-
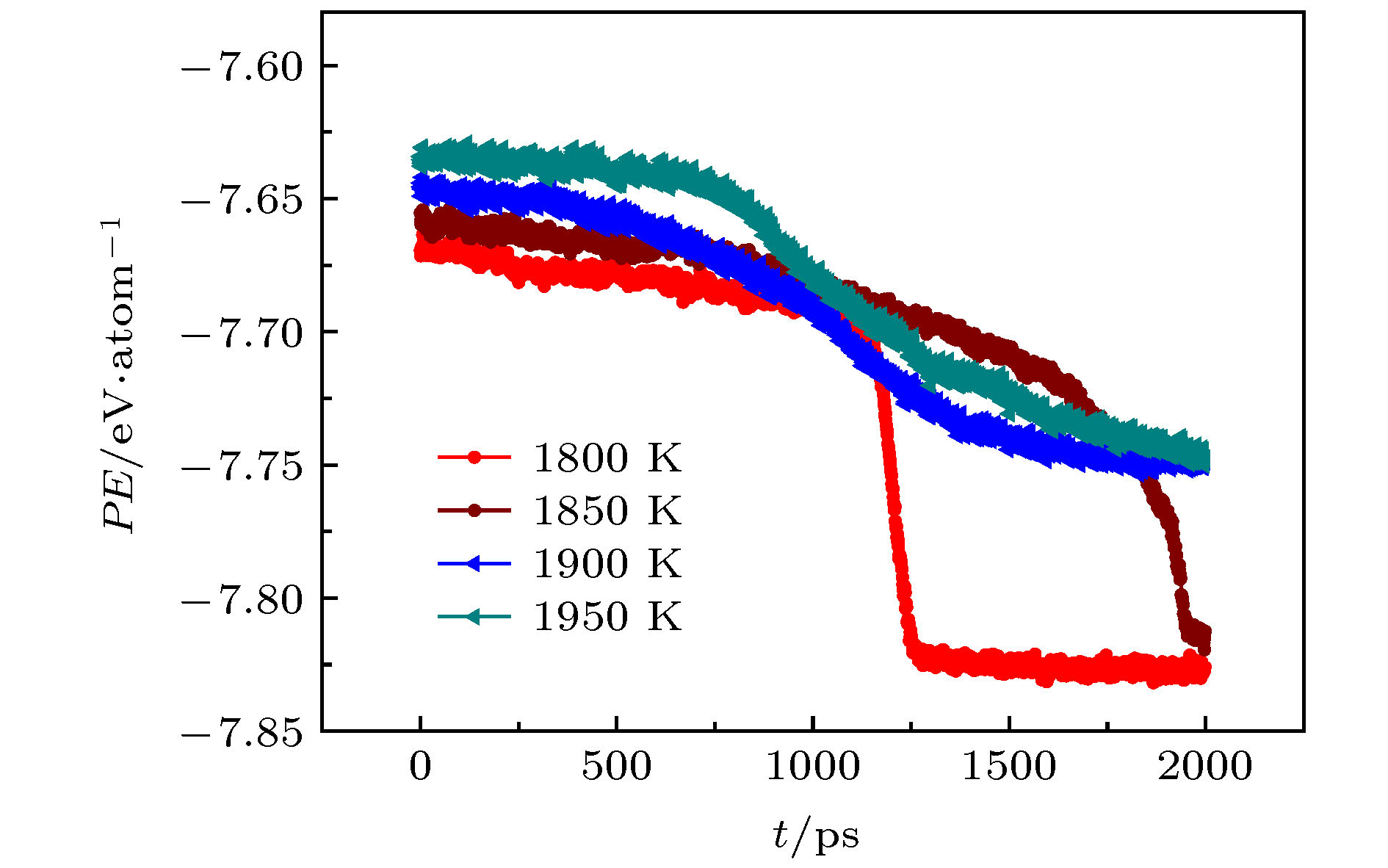
The morphology and physical properties of crystal as well as the glass-forming ability (GFA) of metals are closely related to the evolution pathway of atomic structures in the early stage of nucleation in supercooled liquids. Therefore, the study of the evolution of atomic structures in the isothermal crystallization process of supercooled liquids, is of great significance not only for predicting and accurately controlling the crystal nucleation and growth, but also for understanding the local atomic structural origin of the GFA. In the present work, the atomic-level mechanism for isothermal crystallization in the supercooled liquid tantalum is studied by molecular dynamics (MD) simulation. The microstructural evolution of metal Ta system is characterized and analyzed by using the potential energy per atom (PE), the pair distribution function (PDF) g(r), and the largest standard cluster (LSC). Two crystallization paths of Ta supercooled liquid can be observed during isothermal relaxations. For each pathway the incubation time of the formation critical nucleus increases with annealing temperature (T) rising. At 1800 K ≤ T ≤ 1850 K, the crystallization of supercooled liquid Ta conforms to the Ostwald's step rule: first, Z12 (i.e. icosahedron) and Z14 (Kasper cluster with 14 coordination number) clusters in supercooled liquids are hinged into medium-range order (i.e., Z-MRO); then the Z-MRO are merged and ordered into A15 crystal phase; finally, BCC crystal nucleus inside of the A15 phase grows rapidly into BCC single crystal at the cost of the atoms in A15 phase. While at 1900 K ≤ T ≤ 1950 K, Ta supercooled liquid is directly transformed into A15 phase. The A15 crystal phase is mainly formed by the continuous merging of the largest Z-MRO with the small Z-MRO, which is similar to the picture of the classical nucleation theory (CNT). However, whether the phase transition from A15 to BCC will occur above 1900 K remains to be further confirmed by a longer-time MD simulation. Relative to the supercooled liquids of monoatomic metals with lower melting point, the good GFA of Ta may originate from the slowly growing A15 crystal nucleus in its supercooled liquid.
[1] 王锦程, 郭灿, 张琪, 唐赛, 李俊杰, 王志军 2018 金属学报 54 204
 Google Scholar
Google Scholar
Wang J C, Guo C, Zhang Q, Tang S, Li J J, Wang Z J 2018 Acta Metall. Sin. 54 204
 Google Scholar
Google Scholar
[2] 刘丽霞, 侯兆阳, 刘让苏 2012 61 056101
 Google Scholar
Google Scholar
Liu L X, Hou Z Y, Liu R S 2012 Acta Phys. Sin. 61 056101
 Google Scholar
Google Scholar
[3] Shibuta Y, Sakane S, Takaki T, Ohno M 2016 Acta Mater. 105 328
 Google Scholar
Google Scholar
[4] Sun Y, Song H J, Zhang F, Yang L, Ye Z, Mendelev M I, Wang C Z, Ho K M 2018 Phys. Rev. Lett. 120 085703
 Google Scholar
Google Scholar
[5] Okita S, Verestek W, Sakane S, Takaki T, Ohno M, Shibuta Y 2017 J. Cryst. Growth 474 140
 Google Scholar
Google Scholar
[6] Herlach D M, Kobold R, Klein S 2018 JOM 70 726
 Google Scholar
Google Scholar
[7] Sosso G C, Chen J, Cox S J, Fitzner M, Pedevilla P, Zen A, Michaelides A 2016 Chem. Rev. 116 7078
 Google Scholar
Google Scholar
[8] Leines G D, Drautz R, Rogal J 2017 J. Chem. Phys. 146 154702
 Google Scholar
Google Scholar
[9] Turnbull D 1969 Contemp. Phys. 10 473
 Google Scholar
Google Scholar
[10] Wen D D, Deng Y H, Dai X Y, Tian Z A, Peng P 2019 Philos. Mag. 99 2904
 Google Scholar
Google Scholar
[11] Chung S Y, Kim Y M, Kim J G, Kim Y J 2009 Nat. Phys. 5 68
 Google Scholar
Google Scholar
[12] Zhong L, Wang J W, Sheng H W, Zhang Z, Mao S X 2014 Nature 512 177
 Google Scholar
Google Scholar
[13] Plimpton S 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[14] [15] Wu Z W, Li M Z, Wang W H, Song W J, Liu K X 2013 J. Chem. Phys. 138 074502
 Google Scholar
Google Scholar
[16] Wu Z, Mo Y, Lang L, Yu A, Xie Q, Liu R, Tian Z 2018 Phys.Chem.Chem.Phys. 20 28088
 Google Scholar
Google Scholar
[17] Zhang J C, Chen C, Pei Q X, Wan Q, Zhang W X, Sha Z D 2015 Mater. Des. 77 1
 Google Scholar
Google Scholar
[18] Tian Z A, Liu R S, Dong K J, Yu A B 2011 Europhys. Lett. 96 36001
 Google Scholar
Google Scholar
[19] Cheng Y Q, Ma E 2011 Prog. Mater. Sci. 56 379
 Google Scholar
Google Scholar
[20] Wen D D, Deng Y H, Liu J, Tian Z A, Peng P 2017 Comput. Mater. Sci. 140 275
 Google Scholar
Google Scholar
[21] Gabriele C S, Ji C, Stephen J C, Martin F, Philipp P, Andrea Z, Angelos M 2016 Chemical Review 116 7078
[22] 文大东, 彭平, 蒋元祺, 田泽安, 刘让苏 2013 62 196101
 Google Scholar
Google Scholar
Wen D D, Peng P, Jiang Y Q, Tian Z A, Liu R S 2013 Acta Phys. Sin. 62 196101
 Google Scholar
Google Scholar
[23] Cortella L, Vinet B 1993 Phys. Rev. Lett. 70 1469
 Google Scholar
Google Scholar
[24] Wolde ten P R, Montero M J R, Frenke D 1995 Phys. Rev. Lett. 75 2714
 Google Scholar
Google Scholar
[25] John A M, James F B, Robert E R, Per S, Frederick H S, Lin H Y 2002 J. Phys. Condens. Matter 14 2825
 Google Scholar
Google Scholar
[26] Jakse N, Bacq O L, Pasturel A 2004 Phys. Rev. B 70 174203
 Google Scholar
Google Scholar
[27] Schenk T, Moritz D H, Simonet V, Bellissent R, Herlach D M 2002 Phys. Rev. Lett. 89 075507
 Google Scholar
Google Scholar
[28] Zhang Q, Wang J C, Tang S, Wang Y J, Li J J, Zhou W Q, Wang Z J 2019 Phys. Chem. Chem. Phys. 21 4122
 Google Scholar
Google Scholar
[29] Wu Z Z, Mo Y F, Lang L, Yu A B, Xie Q, Liu R S, Tian Z A 2018 Phys. Chem. Chem. Phys. 20 28088
-
图 2 金属Ta体系的双体分布函数g(r)和结构因子S(q) (a) 300 K时体系的g(r) 曲线; (b) 300 K时模拟体系的结构因子S(q); (c)不同温度下体系晶化前后的g(r)曲线
Figure 2. The g(r) and S(q) curves of metal Ta system at several selected temperatures: (a) Comparison of g(r) for Ta metallic glass at 300 K between present MD simulation and ab initio MD results; (b) comparison of S(q) for Ta metallic glass at 300 K between present MD simulation and experimental values; (c) the g(r) curves of metal Ta system for t = 0 ps and 2000 ps at different temperatures.
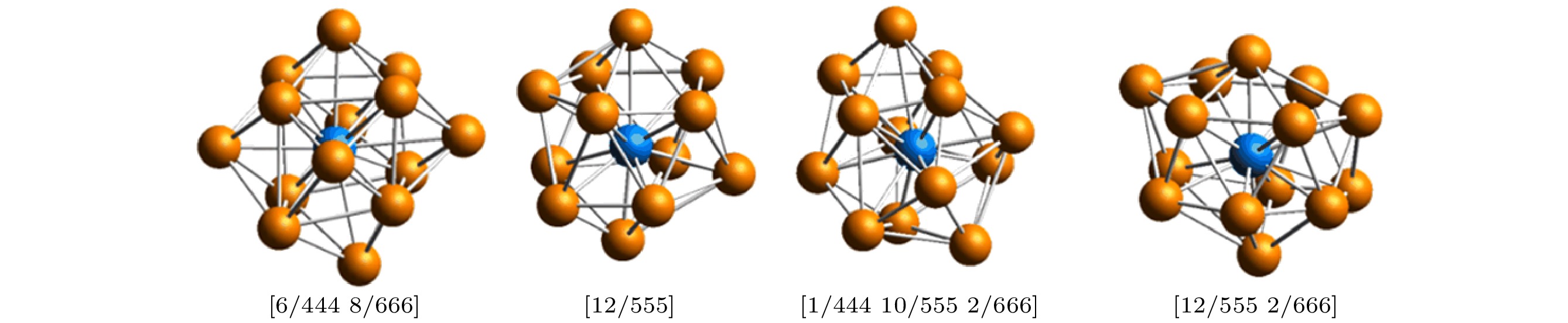
图 3 最大标准团簇(LSC)拓扑结构示意图 (a)最大标准团簇(LSC); (b), (d) 共有近邻子团簇(CNS); (c), (e) 共有近邻(CNN) (小球上的数字代表原子在当前模拟系统中的编号)
Figure 3. Topology of a largest standard cluster (LSC): (a) A [12/555 2/666] Kasper cluster composed of a central atom (labeled 7031) and 14 neighbors; (b) a common neighbor subcluster (CNS) of 666 composed of a bonded reference pair (labeled 7031 and 8877) and 6 common near neighbors (CNNs); (c) the topology of the 6 CNNs; (d) the CNS of 555 and (e) the topology of its 5 CNNs. The number on the ball represents the ID of atoms in the current simulation system.
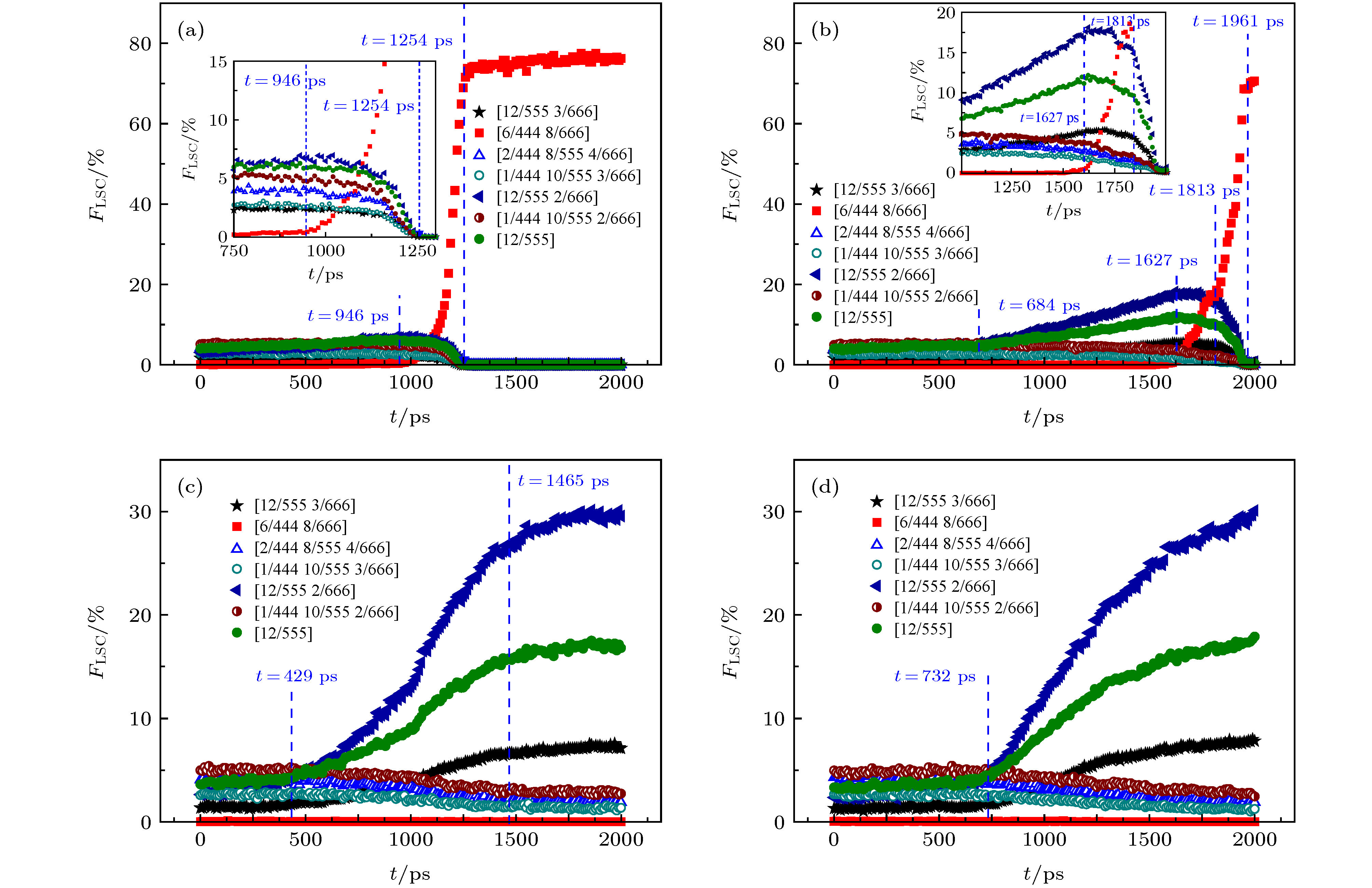
图 5 钽过冷液体结晶过程中典型LSC的比例
$ {F}_{\rm{LSC}} $ 随时间t的演化 (a) 1800 K; (b) 1850 K; (c) 1900 K; (d) 1950 K. (内插小图是局部放大)Figure 5. The evolution of the fraction
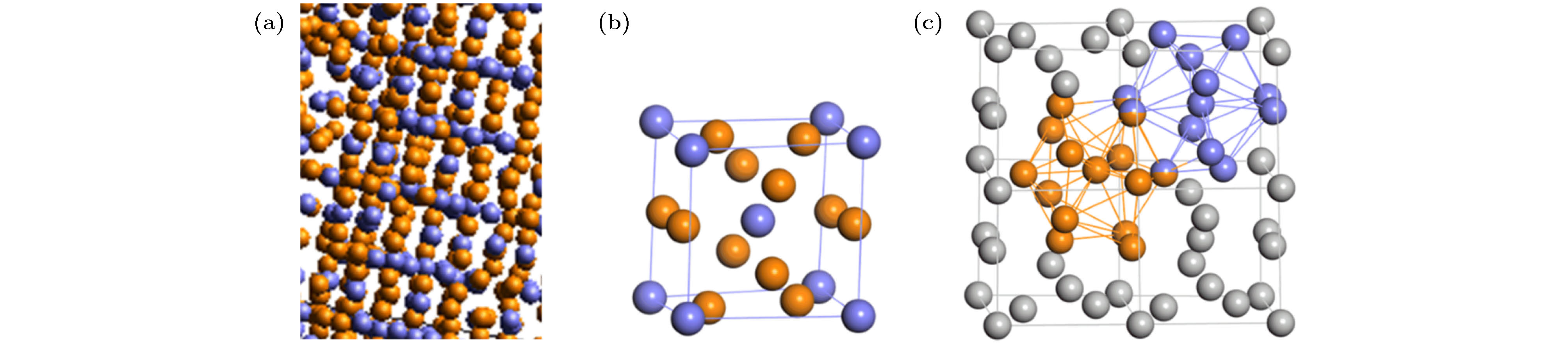
$ {F}_{\rm{LSC}} $ of typical LSCs with time t during the crystallization process of supercooled liquid tantalum: (a) 1800 K; (b) 1850 K; (c) 1900 K; (d) 1950 K. The inset is the zoom.图 6 A15相结构示意图 (a)1800 K金属Ta体系中的A15相晶体结构(t = 2000 ps); (b)A15晶体相的单胞结构(浅蓝色球表示二十面体团簇原子, 橙色球表示Z14团簇原子); (c) A15相超晶胞(
$ 2\times 2\times 1 $ )中的Z14团簇(橙色) 和二十面体团簇(浅蓝色)示意图(双手球代表共享原子)Figure 6. Schematic diagram of A15 phase structure: (a) A15 phase crystal structure in metal Ta system at 1800 K (t = 2000 ps); (b) unit cell of A15 crystal phase (blue balls represent the atoms of icosahedra, while orange ball represents the atom of Z14 clusters); (c) schematic diagram of Z14 cluster (orange) and icosahedron (light blue) in a supercell (2 × 2 × 1) of A15 phase (double handball represents the shared atom).
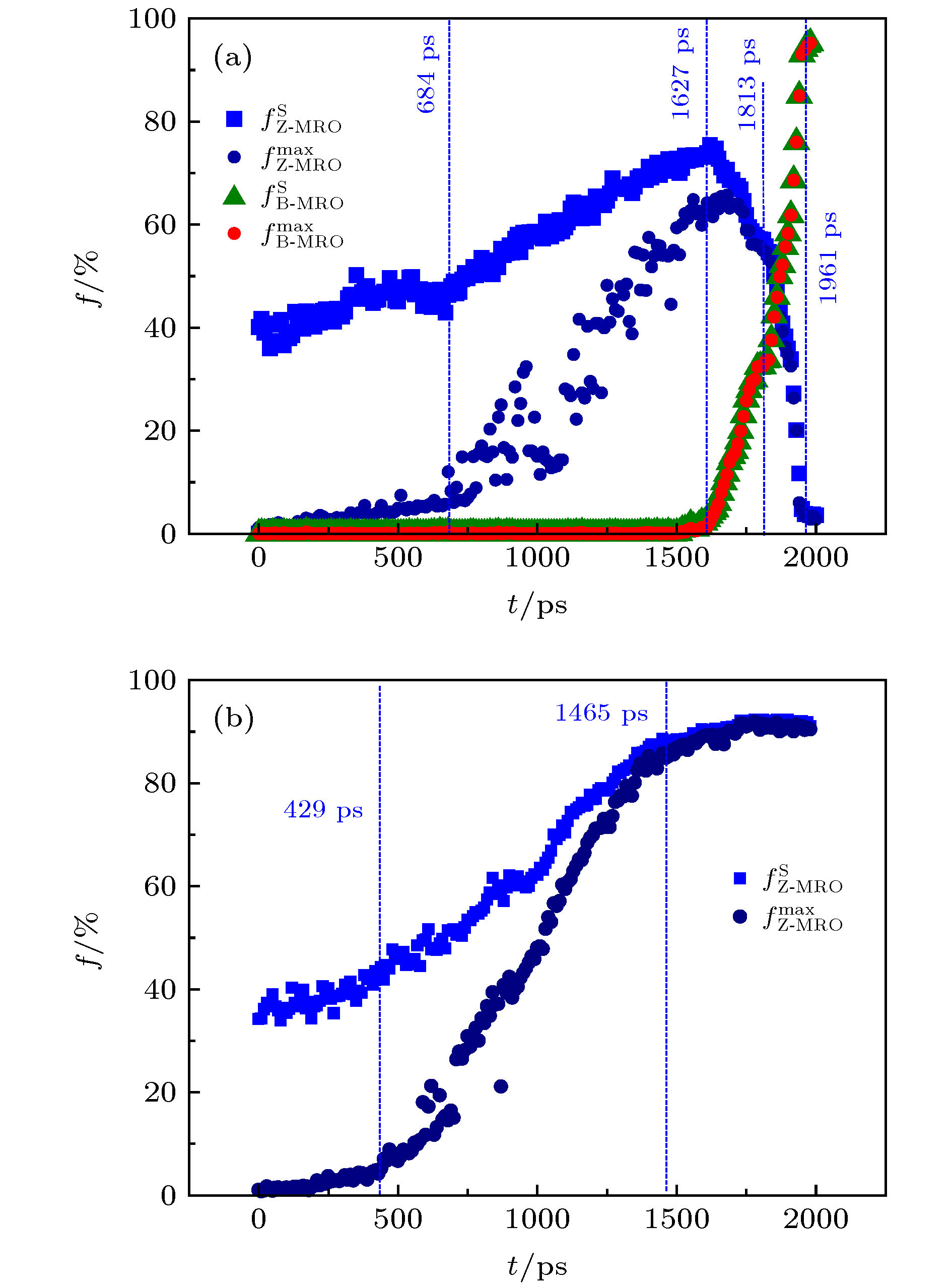
图 7 钽过冷液体的中程序(MRO)原子分数f随时间t的演化 (a) 1850 K; (b) 1900 K
Figure 7. The time t evolution of the atomic fraction f of medium-range order (MRO) during the crystallization of supercooled liquid tantalum: (a) 1850 K; (b) 1900 K. The
$ {\rm{B}} - {\rm{MRO}}$ and${\rm{Z}} - {\rm{MRO}} $ respectively represents the MRO of BCC and the MRO composed by Z12 and Z14 clusters, while the$ {\rm{max}} $ and$ {\rm{S}} $ denotes the maximum MRO and the total MROs, respectively.图 8 Ta过冷液体晶化过程中的B-MRO和Z-MRO中心原子的空间分布 (a) 1850 K; (b) 1900 K. (其中浅蓝色球代表Z12团簇原子, 橙色球代表Z14团簇原子, 绿色球代表BCC团簇原子)
Figure 8. Spatial distribution of central atoms of B-MRO and Z-MRO during crystallization of supercooled liquid Ta: (a) 1850 K; (b) 1900 K. (The light blue spheres represent Z12 cluster atoms, the orange spheres represent Z14 ones, and the green spheres denote BCC atoms)
-
[1] 王锦程, 郭灿, 张琪, 唐赛, 李俊杰, 王志军 2018 金属学报 54 204
 Google Scholar
Google Scholar
Wang J C, Guo C, Zhang Q, Tang S, Li J J, Wang Z J 2018 Acta Metall. Sin. 54 204
 Google Scholar
Google Scholar
[2] 刘丽霞, 侯兆阳, 刘让苏 2012 61 056101
 Google Scholar
Google Scholar
Liu L X, Hou Z Y, Liu R S 2012 Acta Phys. Sin. 61 056101
 Google Scholar
Google Scholar
[3] Shibuta Y, Sakane S, Takaki T, Ohno M 2016 Acta Mater. 105 328
 Google Scholar
Google Scholar
[4] Sun Y, Song H J, Zhang F, Yang L, Ye Z, Mendelev M I, Wang C Z, Ho K M 2018 Phys. Rev. Lett. 120 085703
 Google Scholar
Google Scholar
[5] Okita S, Verestek W, Sakane S, Takaki T, Ohno M, Shibuta Y 2017 J. Cryst. Growth 474 140
 Google Scholar
Google Scholar
[6] Herlach D M, Kobold R, Klein S 2018 JOM 70 726
 Google Scholar
Google Scholar
[7] Sosso G C, Chen J, Cox S J, Fitzner M, Pedevilla P, Zen A, Michaelides A 2016 Chem. Rev. 116 7078
 Google Scholar
Google Scholar
[8] Leines G D, Drautz R, Rogal J 2017 J. Chem. Phys. 146 154702
 Google Scholar
Google Scholar
[9] Turnbull D 1969 Contemp. Phys. 10 473
 Google Scholar
Google Scholar
[10] Wen D D, Deng Y H, Dai X Y, Tian Z A, Peng P 2019 Philos. Mag. 99 2904
 Google Scholar
Google Scholar
[11] Chung S Y, Kim Y M, Kim J G, Kim Y J 2009 Nat. Phys. 5 68
 Google Scholar
Google Scholar
[12] Zhong L, Wang J W, Sheng H W, Zhang Z, Mao S X 2014 Nature 512 177
 Google Scholar
Google Scholar
[13] Plimpton S 1995 J. Comput. Phys. 117 1
 Google Scholar
Google Scholar
[14] [15] Wu Z W, Li M Z, Wang W H, Song W J, Liu K X 2013 J. Chem. Phys. 138 074502
 Google Scholar
Google Scholar
[16] Wu Z, Mo Y, Lang L, Yu A, Xie Q, Liu R, Tian Z 2018 Phys.Chem.Chem.Phys. 20 28088
 Google Scholar
Google Scholar
[17] Zhang J C, Chen C, Pei Q X, Wan Q, Zhang W X, Sha Z D 2015 Mater. Des. 77 1
 Google Scholar
Google Scholar
[18] Tian Z A, Liu R S, Dong K J, Yu A B 2011 Europhys. Lett. 96 36001
 Google Scholar
Google Scholar
[19] Cheng Y Q, Ma E 2011 Prog. Mater. Sci. 56 379
 Google Scholar
Google Scholar
[20] Wen D D, Deng Y H, Liu J, Tian Z A, Peng P 2017 Comput. Mater. Sci. 140 275
 Google Scholar
Google Scholar
[21] Gabriele C S, Ji C, Stephen J C, Martin F, Philipp P, Andrea Z, Angelos M 2016 Chemical Review 116 7078
[22] 文大东, 彭平, 蒋元祺, 田泽安, 刘让苏 2013 62 196101
 Google Scholar
Google Scholar
Wen D D, Peng P, Jiang Y Q, Tian Z A, Liu R S 2013 Acta Phys. Sin. 62 196101
 Google Scholar
Google Scholar
[23] Cortella L, Vinet B 1993 Phys. Rev. Lett. 70 1469
 Google Scholar
Google Scholar
[24] Wolde ten P R, Montero M J R, Frenke D 1995 Phys. Rev. Lett. 75 2714
 Google Scholar
Google Scholar
[25] John A M, James F B, Robert E R, Per S, Frederick H S, Lin H Y 2002 J. Phys. Condens. Matter 14 2825
 Google Scholar
Google Scholar
[26] Jakse N, Bacq O L, Pasturel A 2004 Phys. Rev. B 70 174203
 Google Scholar
Google Scholar
[27] Schenk T, Moritz D H, Simonet V, Bellissent R, Herlach D M 2002 Phys. Rev. Lett. 89 075507
 Google Scholar
Google Scholar
[28] Zhang Q, Wang J C, Tang S, Wang Y J, Li J J, Zhou W Q, Wang Z J 2019 Phys. Chem. Chem. Phys. 21 4122
 Google Scholar
Google Scholar
[29] Wu Z Z, Mo Y F, Lang L, Yu A B, Xie Q, Liu R S, Tian Z A 2018 Phys. Chem. Chem. Phys. 20 28088
Catalog
Metrics
- Abstract views: 8296
- PDF Downloads: 101
- Cited By: 0














 DownLoad:
DownLoad: