-
Silica glasses are composed of multi-oxides, apart from the major component silica. Though it is a general practice in the industries to prepare glasses at specific oxide ratios, the composition rule is largely missing, complicated by the implication of multi-oxides. Necessarily, their interpretation is rooted in chemical units, on which the specific compositions depend. However, in silica glasses the inter-atomic bonding network is continuous and there is no weak bonds, like the inter-molecular ones in molecular compounds, to define molecular entities that carry the chemical information of the materials. As the first stage towards understanding the composition rule, the present paper introduces a new method, so-called the cluster-plus-glue-atom model, to unveil the molecule-like structural units of the glass-relevant oxides. It is pointed out that their respective contributions to the construction of glass networks originate from their characteristic cluster structures, and from which molecule-like structural units are proposed that represent the smallest structural units of these oxides. Oxides participating in the glass network formation mainly present triangular or tetrahedral clusters which are required for a three-dimensional glassy network. For example, the basic network former SiO2 is formulated as [Si-O4]Si and contains 32 valence electrons. The intermediate oxides are characterized by the simultaneous formation of both octahedra and tetrahedra. The network modifiers present mainly cubes and octahedra. It is confirmed that the molecule-like structural units of the glass-formation oxides all meet octet rule (that is, the total number of valence electrons contained in each structural unit is an integer multiple of 8), just like common molecules. The proposed concept of molecular structural units sheds a new light on understanding the composition rule of silicate glasses and can eventually solve the long-standing problem of composition design of silica glasses.
[1] 王建成 2015 中国工业评论 0 4
Wang J C 2015 China Industry Review 0 4
[2] 马艳平 2019 博士学位论文 (大连: 大连理工大学)
Ma Y P 2019 Ph. D. Dissertation (Dalian: Dalian University of Technology) (in Chinese)
[3] Ma Y P, Dong D D, Wu A M, Dong C 2018 Inorg. Chem. 57 710
 Google Scholar
Google Scholar
[4] Nuernberg F, Kuehn B, Rollmann K Metrology of Fused Silica Laser-Induced Damage in Optical Materials Boulder, Colorado, United States, September 25 2016, p10014
[5] Salmon P S 2002 Nat. Mater. 1 7
 Google Scholar
Google Scholar
[6] Elliot S R 1984 Physics of Amorphous Materials (London: Longman) pp20–27
[7] Scholze H 1992 Appl. Opt. 31 31
[8] Dong C, Wang Z J, Zhang S, Wang Y M 2020 Int. Mater. Rev. 65 286
 Google Scholar
Google Scholar
[9] 董闯, 董丹丹, 王清 2018 金属学报 54 293
 Google Scholar
Google Scholar
Dong C, Dong D D, Wang Q 2018 Acta Mater 54 293
 Google Scholar
Google Scholar
[10] Dong C, Wang Q, Qiang J B, Wang Y M, Jiang N, Han G, Li Y H, Wu J, Xia J H 2007 Phys. D: Appl. Phys. 40 273
 Google Scholar
Google Scholar
[11] Han G, Qian J, Li F, Yuan L, Quan S G, Wang Q, Wang Y M, Dong C, Peter Häusslerl 2011 Acta. Mater. 59 5917
 Google Scholar
Google Scholar
[12] Du J, We B, Melnik R, Yoshiyuki K 2014 Acta. Mater. 75 113
 Google Scholar
Google Scholar
[13] Chen J X, Wang Q, Wang Y M, Qiang J B, Dong C 2010 Phil. Mag. Lett. 90 683
 Google Scholar
Google Scholar
[14] Dong D D, Zhang S, Wang Z J, Dong C, Häusslerl P 2016 Mater. Des. 96 115
 Google Scholar
Google Scholar
[15] 董丹丹 2017 博士学位论文 (大连: 大连理工大学)
Dong D D 2017 Ph. D. Dissertation (Dalian: Dalian University of Technology) (in Chinese)
[16] 张爽 2019 博士学位论文 (大连: 大连理工大学)
Zhang S 2019 Ph. D. Dissertation (Dalian: Dalian University of technology) (in Chinese)
[17] Lewis G N 1916 J. Am. Chem. Soc. 38 762
 Google Scholar
Google Scholar
[18] Miracle D, Sanders W, Senkov O N 2003 Philos. Mag. 83 2409
 Google Scholar
Google Scholar
[19] Villars P, Calvert L D 1985 Pearson’s Handbook of Crystallographic Data For Intermetallic Phases (Ohio, USA: American Society for Metals) pp2579−2580
[20] 周艳艳, 张希艳 2014 玻璃化学) (北京: 化学工业出版社) 第220−225页
Zhou Y Y, Zhang X Y 2014 Glass Chemistry (Beijing: Chemical Industry Press) pp220−225
[21] 田英良, 孙诗兵 2009 新编玻璃工艺学 (北京: 中国轻工业出版社) 第25—30页
TianY L, Sun S B 2009 New Glass Technology (Version 1) (Beijing: China Light Industry Press) pp25–30
[22] Özgür Ü, Alivov Y I, Liu C, Teke A, Reshchikov M A, Doğan S, Avrutin V, Chong S J, Morkoç H 2005 Appl. Phys. 98 041301
 Google Scholar
Google Scholar
[23] 余小红 2015 博士学位论文 (武汉: 中国地质大学)
Yu X H 2015 Ph. D. Dissertation (Wuhan: China University of Geosciences) (in Chinese)
[24] 戴培赞, 戚凭, 姜富城, 鲁萌萌 2006 青岛大学学报 19 22
Dai P Z, Qi P, Jiang F C, Lu M M 2006 Journal of Qingdao University 19 22
[25] Hazen R M, Finger L W 1986 J. Appl. Phys. 59 372 8
[26] Boettger J C, Wills J M 1996 Phys. Rev. B 54 8965
 Google Scholar
Google Scholar
-
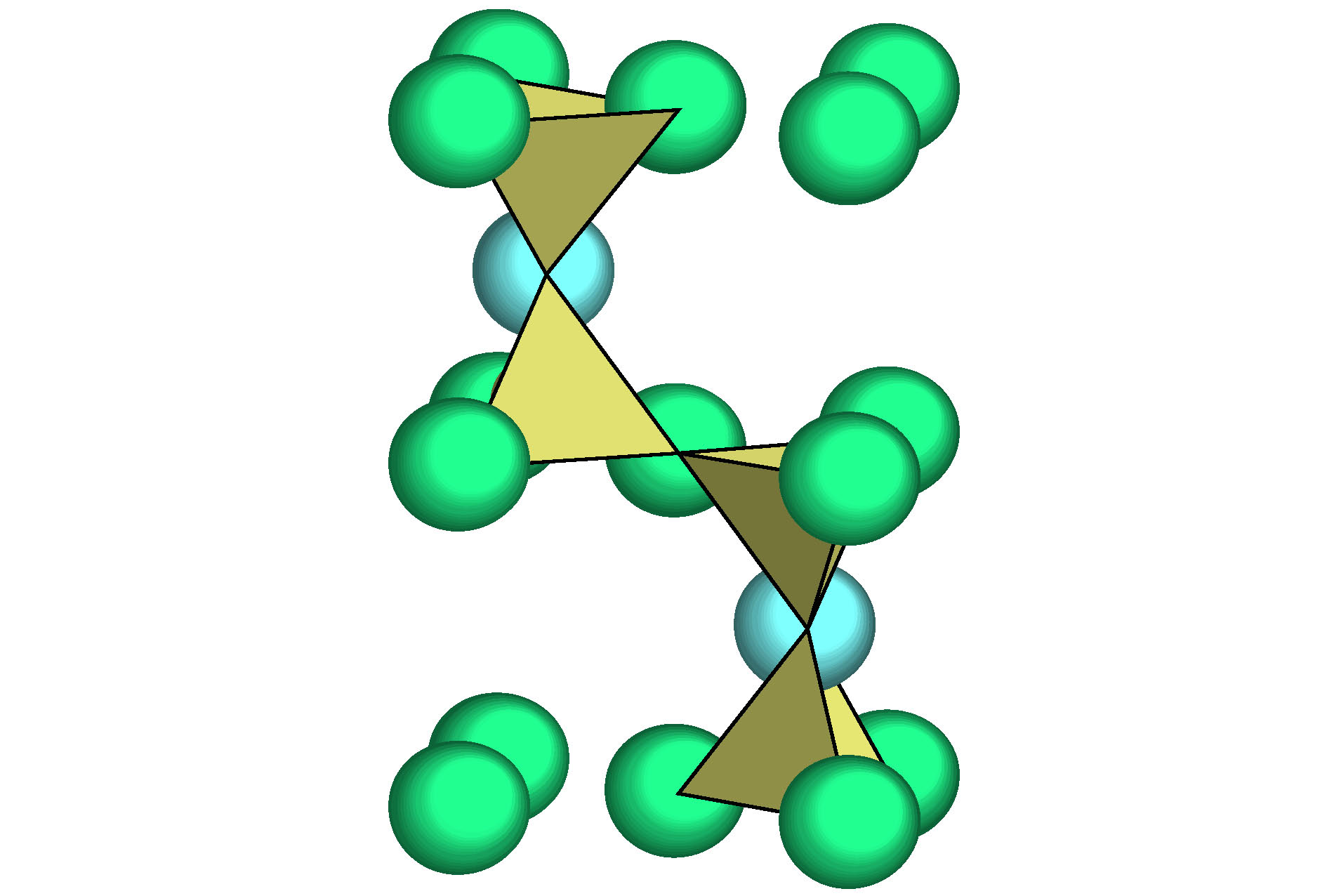
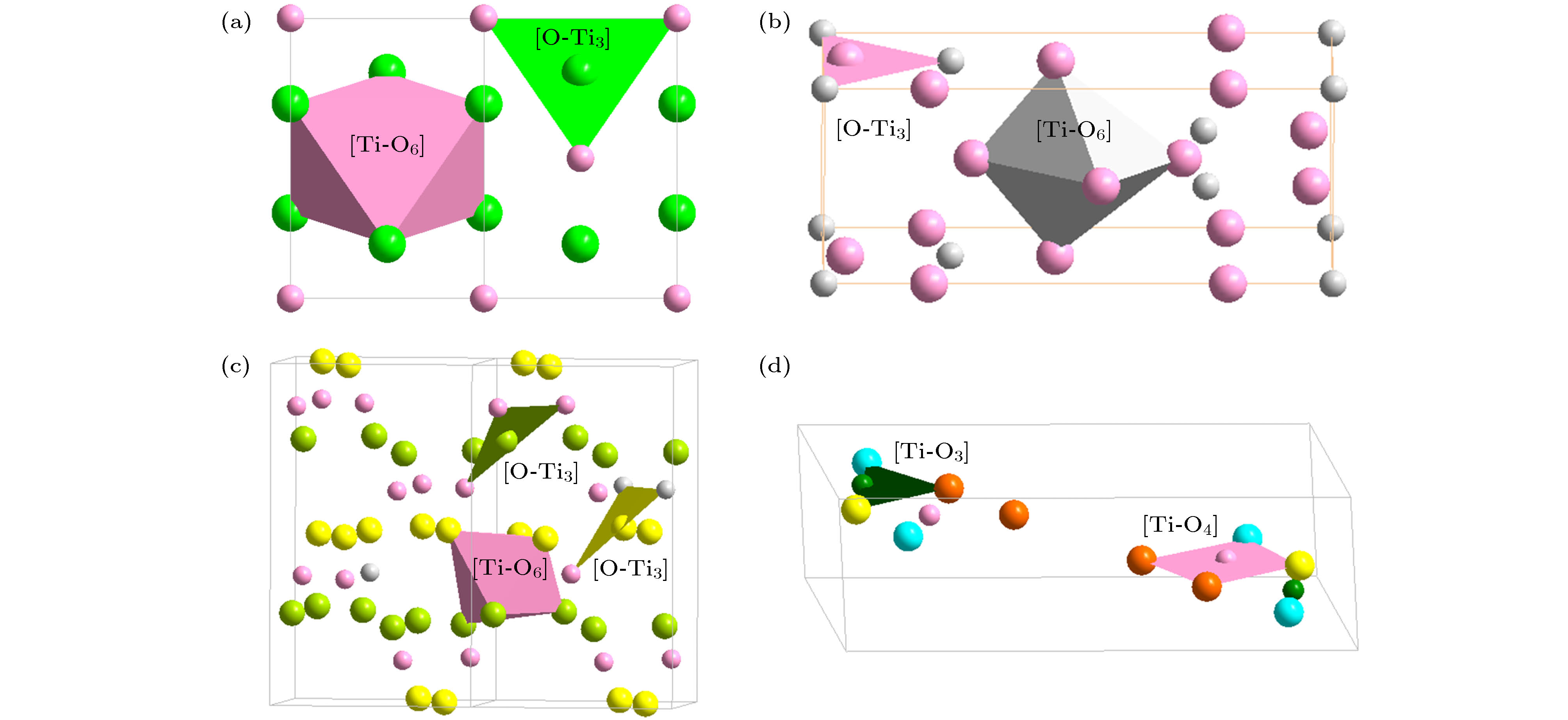
O2Si Structure type O2Si Pearson symbol hP12 Space group P63/mmc No.194 a = 0.5052(9) nm c = 0.827(2) nm γ = 120° O1 2c $ {\bar 6}m2$ x = 1/3 y = 2/3 z = 1/4 occ. = 1 Si 4f 3m. x = 1/3 y = 2/3 z = 0.0620 occ. = 1 O2 6g .2/m x = 1/2 y = 0 z = 0 occ. = 1 表 2 硅酸盐玻璃相关氧化物的类分子结构单元, 分为中心为阳离子和阴离子O两种; 依托于主团簇的类分子结构单元用黑体标出
Table 2. Molecule-like structural units of glass-relevant oxides. Molecule-like structural unit, based on principal clusters, are bolded.
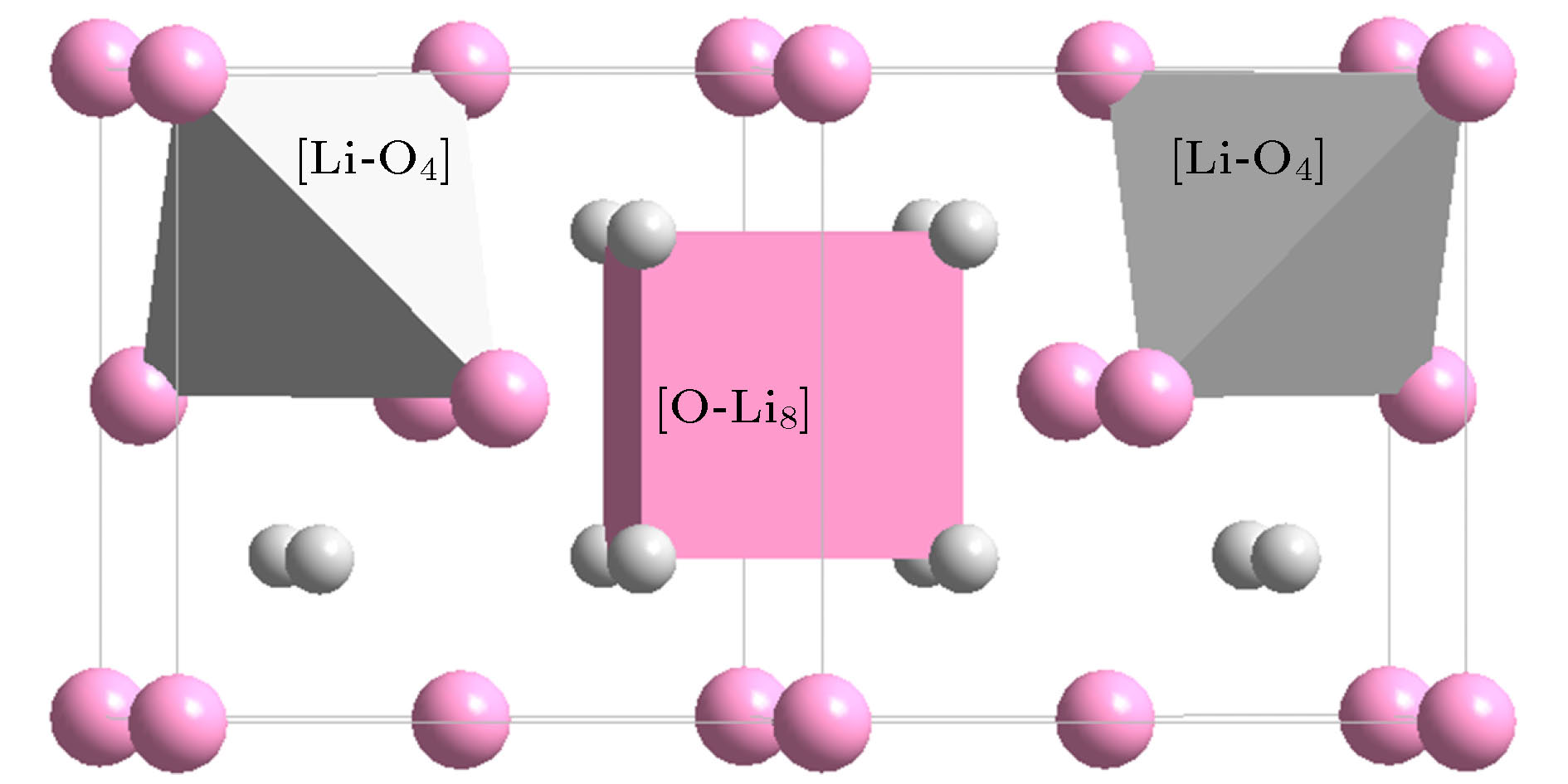
Classification Oxide (space group) Cationic structural unit (e/u) Anion structural unit (e/u) Network formation β-SiO2 (P63/mmc) [Si-O4]Si = 2{SiO2} (32) [O-Si2]O3= 2{SiO2} (32) B2O3 (P31) [B-O3]B = {B2O3} (24) [O-B2]O2 = {B2O3} (24) B2O3 (Cmc21) [B-O4]B3O2 = 2{B2O3} (48) [O-B2]O2 = {B2O3} (24) [O-B3]O5B1 = 2{B2O3} (48) GeO2 (P41212) [Ge-O4]Ge = 2{GeO2} (32) [O-Ge2]O3 = 2{GeO2} (32) P2O5 (Pnma) [P-O4]P1O1 = {P2O5} (40) [O-P2]O4 = {P2O5} (40) [O-P1]P1O4 = {P2O5} (40) Network outside body (Li, Na, K)2O
(anti-fluorite$ Fm{\bar 3}m$)[(Li, Na, K)-O4](Li, Na, K)7 =
4{(Li, Na, K)O2} (32)[O-(Li, Na, K)8]O3 =
4{(Li, Na, K)O2} (32)(Mg, Ca, Ba)O
(Halite, $ Fm{\bar 3}m$)[(Mg, Ca, Ba)-O6](Mg, Ca, Ba)5 =
6{(Mg, Ca, Ba)O} (48)[O-(Mg, Ca, Ba)6]O5 =
4{(Mg, Ca, Ba)O} (48)ZrO2 (rutile, P42/mnm) [Zr-O6]Zr2 = 3{ZrO2} (48) [O-Zr3]O5 = 3{ZrO2} (48) Network intermediate (Be, Zn)O
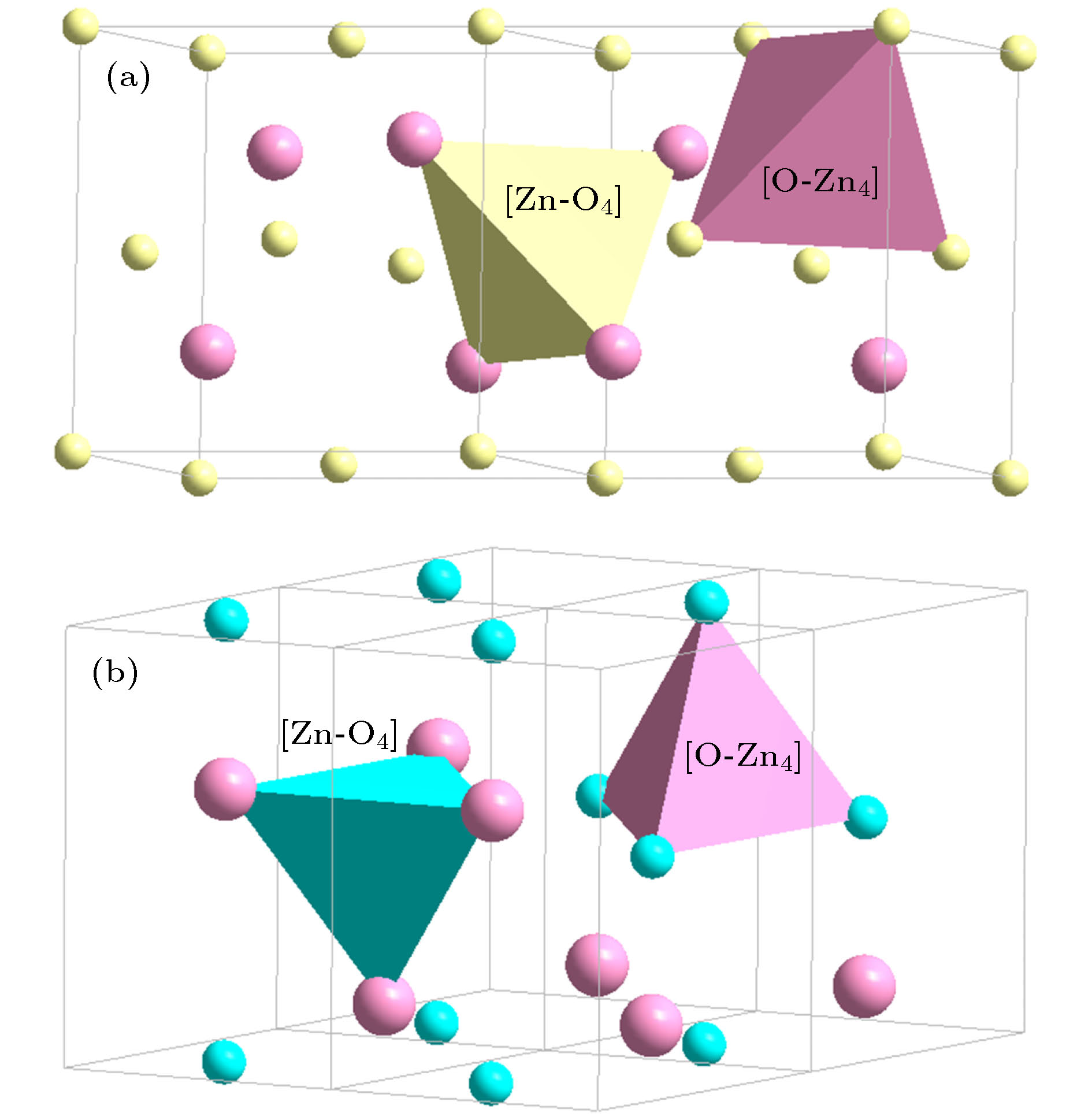
(sphalerite, P43m)[(Be, Zn)-O4](Be, Zn)3 = 4{(Be, Zn)O} (32) [O-(Be, Zn)4]O3 = 4{(Be, Zn)O} (32) (Be, Zn)O
(Wurtzite, P63mc)[Zn-O4]Zn3 = 4{ZnO} (32) [O-Zn4]O3 = 4{ZnO} (32) (Be, Zn)O
(Halite, $ Fm{\bar 3}m$)[Zn-O6]Zn5 = 6{ZnO} (48) [O-Zn6]O5 = 6{ZnO} (48) α-Al2O3 ($ R{\bar 3}c$) [Al-O6]Al3 = 2{Al2O3} (48) [O-Al4]O5 = 2{Al2O3} (48) γ-Al2O3 (Spinel, $ Fd{\bar 3}m$) [Al-O4]O2Al3 = 2{Al2O3} (48) [O-Al4]O5 = 2{Al2O3} (48) [Al-O6]Al3 = 2{Al2O3} (48) Ga2O3 (α-Al2O3, $ R{\bar 3}c$) [Ga-O6]Ga3 = 2{Ga2O3} (48) [O-Ga4]O5 = 2{Ga2O3} (48) Ga2O3 (Spinel, $ Fd{\bar 3}m$) [Ga-O4]O2Ga3 = 2{Ga2O3} (48) [O-Ga4]O5 = 2{Ga2O3} (48) [Ga-O6]Ga3 = 2{Ga2O3} (48) Fe2O3 (α-Al2O3, $ R{\bar 3}c$) [Fe-O6]Fe3 = 2{Fe2O3} (48) [O-Fe4]O5 = 2{Fe2O3} (48) Fe3O4 (Spinel, $ Fd{\bar 3}m$) [Fe-O4]Fe2 = {Fe3O4} (32) [O-Fe4]O7Fe2 = 2{Fe3O4} (64) [Fe-O6]Fe5O2 = 2{Fe3O4} (64) TiO2 (Rutile, P42/mnm) [Ti-O6]Ti2 = 3{TiO2} (48) [O-Ti3]O5 = 3{TiO2} (48) TiO2 (Anatase, I41/amd) [Ti-O6]Ti2 = 3{TiO2} (48) [O-Ti3]O5 = 3{TiO2} (48) TiO2 (Brookite, Pbca) [Ti-O6]Ti2 = 3{TiO2} (48) [O-Ti3]O5 = 3{TiO2} (48) TiO2 (P21/m) [Ti-O4]Ti = 2{TiO2} (32) [O-Ti2]O3 = 2{TiO2} (32) [Ti-O3]TiO = 2{TiO2} (32) [O-Ti]O3Ti = 2{TiO2} (32) -
[1] 王建成 2015 中国工业评论 0 4
Wang J C 2015 China Industry Review 0 4
[2] 马艳平 2019 博士学位论文 (大连: 大连理工大学)
Ma Y P 2019 Ph. D. Dissertation (Dalian: Dalian University of Technology) (in Chinese)
[3] Ma Y P, Dong D D, Wu A M, Dong C 2018 Inorg. Chem. 57 710
 Google Scholar
Google Scholar
[4] Nuernberg F, Kuehn B, Rollmann K Metrology of Fused Silica Laser-Induced Damage in Optical Materials Boulder, Colorado, United States, September 25 2016, p10014
[5] Salmon P S 2002 Nat. Mater. 1 7
 Google Scholar
Google Scholar
[6] Elliot S R 1984 Physics of Amorphous Materials (London: Longman) pp20–27
[7] Scholze H 1992 Appl. Opt. 31 31
[8] Dong C, Wang Z J, Zhang S, Wang Y M 2020 Int. Mater. Rev. 65 286
 Google Scholar
Google Scholar
[9] 董闯, 董丹丹, 王清 2018 金属学报 54 293
 Google Scholar
Google Scholar
Dong C, Dong D D, Wang Q 2018 Acta Mater 54 293
 Google Scholar
Google Scholar
[10] Dong C, Wang Q, Qiang J B, Wang Y M, Jiang N, Han G, Li Y H, Wu J, Xia J H 2007 Phys. D: Appl. Phys. 40 273
 Google Scholar
Google Scholar
[11] Han G, Qian J, Li F, Yuan L, Quan S G, Wang Q, Wang Y M, Dong C, Peter Häusslerl 2011 Acta. Mater. 59 5917
 Google Scholar
Google Scholar
[12] Du J, We B, Melnik R, Yoshiyuki K 2014 Acta. Mater. 75 113
 Google Scholar
Google Scholar
[13] Chen J X, Wang Q, Wang Y M, Qiang J B, Dong C 2010 Phil. Mag. Lett. 90 683
 Google Scholar
Google Scholar
[14] Dong D D, Zhang S, Wang Z J, Dong C, Häusslerl P 2016 Mater. Des. 96 115
 Google Scholar
Google Scholar
[15] 董丹丹 2017 博士学位论文 (大连: 大连理工大学)
Dong D D 2017 Ph. D. Dissertation (Dalian: Dalian University of Technology) (in Chinese)
[16] 张爽 2019 博士学位论文 (大连: 大连理工大学)
Zhang S 2019 Ph. D. Dissertation (Dalian: Dalian University of technology) (in Chinese)
[17] Lewis G N 1916 J. Am. Chem. Soc. 38 762
 Google Scholar
Google Scholar
[18] Miracle D, Sanders W, Senkov O N 2003 Philos. Mag. 83 2409
 Google Scholar
Google Scholar
[19] Villars P, Calvert L D 1985 Pearson’s Handbook of Crystallographic Data For Intermetallic Phases (Ohio, USA: American Society for Metals) pp2579−2580
[20] 周艳艳, 张希艳 2014 玻璃化学) (北京: 化学工业出版社) 第220−225页
Zhou Y Y, Zhang X Y 2014 Glass Chemistry (Beijing: Chemical Industry Press) pp220−225
[21] 田英良, 孙诗兵 2009 新编玻璃工艺学 (北京: 中国轻工业出版社) 第25—30页
TianY L, Sun S B 2009 New Glass Technology (Version 1) (Beijing: China Light Industry Press) pp25–30
[22] Özgür Ü, Alivov Y I, Liu C, Teke A, Reshchikov M A, Doğan S, Avrutin V, Chong S J, Morkoç H 2005 Appl. Phys. 98 041301
 Google Scholar
Google Scholar
[23] 余小红 2015 博士学位论文 (武汉: 中国地质大学)
Yu X H 2015 Ph. D. Dissertation (Wuhan: China University of Geosciences) (in Chinese)
[24] 戴培赞, 戚凭, 姜富城, 鲁萌萌 2006 青岛大学学报 19 22
Dai P Z, Qi P, Jiang F C, Lu M M 2006 Journal of Qingdao University 19 22
[25] Hazen R M, Finger L W 1986 J. Appl. Phys. 59 372 8
[26] Boettger J C, Wills J M 1996 Phys. Rev. B 54 8965
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 24833
- PDF Downloads: 249
- Cited By: 0















 DownLoad:
DownLoad: