-
The effects of Re on the microstructure and mechanical properties of
$ {\gamma '}$ phase Ni3Al intermetallics are investigated by using first-principles method based on density functional theory and generalized gradient approximation. It shows that in most stoichiometric ranges, the dissolution energy of Re replacing Al site is smaller than that of Re replacing Ni site. That means the energetically-favorable site of Re in Ni3Al is Al replace site. We further investigate the interaction between two Re atoms in Ni3Al. The larger the distance between two Re atoms, the more stable the system becomes, showing that Re atoms are dispersed in Ni3Al instead of being aggregated. Re doping causes a small increase in the lattice constant of Ni3Al intermetallics without causing serious lattice deformation. Analyses on differential charge density and state density show that Re atom bonds with neighboring atoms, especially with Ni atoms, and reduces the surrounding Ni—Al bond energy. Analyses on local state density show that Re atom has orbital interaction with the neighboring Ni and Al atoms, and the interaction with Ni is larger, which concerns the 5d orbit of Re and the 3d orbit of Ni. The effect of Re on the mechanical properties of Ni3Al intermetallics is also investigated. The elastic constants calculating results together with empirical criteria indicate the presence of Re atom (corresponding concentration is 0.93%) can cause increase of the stiffness and hardness of Ni3Al. The Cauchy pressure value shows a slight improvement in toughness. The increase of Re doping concentration (the concentration of Re in Ni3Al is 1.85%) can cause increase of the lattice constant, stiffness and hardness and decrease of the ductility of Ni3Al. In order to correct the temperature of the results obtained by first-principles method, the influence of temperature on the mechanical properties of Ni3Al has been further investigated through Phonopy calculation. The influence of temperature on the coefficient of thermal expansion and bulk modulus of elasticity is obtained by quasi harmonic approximation. The results show that the addition of Re slightly enhances the entropy of Ni3Al, but causes decrease of its Helmholtz free energy on a small extent. At high temperature, doping of Re greatly strengthens the bulk modulus of Ni3Al but decreases the thermal expansion coefficient of which. Results of the current research can provide theoretical data for improving the mechanical properties of single crystal turbine blades of aero-engines.-
Keywords:
- Ni3Al intermetallics /
- rare metal element Re /
- electronic structure /
- mechanical properties
[1] 方昌德 2004 航空发动机 30 1
 Google Scholar
Google Scholar
Fang C D 2004 Aeroengine 30 1
 Google Scholar
Google Scholar
[2] 秦琴, 毛子荐, 刘昭凡 2017 工具技术 51 3
 Google Scholar
Google Scholar
Qin Q, Mao Z J, Liu Z F 2017 Tool Eng. 51 3
 Google Scholar
Google Scholar
[3] 徐颂波 1997 上海有色金属 18 88
Xu S B 1997 Shanghai Nonferro. Met. 18 88
[4] 陈金栌, 朱定一, 林登宜 2006 材料导报 20 35
 Google Scholar
Google Scholar
Chen J L, Zhu D Y, Lin D Y 2006 Mater. Rev. 20 35
 Google Scholar
Google Scholar
[5] 赵希宏, 韩雅芳, 谭永宁, 殷克勤, 余乾, 肖程波 1997 材料工程 42 13
Zhao X H, Han Y F, Tan Y N, Yin K Q, Yu Q, Xiao C B 1997 J. Mater. Eng. 42 13
[6] Lucaci M, Orban R L, Patroi D, Hodorogea S, Bibicu I, Lungu M 2007 Adv. Mater. Res. 23 67
 Google Scholar
Google Scholar
[7] Kumar A, Chernatynskiy A, Hong M, Phillpot S, Sinnott S 2015 Comput. Mater. Sci. 101 39
 Google Scholar
Google Scholar
[8] Wu Q, Li S S 2012 Comput. Mater. Sci. 53 1
 Google Scholar
Google Scholar
[9] Masahashi N, Takasugi T, Izumi O 1988 Acta Metall. 36 1823
 Google Scholar
Google Scholar
[10] Masahashi N, Takasugi T, Izumi O 1987 J. Mater. Sci. 22 2599
 Google Scholar
Google Scholar
[11] 张永刚, 韩雅芳, 陈国良, 郭建亭, 万晓景, 冯涤 2001 金属间化合物结构材料 (北京: 国防工业出版社) 第577−578页
Zhang Y G, Han Y F, Chen G L, Guo J T, Wan X J, Feng D 2001 Structural Intermetallics (Beijing: National Defense Industry Press) pp577−578 (in Chinese)
[12] 于松, 王崇愚, 于涛 2007 56 3212
 Google Scholar
Google Scholar
Yu S, Wang C Y, Yu T 2007 Acta Phys. Sin. 56 3212
 Google Scholar
Google Scholar
[13] 刘争光 2014 博士学位论文 (北京: 钢铁研究总院)
Liu Z G 2014 Ph. D. Dissertation (Beijing: Central Iron & Steel Research Institute) (in Chinese)
[14] Gong W, Zhao W Y, Miao N H, Zhou J, Sun Z M, Li S S, Gong S K 2018 Comput. Mater. Sci. 144 23
 Google Scholar
Google Scholar
[15] 胡雪兰 2009 博士学位论文 (北京: 北京航空航天大学)
Hu X L 2009 Ph. D. Dissertation (Beijing: Beihang University) (in Chinese)
[16] 刘悦林 2009 博士学位论文 (北京: 北京航空航天大学)
Liu Y L 2009 Ph. D. Dissertation (Beijing: Beihang University) (in Chinese)
[17] Liu S L, Wang C Y, Yu T, Liu Z G 2015 Comput. Mater. Sci. 97 102
 Google Scholar
Google Scholar
[18] Yu X X, Wang C Y, Zhang X N, Yan P, Zhang Z 2014 J. Alloys Compd. 582 299
 Google Scholar
Google Scholar
[19] Wang S Y, Wang C Y, Sun J H, Duan W H, Zhao D L 2002 Phys. Rev. B 65 035101
 Google Scholar
Google Scholar
[20] Zhou Y, Mao Z G, Booth-Morrison C, Seidman D N 2008 Appl. Phys. Lett. 93 71905
 Google Scholar
Google Scholar
[21] Yang X Y, Hu W Y 2014 J. Appl. Phys. 115 1679
[22] Liu S L, Wang C Y, Yu T 2015 Comput. Mater. Sci. 110 261
 Google Scholar
Google Scholar
[23] Prikhodko S V, Yang H, Ardell A J 1999 Metall. Mater. Trans. A 30 2403
 Google Scholar
Google Scholar
[24] Li P, Li Q Q, Jin T, Zhou Y Z, Li J G, Sun X F, Zhang Z F 2014 Mater. Sci. Eng., A 603 84
 Google Scholar
Google Scholar
[25] 赵海根, 李树索, 裴延玲, 宫声凯, 徐惠彬 2015 金属学报 51 1279
Zhao H G, Li S S, Pei Y L, Gong S K, Xu H B 2015 Acta Metall. Sin. 51 1279
[26] Pugh S F 1954 Philos. Mag. 45 823
 Google Scholar
Google Scholar
[27] Pettifor D G 1992 Mater. Sci. Technol. 8 345
 Google Scholar
Google Scholar
[28] 黄昆 2014 固体物理学 (第二版) (北京: 北京大学出版社) 第54−55页
Huang K 2014 Solid State Physics (2nd Ed.) (Beijing: Peking University Press) pp54−55 (in Chinese)
[29] 胡赓祥, 蔡珣, 戎咏华 2010 材料科学基础 (第三版) (上海: 上海交通大学出版社) 第169−170页
Hu G X, Cai X, Rong Y H 2010 Fundamentals of Materials Science (3rd Ed.) (Shanghai: Shanghai Jiao Tong University Press) pp169−170 (in Chinese)
-
图 3 Re替代前后掺杂位置近邻的化学键 (a) Re替代前Al原子与近邻Ni所成的化学键; (b) Re替代后与近邻Ni所成的化学键; (c) Re替代前掺杂点位附近Ni—Ni键和Ni—Al键的情况; (d) Re替代后掺杂点位附近Ni—Ni键和Ni—Al键的情况
Figure 3. The nearby bonds around doping position before or after Re substitutes: (a) The bonds formed between Al and neighboring Ni before Re substitutes; (b) the bonds formed between Re and neighboring Ni after Re substitutes; (c) the nearby Ni—Ni bonds and Ni—Al bonds around doping position before Re substitutes; (d) the nearby Ni—Ni bonds and Ni—Al bonds around doping position after Re substitutes.
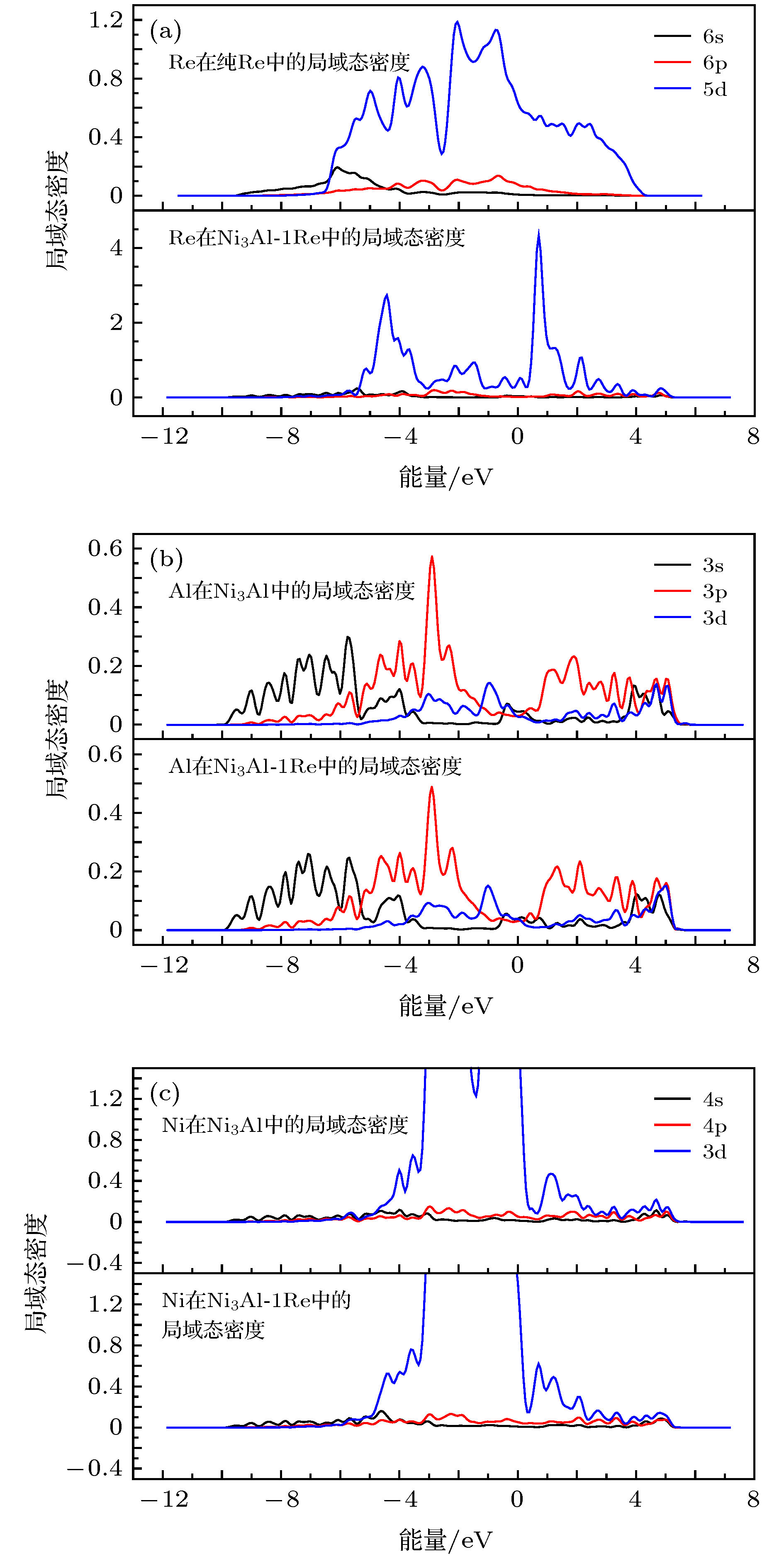
图 6 Re, 与Re近邻的Al和与Re近邻的Ni的局域态密度 (a) Re原子在纯Re和在Ni3Al-1Re中的局域态密度; (b)与Re近邻的Al在Ni3Al和Ni3Al-1Re中的局域态密度; (c)与Re近邻的Ni在Ni3Al和Ni3Al-1Re中的局域态密度
Figure 6. Local density of states(LDOS) of Re, Al(next to Re) and Ni(next to Re): (a) LDOS of Re in pure Re and in Ni3Al-1Re; (b) LDOS of Al(next to Re) in Ni3Al and in Ni3Al-1Re; (c) LDOS of Ni(next to Re) in Ni3Al and in Ni3Al-1Re.
图 7 Ni3Al和Ni3Al-1Re的热力学性质随温度的变化曲线 (a) Ni3Al和Ni3Al-1Re的熵随温度的变化曲线; (b) Ni3Al和Ni3Al-1Re的亥姆霍兹自由能随温度的变化曲线; (c) Ni3Al和Ni3Al-1Re的定体热容随温度的变化曲线; (d) Ni3Al和Ni3Al-1Re的体弹性模量随温度的变化曲线; (e) Ni3Al和Ni3Al-1Re的热膨胀系数随温度的变化曲线
Figure 7. Thermal properties of Ni3Al and Ni3Al-1Re as a function of temperature: (a) Entropy of Ni3Al and Ni3Al-1Re as a function of temperature; (b) Helmholtz free energy of Ni3Al and Ni3Al-1Re as a function of temperature; (c)
${C_V}$ of Ni3Al and Ni3Al-1Re as a function of temperature; (d) B of Ni3Al and Ni3Al-1Re as a function of temperature; (e)$\alpha $ of Ni3Al and Ni3Al-1Re as a function of temperature.表 1 Ni3Al超晶胞能量与2个Re原子间距的关系
Table 1. Relation between the energy of Ni3Al supercell and the distance between 2 Re atoms.
相对位
置情况2个Re原子
相距a2个Re原子
相距$\sqrt 2 a$2个Re原子
相距$\sqrt 3 a$晶胞能量/eV –604.81 –604.85 –604.88 表 2 Ni3Al弹性常数和弹性模量的计算值与其他研究的计算值和实验值的对比以及Ni3Al-1Re, Ni3Al-2Re弹性常数等物理量的计算值
Table 2. Comparison of calculated values of elastic constant and modulus of Ni3Al with other calculated, experimental values and calculated values of some physical parameters of Ni3Al-1Re and Ni3Al-2Re.
c11/GPa c12/GPa c44/GPa B/GPa G/GPa E/GPa c12 – c44 G/B Ni3Al 233.2 152.6 118.1 179.5 87.0 224.7 34.5 0.48 Calc1[11] $227 \pm 5$ $148 \pm 5$ $120 \pm 2$ — — — — — Calc2[22] 242.6 149.3 130.3 180.4 86.4 223.0 — — Expt1[11] 220.1 146.0 123.6 171.0 79.0 205.0 — — Expt2[23] 225.0 149.0 124.0 174.3 77.3 202.1 — — Ni3Al-1Re 236.7 153.7 118.8 181.4 87.9 227.0 34.9 0.48 Ni3Al-2Re 241.8 154.5 120.8 183.6 89.9 231.9 33.7 0.49 -
[1] 方昌德 2004 航空发动机 30 1
 Google Scholar
Google Scholar
Fang C D 2004 Aeroengine 30 1
 Google Scholar
Google Scholar
[2] 秦琴, 毛子荐, 刘昭凡 2017 工具技术 51 3
 Google Scholar
Google Scholar
Qin Q, Mao Z J, Liu Z F 2017 Tool Eng. 51 3
 Google Scholar
Google Scholar
[3] 徐颂波 1997 上海有色金属 18 88
Xu S B 1997 Shanghai Nonferro. Met. 18 88
[4] 陈金栌, 朱定一, 林登宜 2006 材料导报 20 35
 Google Scholar
Google Scholar
Chen J L, Zhu D Y, Lin D Y 2006 Mater. Rev. 20 35
 Google Scholar
Google Scholar
[5] 赵希宏, 韩雅芳, 谭永宁, 殷克勤, 余乾, 肖程波 1997 材料工程 42 13
Zhao X H, Han Y F, Tan Y N, Yin K Q, Yu Q, Xiao C B 1997 J. Mater. Eng. 42 13
[6] Lucaci M, Orban R L, Patroi D, Hodorogea S, Bibicu I, Lungu M 2007 Adv. Mater. Res. 23 67
 Google Scholar
Google Scholar
[7] Kumar A, Chernatynskiy A, Hong M, Phillpot S, Sinnott S 2015 Comput. Mater. Sci. 101 39
 Google Scholar
Google Scholar
[8] Wu Q, Li S S 2012 Comput. Mater. Sci. 53 1
 Google Scholar
Google Scholar
[9] Masahashi N, Takasugi T, Izumi O 1988 Acta Metall. 36 1823
 Google Scholar
Google Scholar
[10] Masahashi N, Takasugi T, Izumi O 1987 J. Mater. Sci. 22 2599
 Google Scholar
Google Scholar
[11] 张永刚, 韩雅芳, 陈国良, 郭建亭, 万晓景, 冯涤 2001 金属间化合物结构材料 (北京: 国防工业出版社) 第577−578页
Zhang Y G, Han Y F, Chen G L, Guo J T, Wan X J, Feng D 2001 Structural Intermetallics (Beijing: National Defense Industry Press) pp577−578 (in Chinese)
[12] 于松, 王崇愚, 于涛 2007 56 3212
 Google Scholar
Google Scholar
Yu S, Wang C Y, Yu T 2007 Acta Phys. Sin. 56 3212
 Google Scholar
Google Scholar
[13] 刘争光 2014 博士学位论文 (北京: 钢铁研究总院)
Liu Z G 2014 Ph. D. Dissertation (Beijing: Central Iron & Steel Research Institute) (in Chinese)
[14] Gong W, Zhao W Y, Miao N H, Zhou J, Sun Z M, Li S S, Gong S K 2018 Comput. Mater. Sci. 144 23
 Google Scholar
Google Scholar
[15] 胡雪兰 2009 博士学位论文 (北京: 北京航空航天大学)
Hu X L 2009 Ph. D. Dissertation (Beijing: Beihang University) (in Chinese)
[16] 刘悦林 2009 博士学位论文 (北京: 北京航空航天大学)
Liu Y L 2009 Ph. D. Dissertation (Beijing: Beihang University) (in Chinese)
[17] Liu S L, Wang C Y, Yu T, Liu Z G 2015 Comput. Mater. Sci. 97 102
 Google Scholar
Google Scholar
[18] Yu X X, Wang C Y, Zhang X N, Yan P, Zhang Z 2014 J. Alloys Compd. 582 299
 Google Scholar
Google Scholar
[19] Wang S Y, Wang C Y, Sun J H, Duan W H, Zhao D L 2002 Phys. Rev. B 65 035101
 Google Scholar
Google Scholar
[20] Zhou Y, Mao Z G, Booth-Morrison C, Seidman D N 2008 Appl. Phys. Lett. 93 71905
 Google Scholar
Google Scholar
[21] Yang X Y, Hu W Y 2014 J. Appl. Phys. 115 1679
[22] Liu S L, Wang C Y, Yu T 2015 Comput. Mater. Sci. 110 261
 Google Scholar
Google Scholar
[23] Prikhodko S V, Yang H, Ardell A J 1999 Metall. Mater. Trans. A 30 2403
 Google Scholar
Google Scholar
[24] Li P, Li Q Q, Jin T, Zhou Y Z, Li J G, Sun X F, Zhang Z F 2014 Mater. Sci. Eng., A 603 84
 Google Scholar
Google Scholar
[25] 赵海根, 李树索, 裴延玲, 宫声凯, 徐惠彬 2015 金属学报 51 1279
Zhao H G, Li S S, Pei Y L, Gong S K, Xu H B 2015 Acta Metall. Sin. 51 1279
[26] Pugh S F 1954 Philos. Mag. 45 823
 Google Scholar
Google Scholar
[27] Pettifor D G 1992 Mater. Sci. Technol. 8 345
 Google Scholar
Google Scholar
[28] 黄昆 2014 固体物理学 (第二版) (北京: 北京大学出版社) 第54−55页
Huang K 2014 Solid State Physics (2nd Ed.) (Beijing: Peking University Press) pp54−55 (in Chinese)
[29] 胡赓祥, 蔡珣, 戎咏华 2010 材料科学基础 (第三版) (上海: 上海交通大学出版社) 第169−170页
Hu G X, Cai X, Rong Y H 2010 Fundamentals of Materials Science (3rd Ed.) (Shanghai: Shanghai Jiao Tong University Press) pp169−170 (in Chinese)
Catalog
Metrics
- Abstract views: 14879
- PDF Downloads: 212
- Cited By: 0
















 DownLoad:
DownLoad: