-
光片荧光显微术(light-sheet fluorescence microscopy, LSFM)采用薄片光束从侧面激发样品, 在垂直于光片方向上进行成像, 具有成像速度快、光学层析能力强以及光漂白和光毒性低等优点, 适用于对较大活体生物样品进行高质量、长时间三维动态观测. 然而, 传统高斯光束LSFM存在分辨率低和成像视场小的问题. 本文在双边照明LSFM的基础上, 结合虚拟单像素成像解卷积技术, 提出了一种大视场高分辨双边照明LSFM, 实现了视场和分辨率的同时提升. 设计和搭建了双边照明LSFM, 开展了荧光珠和转基因斑马鱼样品的三维光切片显微成像实验, 实验结果证明了系统的三维高分辨成像能力, 对于大视场、高分辨LSFM的发展和应用具有重要意义.In light-sheet fluorescence microscopy (LSFM) a thin light sheet is used to excite the specimen from the side and imaging is performed in the direction perpendicular to the light-sheet. It has the advantages of fast imaging speed, high optical sectioning capability and low photobleaching and phototoxicity to samples. Therefore, it is suitable for high-quality, long-term three-dimensional dynamic observation of large living biological samples. However, the traditional Gaussian light sheet illumination microscopy technology has the problems of small imaging field of view and low spatial resolution. Based on the existing dual-sided illumination LSFM, a large field of view and high resolution LSFM combined with virtual single-pixel imaging deconvolution is presented in this paper, which improves the field of view and resolution of LSFM simultaneously. The relevant microscope is designed and built, and three-dimensional optical sectioning imaging experiments on fluorescent beads and transgenic zebrafish standard samples are carried out. The experimental results prove the three-dimensional high resolution imaging capability of the microscope, which is of great significance in developing the large field of view and high resolution LSFM.
-
Keywords:
- light-sheet fluorescence microscopy /
- virtual single-pixel imaging /
- deconvolution /
- three-dimensional imaging
[1] Power R M, Huisken J 2017 Nat. Methods 14 360
 Google Scholar
Google Scholar
[2] Royer L A, Lemon W C, Chhetri R K, Wan Y N, Coleman M, Myers E W, Keller P J 2016 Nat. Biotechnol. 34 1267
 Google Scholar
Google Scholar
[3] Voie A H, Burns D H, Spelman F A 1993 J. Microsc-Oxford 170 229
 Google Scholar
Google Scholar
[4] Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer E H K 2004 Science 305 1007
 Google Scholar
Google Scholar
[5] Swoger J, Huisken J, Stelzer E H K 2003 Opt. Lett. 28 1654
 Google Scholar
Google Scholar
[6] Krzic U, Gunther S, Saunders T E, Streichan S J, Hufnagel L 2012 Nat. Methods 9 730
 Google Scholar
Google Scholar
[7] Keller P J, Schmidt A D, Wittbrodt J, Stelzer E H K 2008 Science 322 1065
 Google Scholar
Google Scholar
[8] Truong T V, Supatto W, Koos D S, Choi J M, Fraser S E 2011 Nat. Methods 8 757
 Google Scholar
Google Scholar
[9] Liu S, Nie J, Li Y S, Yu T T, Zhu D, Fei P 2017 J. Innovative Opt. Health. Sci. 10 1743006
 Google Scholar
Google Scholar
[10] Planchon T A, Gao L, Milkie D E, Davidson M W, Galbraith J A, Galbraith C G, Betzig E 2011 Nat. Methods 8 417
 Google Scholar
Google Scholar
[11] Gao L, Shao L, Chen B C, Betzig E 2014 Nat. Protoc. 9 1083
 Google Scholar
Google Scholar
[12] Vettenburg T, Dalgarno H I C, Nylk J, Coll-Lladό C, Ferrier D E K, Čižmár T, Gunn-Moore F J, Dholakia K 2014 Nat. Methods 11 541
 Google Scholar
Google Scholar
[13] Yang Z Y, Prokopas M, Nylk J, Coll-Lladό C, Gunn-Moore F J, Ferrier D E K, Vettenburg T, Dholakia K 2014 Biomed. Opt. Express 5 3434
 Google Scholar
Google Scholar
[14] Jia H, Yu X H, Yang Y L, Zhou X, Yan S H, Liu C, Lei M, Yao B L 2019 J. Biophotonics 12 e201800094
 Google Scholar
Google Scholar
[15] Gao L 2015 Opt. Express 23 6102
 Google Scholar
Google Scholar
[16] Westphal V, Rizzoli S O, Lauterbach M A, Kamin D, Jahn R, Hell S W 2008 Science 320 246
 Google Scholar
Google Scholar
[17] Li D, Shao L, Chen B C, Zhang X, Zhang M S, Moses B, Milkie D E, Beach J R, Hammer J A, Pasham M, Kirchhausen T, Baird M A, Davidson M W, Xu P Y, Betzig E 2015 Science 349 aab3500
 Google Scholar
Google Scholar
[18] Betzig E, Patterson G H, Sougrat R, Lindwasser O W, Olenych S, Bonifacino J S, Davidson M W, Lippincott-Schwartz J, Hess H F 2006 Science 313 1642
 Google Scholar
Google Scholar
[19] Rust M J, Bates M, Zhuang X W 2006 Nat. Methods 3 793
 Google Scholar
Google Scholar
[20] Dertinger T, Colyer R, Iyer G, Weiss S, Enderlein J 2009 Proc. Natl. Acad. Sci. U. S. A. 106 22287
 Google Scholar
Google Scholar
[21] Zanacchi F C, Lavagnino Z, Donnorso M P, Del Bue A, Furia L, Faretta M, Diaspro A 2011 Nat. Methods 8 1047
 Google Scholar
Google Scholar
[22] Liu Z, Lavis L D, Betzig E 2015 Mol. Cell 58 644
 Google Scholar
Google Scholar
[23] Legant W R, Shao L, Grimm J B, Brown T A, Milkie D E, Avants B B, Lavis L D, Betzig E 2016 Nat. Methods 13 359
 Google Scholar
Google Scholar
[24] Chen B C, Legant W R, Wang K, Shao L, Milkie D E, Davidson M W, Janetopoulos C, Wu X F S, Hammer J A, Liu Z, English B P, Mimori-Kiyosue Y, Romero D P, Ritter A T, Lippincott-Schwartz J, Fritz-Laylin L, Mullins R D, Mitchell D M, Bembenek J N, Reymann A C, Bohme R, Grill S W, Wang J T, Seydoux G, Tulu U S, Kiehart D P, Betzig E 2014 Science 346 439
 Google Scholar
Google Scholar
[25] Zhang W, Li S W, Yang Z G, Yu B, Lin D Y, Xiong J, Qu J L 2020 Biomed. Opt. Express 11 3648
 Google Scholar
Google Scholar
-
图 3 用于系统成像分辨率标定的直径100 nm荧光珠图像及其尺寸统计直方图 (a) 未经v-SPI处理的图像; (b) 图(a)中荧光珠沿X方向强度曲线FWHM的直方图分布(N = 25); (c) 经v-SPI处理的图像; (d) 图(c)中相同荧光珠沿X方向强度曲线FWHM的直方图分布;
$ \overline{X} $ 为平均值Fig. 3. Images of 100-nm-diameter fluorescent beads for system resolution calibration: (a) Image without v-SPI processing; (b) the corresponding histogram distribution of the intensity profile FWHM from 25 fluorescent beads in X direction; (c) image with v-SPI processing; (d) the corresponding FWHM histogram distribution from the same beads in X direction;
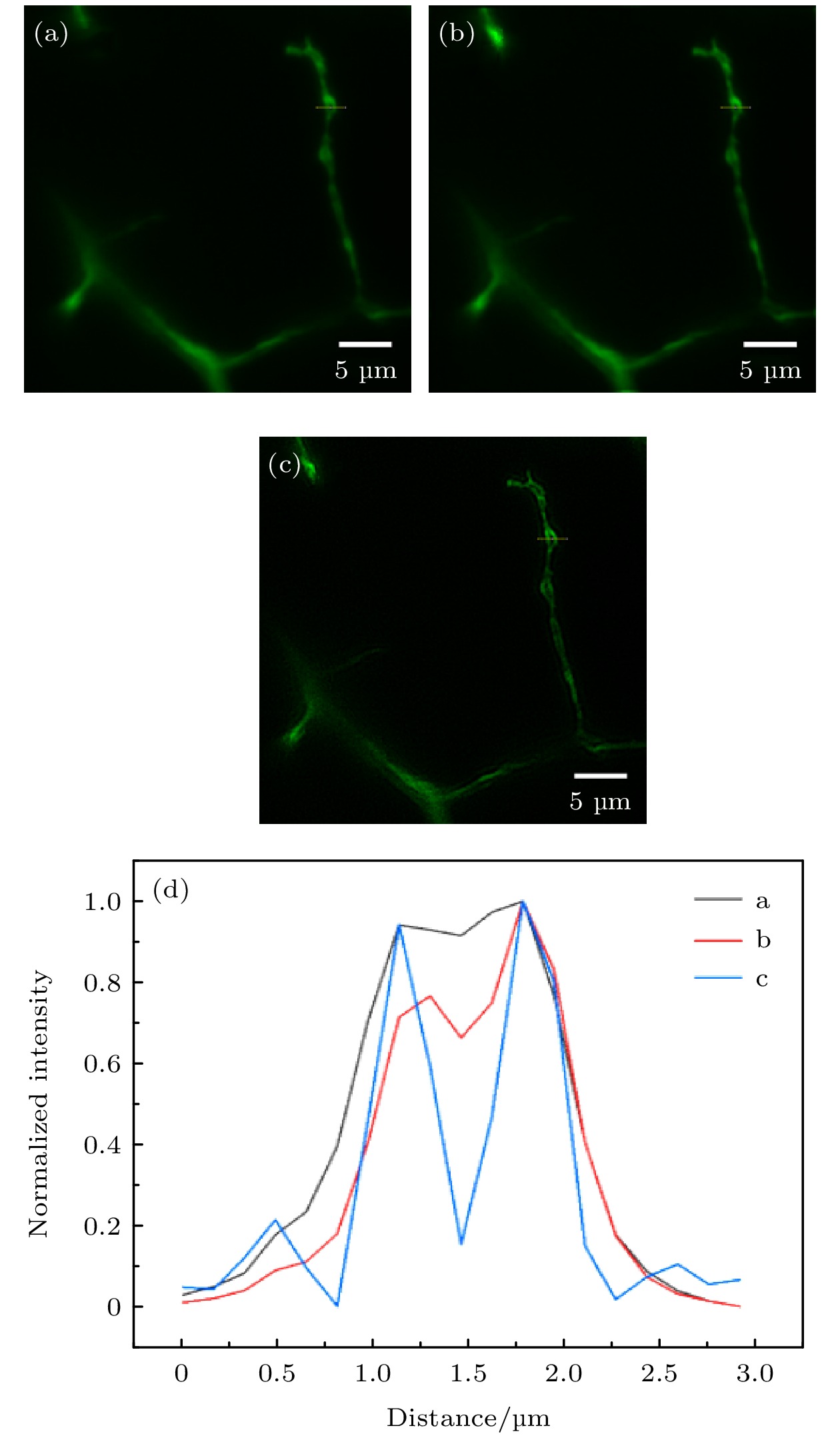
$ \overline{X} $ is the average value.图 5 斑马鱼运动神经元成像 (a) 未经特殊处理的图像; (b) RL解卷积处理的图像; (c) v-SPI处理图像; (d) 图(a)−(c)中黄色色实线标记位置的归一化强度曲线
Fig. 5. Images of motoneurons in a zebrafish: (a) Image without special processing; (b) image with RL deconvolution; (c) image with v-SPI processing; (d) normalized intensity profiles along the yellow solid lines in panels (a)−(c).
-
[1] Power R M, Huisken J 2017 Nat. Methods 14 360
 Google Scholar
Google Scholar
[2] Royer L A, Lemon W C, Chhetri R K, Wan Y N, Coleman M, Myers E W, Keller P J 2016 Nat. Biotechnol. 34 1267
 Google Scholar
Google Scholar
[3] Voie A H, Burns D H, Spelman F A 1993 J. Microsc-Oxford 170 229
 Google Scholar
Google Scholar
[4] Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer E H K 2004 Science 305 1007
 Google Scholar
Google Scholar
[5] Swoger J, Huisken J, Stelzer E H K 2003 Opt. Lett. 28 1654
 Google Scholar
Google Scholar
[6] Krzic U, Gunther S, Saunders T E, Streichan S J, Hufnagel L 2012 Nat. Methods 9 730
 Google Scholar
Google Scholar
[7] Keller P J, Schmidt A D, Wittbrodt J, Stelzer E H K 2008 Science 322 1065
 Google Scholar
Google Scholar
[8] Truong T V, Supatto W, Koos D S, Choi J M, Fraser S E 2011 Nat. Methods 8 757
 Google Scholar
Google Scholar
[9] Liu S, Nie J, Li Y S, Yu T T, Zhu D, Fei P 2017 J. Innovative Opt. Health. Sci. 10 1743006
 Google Scholar
Google Scholar
[10] Planchon T A, Gao L, Milkie D E, Davidson M W, Galbraith J A, Galbraith C G, Betzig E 2011 Nat. Methods 8 417
 Google Scholar
Google Scholar
[11] Gao L, Shao L, Chen B C, Betzig E 2014 Nat. Protoc. 9 1083
 Google Scholar
Google Scholar
[12] Vettenburg T, Dalgarno H I C, Nylk J, Coll-Lladό C, Ferrier D E K, Čižmár T, Gunn-Moore F J, Dholakia K 2014 Nat. Methods 11 541
 Google Scholar
Google Scholar
[13] Yang Z Y, Prokopas M, Nylk J, Coll-Lladό C, Gunn-Moore F J, Ferrier D E K, Vettenburg T, Dholakia K 2014 Biomed. Opt. Express 5 3434
 Google Scholar
Google Scholar
[14] Jia H, Yu X H, Yang Y L, Zhou X, Yan S H, Liu C, Lei M, Yao B L 2019 J. Biophotonics 12 e201800094
 Google Scholar
Google Scholar
[15] Gao L 2015 Opt. Express 23 6102
 Google Scholar
Google Scholar
[16] Westphal V, Rizzoli S O, Lauterbach M A, Kamin D, Jahn R, Hell S W 2008 Science 320 246
 Google Scholar
Google Scholar
[17] Li D, Shao L, Chen B C, Zhang X, Zhang M S, Moses B, Milkie D E, Beach J R, Hammer J A, Pasham M, Kirchhausen T, Baird M A, Davidson M W, Xu P Y, Betzig E 2015 Science 349 aab3500
 Google Scholar
Google Scholar
[18] Betzig E, Patterson G H, Sougrat R, Lindwasser O W, Olenych S, Bonifacino J S, Davidson M W, Lippincott-Schwartz J, Hess H F 2006 Science 313 1642
 Google Scholar
Google Scholar
[19] Rust M J, Bates M, Zhuang X W 2006 Nat. Methods 3 793
 Google Scholar
Google Scholar
[20] Dertinger T, Colyer R, Iyer G, Weiss S, Enderlein J 2009 Proc. Natl. Acad. Sci. U. S. A. 106 22287
 Google Scholar
Google Scholar
[21] Zanacchi F C, Lavagnino Z, Donnorso M P, Del Bue A, Furia L, Faretta M, Diaspro A 2011 Nat. Methods 8 1047
 Google Scholar
Google Scholar
[22] Liu Z, Lavis L D, Betzig E 2015 Mol. Cell 58 644
 Google Scholar
Google Scholar
[23] Legant W R, Shao L, Grimm J B, Brown T A, Milkie D E, Avants B B, Lavis L D, Betzig E 2016 Nat. Methods 13 359
 Google Scholar
Google Scholar
[24] Chen B C, Legant W R, Wang K, Shao L, Milkie D E, Davidson M W, Janetopoulos C, Wu X F S, Hammer J A, Liu Z, English B P, Mimori-Kiyosue Y, Romero D P, Ritter A T, Lippincott-Schwartz J, Fritz-Laylin L, Mullins R D, Mitchell D M, Bembenek J N, Reymann A C, Bohme R, Grill S W, Wang J T, Seydoux G, Tulu U S, Kiehart D P, Betzig E 2014 Science 346 439
 Google Scholar
Google Scholar
[25] Zhang W, Li S W, Yang Z G, Yu B, Lin D Y, Xiong J, Qu J L 2020 Biomed. Opt. Express 11 3648
 Google Scholar
Google Scholar
计量
- 文章访问数: 6395
- PDF下载量: 86
- 被引次数: 0














 下载:
下载: