, Xu B, Liao S S, Liu G, Sun B Z, Wu M S 2020 Solid State Commun. 321 114037
 Google Scholar
Google Scholar
[48] Liu J, Shi M C, Lu J W, Anantram M P 2018 Phys. Rev. B 97 054416
 Google Scholar
Google Scholar
[49] Guo Y L, Yuan S J, Wang B, Shi L, Wang J L 2018 J. Mater. Chem. C 6 5716
 Google Scholar
Google Scholar
[50] Kresse G, Furthmuller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[51] Kresse G, Hafner J 1994 Phys. Rev. B: Condens. Matter 49 14251
 Google Scholar
Google Scholar
[52] Kresse G, Furthmiiller J 1996 Science 6 15
[53] Blochl P E 1994 Phys. Rev. B: Condens. Matter 50 17953
 Google Scholar
Google Scholar
[54] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[55] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[56] Perdew J P, Ernzerhof M, Burke K 1996 J. Chem. Phys. 105 9982
 Google Scholar
Google Scholar
[57] Liechtenstein A I, Anisimov V V, Zaanen J 1995 Phys. Rev. B: Condens. Matter 52 R5467
 Google Scholar
Google Scholar
[58] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[59] Goodenough J B 1958 J. Phys. Chem. Solids 6 287
 Google Scholar
Google Scholar
[60] Anderson P W 1959 Phys. Rev. 115 2
 Google Scholar
Google Scholar
-
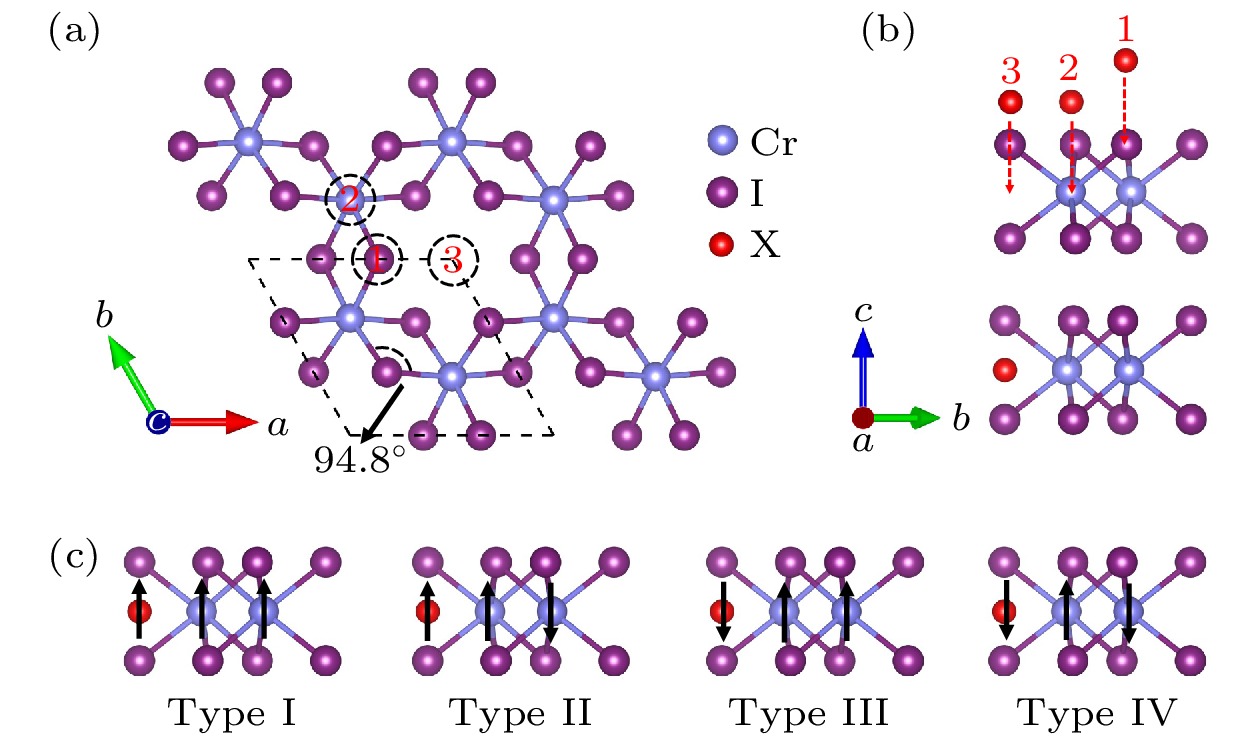
图 1 (a) CrI3单层的原子结构俯视图(3个不同的吸附位用黑色的虚线圆圈表示); (b) CrI3单层的原子结构侧视图(上图中红色球为3个不同的吸附位, 下图中红色球为原子在吸附位3处吸附后的原子优化位置); (c) 四种不同的磁构型结构
Figure 1. (a) Top view of atomic structure diagram of CrI3 monolayer (three different adsorption sites are represented by black dashed circle); (b) side view of atomic structure diagram of CrI3 monolayer (three different adsorption sites are shown with red balls in the top picture, and the optimized site of adsorption atom is shown with red ball in the bottom picture); (c) four different magnetic configurations.
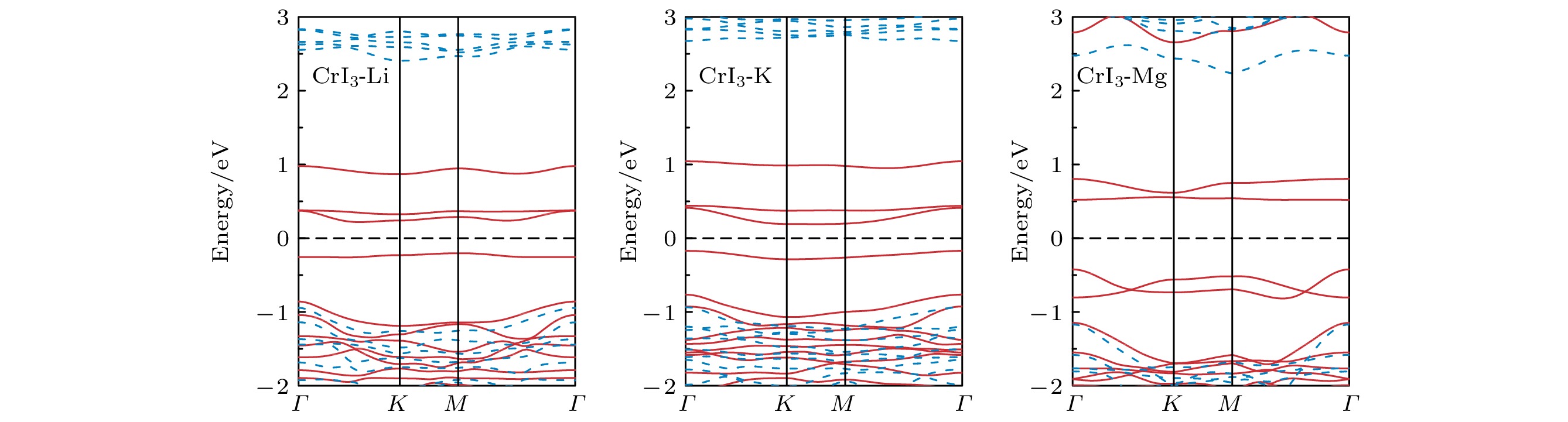
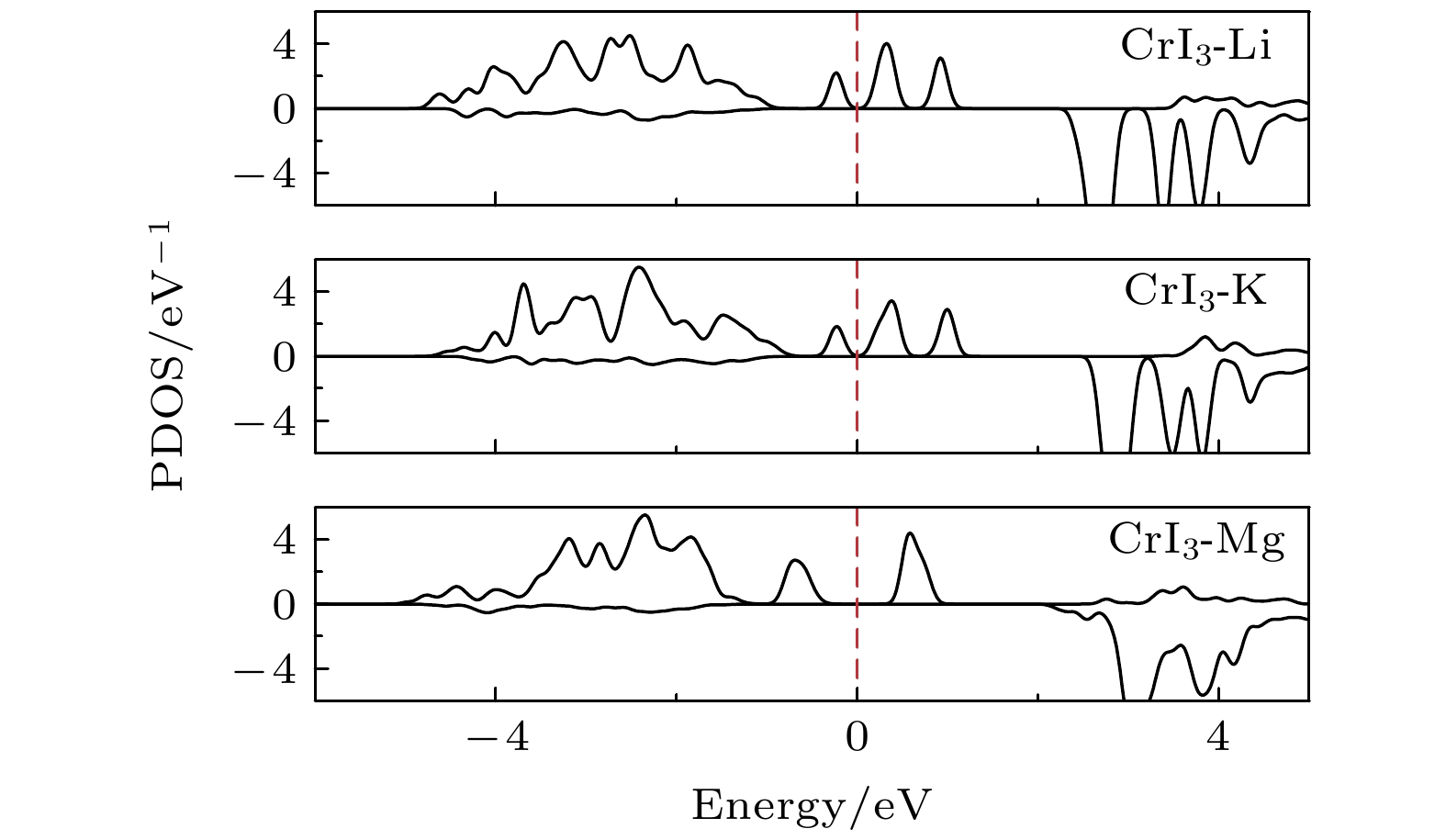
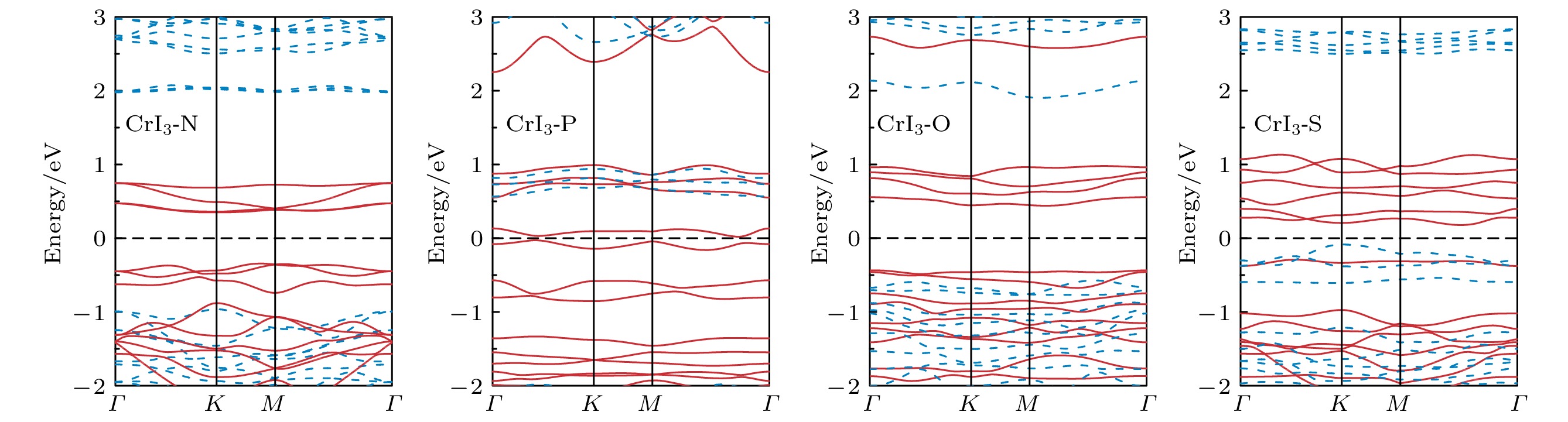
图 2 完美CrI3单层的 (a) 能态密度(红色实线表示Cr-d轨道的分波态密度, 蓝色实线表示I-p轨道的分波态密度)和 (b) 能带结构(红色实线表示上自旋电子的能带, 蓝色虚线表示下自旋电子的能带)
Figure 2. (a) Energy density of states (red solid line for Cr-d projected density of states, blue solid line for I-p projected density of states) and (b) band structure (red solid line for spin up, blue dashed line for spin down) of perfect CrI3 monolayer.
表 1 各种原子位于不同吸附位的CrI3单层优化后的体系能量(E )
Table 1. Energies (E ) of the optimized CrI3 monolayer with various atoms adsorbed at different sites.
原子 E位置1/eV E位置2/eV E位置3/eV Li –32.147 –31.308 –32.152 K –31.411 –30.634 –31.430 Mg –32.156 –31.267 –32.173 Ti — –35.245 –35.472 V –35.293 –34.067 –36.513 Mn –36.923 –35.369 –36.937 Fe –31.450 –33.560 –34.959 Co –30.225 –31.895 –33.242 Ni –29.182 –30.606 –31.603 N –31.274 –32.801 –33.337 P –31.362 –31.664 –31.798 O –32.233 –33.183 –33.240 S –30.657 –30.155 –30.887 表 2 碱(土)金属原子吸附后的CrI3单层的能量(E )和Cr原子的局域磁矩(MCr)
Table 2. Energy (E ) and local magnetic moments (MCr) of CrI3 monolayer adsorbed by alkali (alkaline earth) metal atoms.
原子 E/meV MCr/μB 铁磁 反铁磁 Li 0 59 (4, 3) K 0 52 (4, 3) Mg 0 6 (4, 4) 表 3 过渡金属原子吸附后的CrI3单层的能量(E )和Cr原子的局域磁矩(MCr)
Table 3. Energy (E ) and local magnetic moments (MCr) of CrI3 monolayer adsorbed by transition metal atoms.
原子 E/meV MCr/μB Type Ⅰ Type Ⅱ Type Ⅲ Type Ⅳ Ti 570 0 12 0 (4, –4) V 0 22 30 22 (4, 4) Mn 37 0 38 0 (4, –4) Fe 40 14 0 14 (4, 4) Co 41 40 0 35 (4, 4) Ni 67 38 0 38 (4, 4) 表 4 非金属原子吸附后的CrI3单层的能量(E )和Cr原子的局域磁矩(MCr)
Table 4. Energy (E ) and local magnetic moments (MCr) of CrI3 monolayer adsorbed by non-metal atoms.
原子 E/meV MCr/μB Type Ⅰ Type Ⅱ Type Ⅲ Type Ⅳ N 0 63 37 63 (3, 3) P 0 29 — — (4.5, 4.5) O 0 89 — — (3, 3) S 210 71 0 71 (4, 4) -
[1] Geim A K, Novoselov K S 2007 Nat. Mater. 6 183
 Google Scholar
Google Scholar
[2] Ferrari A C, Meyer J C, Scardaci V, Casiraghi C, Lazzeri M, Mauri F, Piscanec S, Jiang D, Novoselov K S, Roth S, Geim A K 2006 Phys. Rev. Lett. 97 187401
 Google Scholar
Google Scholar
[3] Castro Neto A H, Guinea F, Peres N M R, Novoselov K S, Geim A K 2009 Rev. Mod. Phys. 81 109
 Google Scholar
Google Scholar
[4] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[5] Chen Q, Ouyang Y, Yuan S, Li R, Wang J 2014 ACS Appl. Mater. Inter. 6 16835
 Google Scholar
Google Scholar
[6] Dong L, Kumar H, Anasori B, Gogotsi Y, Shenoy V B 2017 J. Phys. Chem. Lett. 8 422
 Google Scholar
Google Scholar
[7] Ma X C, Wu X, Wang H D, Wang Y C 2018 J. Mater. Chem. A 6 2295
 Google Scholar
Google Scholar
[8] Kadantsev E S, Hawrylak P 2012 Solid State Commun. 152 909
 Google Scholar
Google Scholar
[9] Li F, Wei W, Zhao P, Huang B, Dai Y 2017 J. Phys. Chem. Lett. 8 5959
 Google Scholar
Google Scholar
[10] Mak K F, Lee C, Hone J, Shan J, Heinz T F 2010 Phys. Rev. Lett. 105 136805
 Google Scholar
Google Scholar
[11] Dean C R, Young A F, Meric I, Lee C, Wang L, Sorgenfrei S, Watanabe K, Taniguchi T, Kim P, Shepard K L, Hone J 2010 Nat. Nanotechnol. 5 722
 Google Scholar
Google Scholar
[12] Qiao J, Kong X, Hu Z X, Yang F, Ji W 2014 Nat. Commun. 5 4475
 Google Scholar
Google Scholar
[13] Li L, Yu Y, Ye G J, Ge Q, Ou X, Wu H, Feng D, Chen X H, Zhang Y 2014 Nat. Nanotechnol. 9 372
 Google Scholar
Google Scholar
[14] Wang Q H, Kalantar-Zadeh K, Kis A, Coleman J N, Strano M S 2012 Nat. Nanotechnol. 7 699
 Google Scholar
Google Scholar
[15] Ghorbani-Asl M, Kuc A, Miro P, Heine T 2016 Adv. Mater. 28 853
 Google Scholar
Google Scholar
[16] Radisavljevic B, Radenovic A, Brivio J, Giacometti V, Kis A 2011 Nat. Nanotechnol. 6 147
 Google Scholar
Google Scholar
[17] Tao L, Cinquanta E, Chiappe D, Grazianetti C, Fanciulli M, Dubey M, Molle A, Akinwande D 2015 Nat. Nanotechnol. 10 227
 Google Scholar
Google Scholar
[18] Ma L, Dai J, Zeng X C 2017 Adv. Energy Mater. 7
 Google Scholar
Google Scholar
[19] Jing Y, Zhou Z, Cabrera C R, Chen Z 2014 J. Mater. Chem. A 2
 Google Scholar
Google Scholar
[20] Bonaccorso F, Colombo L, Yu G, Stoller M, Tozzini V, Ferrari A C, Ruoff R S, Pellegrini V 2015 Science 347 1246501
 Google Scholar
Google Scholar
[21] Huang B, Clark G, Navarro-Moratalla E, Klein D R, Cheng R, Seyler K L, Zhong D, Schmidgall E, McGuire M A, Cobden D H, Yao W, Xiao D, Jarillo-Herrero P, Xu X 2017 Nature 546 270
 Google Scholar
Google Scholar
[22] Zhang W B, Qu Q, Zhu P, Lam C H 2015 J. Mater. Chem. C 3 12457
 Google Scholar
Google Scholar
[23] Fu Y K, Sun Y, Luo X 2019 J. Appl. Phys. 125 053901
 Google Scholar
Google Scholar
[24] Wang H B, Fan F R, Zhu S S, Wu H 2016 Europhys. Lett. 114 47001
 Google Scholar
Google Scholar
[25] McGuire M A, Dixit H, Cooper V R, Sales B C 2015 Chem. Mater. 27 612
 Google Scholar
Google Scholar
[26] Webster L, Liang L, Yan J A 2018 Phys. Chem. Chem. Phys. 20 23546
 Google Scholar
Google Scholar
[27] Larson D T, Kaxiras E 2018 Phys. Rev. B 98 085406
 Google Scholar
Google Scholar
[28] Shcherbakov D, Stepanov P, Weber D, Wang Y, Hu J, Zhu Y, Watanabe K, Taniguchi T, Mao Z, Windl W, Goldberger J, Bockrath M, Lau C N 2018 Nano Lett. 18 4214
 Google Scholar
Google Scholar
[29] Chen L B, Chung J H, Gao B, Chen T, Stone M B, Kolesnikov A I, Huang Q Z, Dai P C 2018 Phys. Rev. X 8 041028
 Google Scholar
Google Scholar
[30] Zeng Y, Wang L, Li S, He C, Zhong D, Yao D X 2019 J. Phys. Codens. Mat. 31 395502
 Google Scholar
Google Scholar
[31] Zhou Y G, Wang Z G, Yang P, Zu X T, Yang L, Sun X, Gao F 2012 ACS Nano 6 9727
 Google Scholar
Google Scholar
[32] Zhu S Z, Li T 2016 Phys. Rev. B 93 115401
 Google Scholar
Google Scholar
[33] 吴木生, 徐波, 刘刚, 欧阳楚英 2012 61 227102
 Google Scholar
Google Scholar
Wu M S, Xu B, Liu G, Ouyang C Y 2012 Acta Phys. Sin. 61 227102
 Google Scholar
Google Scholar
[34] Rai H M, Saxena S K, Mishra V, Late R, Kumar R, Sagdeo P R, Jaiswal N K, Srivastava P 2016 RSC Adv. 6 11014
 Google Scholar
Google Scholar
[35] Osada M, Yoguchi S, Itose M, Li B W, Ebina Y, Fukuda K, Kotani Y, Ono K, Ueda S, Sasaki T 2014 Nanoscale 6 14227
 Google Scholar
Google Scholar
[36] Guan J, Yu G, Ding X, Chen W, Shi Z, Huang X, Sun C 2013 Chemphyschem. 14 2841
 Google Scholar
Google Scholar
[37] Du A J, Chen Y, Zhu Z H, Amal R, Lu G Q, Smith S C 2009 J. Am. Chem. Soc. 131 17354
 Google Scholar
Google Scholar
[38] 黄炳铨, 周铁戈, 吴道雄, 张召富, 李百奎 2019 68 246301
 Google Scholar
Google Scholar
Huang B Q, Zhou T G, Wu D X, Zhang Z F, Li B K 2019 Acta Phys. Sin. 68 246301
 Google Scholar
Google Scholar
[39] Kan E, Li M, Hu S, Xiao C, Xiang H, Deng K 2013 J. Phys. Chem. Lett. 4 1120
 Google Scholar
Google Scholar
[40] Barone V, Peralta J E 2008 Nano Lett. 8 2210
 Google Scholar
Google Scholar
[41] Allen M J, Tung V C, Kaner R B 2010 J. Am. Chem. Soc. 110 132
 Google Scholar
Google Scholar
[42] Lee K W, Lee C E 2012 Adv. Mater. 24 2019
 Google Scholar
Google Scholar
[43] 栾晓玮, 孙建平, 王凡嵩, 韦慧兰, 胡艺凡 2019 68 026802
 Google Scholar
Google Scholar
Luan X W, Sun J P, Wang F S, Wei H L, Hu Y F 2019 Acta Phys. Sin. 68 026802
 Google Scholar
Google Scholar
[44] 杨光敏, 梁志聪, 黄海华 2017 66 057301
 Google Scholar
Google Scholar
Yang G M, Liang Z C, Huang H H 2017 Acta Phys. Sin. 66 057301
 Google Scholar
Google Scholar
[45] Zheng F W, Zhao J Z, Liu Z, Li M L, Zhou M, Zhang S B, Zhang P 2018 Nanoscale 10 14298
 Google Scholar
Google Scholar
[46] Gao Y, Wang J, Li Z P, Yang J J, Xia M R, Hao X F, Xu Y H, Gao F M 2019 Phys. Status. Solidi-R. 13 1800410
 Google Scholar
Google Scholar
[47] Qin W J, Xu B, Liao S S, Liu G, Sun B Z, Wu M S 2020 Solid State Commun. 321 114037
 Google Scholar
Google Scholar
[48] Liu J, Shi M C, Lu J W, Anantram M P 2018 Phys. Rev. B 97 054416
 Google Scholar
Google Scholar
[49] Guo Y L, Yuan S J, Wang B, Shi L, Wang J L 2018 J. Mater. Chem. C 6 5716
 Google Scholar
Google Scholar
[50] Kresse G, Furthmuller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[51] Kresse G, Hafner J 1994 Phys. Rev. B: Condens. Matter 49 14251
 Google Scholar
Google Scholar
[52] Kresse G, Furthmiiller J 1996 Science 6 15
[53] Blochl P E 1994 Phys. Rev. B: Condens. Matter 50 17953
 Google Scholar
Google Scholar
[54] Kresse G, Joubert D 1999 Phys. Rev. B 59 1758
[55] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[56] Perdew J P, Ernzerhof M, Burke K 1996 J. Chem. Phys. 105 9982
 Google Scholar
Google Scholar
[57] Liechtenstein A I, Anisimov V V, Zaanen J 1995 Phys. Rev. B: Condens. Matter 52 R5467
 Google Scholar
Google Scholar
[58] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[59] Goodenough J B 1958 J. Phys. Chem. Solids 6 287
 Google Scholar
Google Scholar
[60] Anderson P W 1959 Phys. Rev. 115 2
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 8251
- PDF Downloads: 194
- Cited By: 0














 DownLoad:
DownLoad: