-
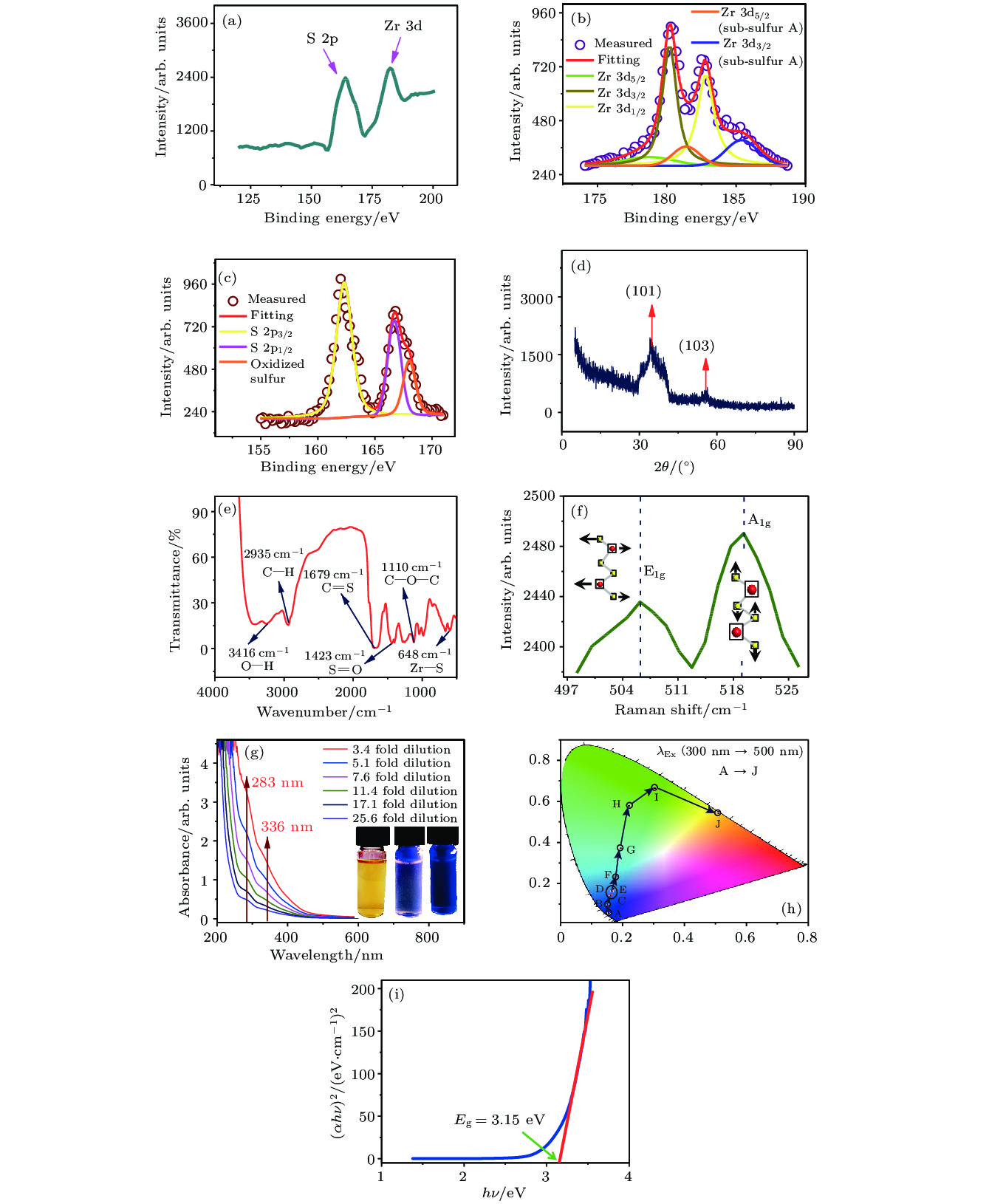
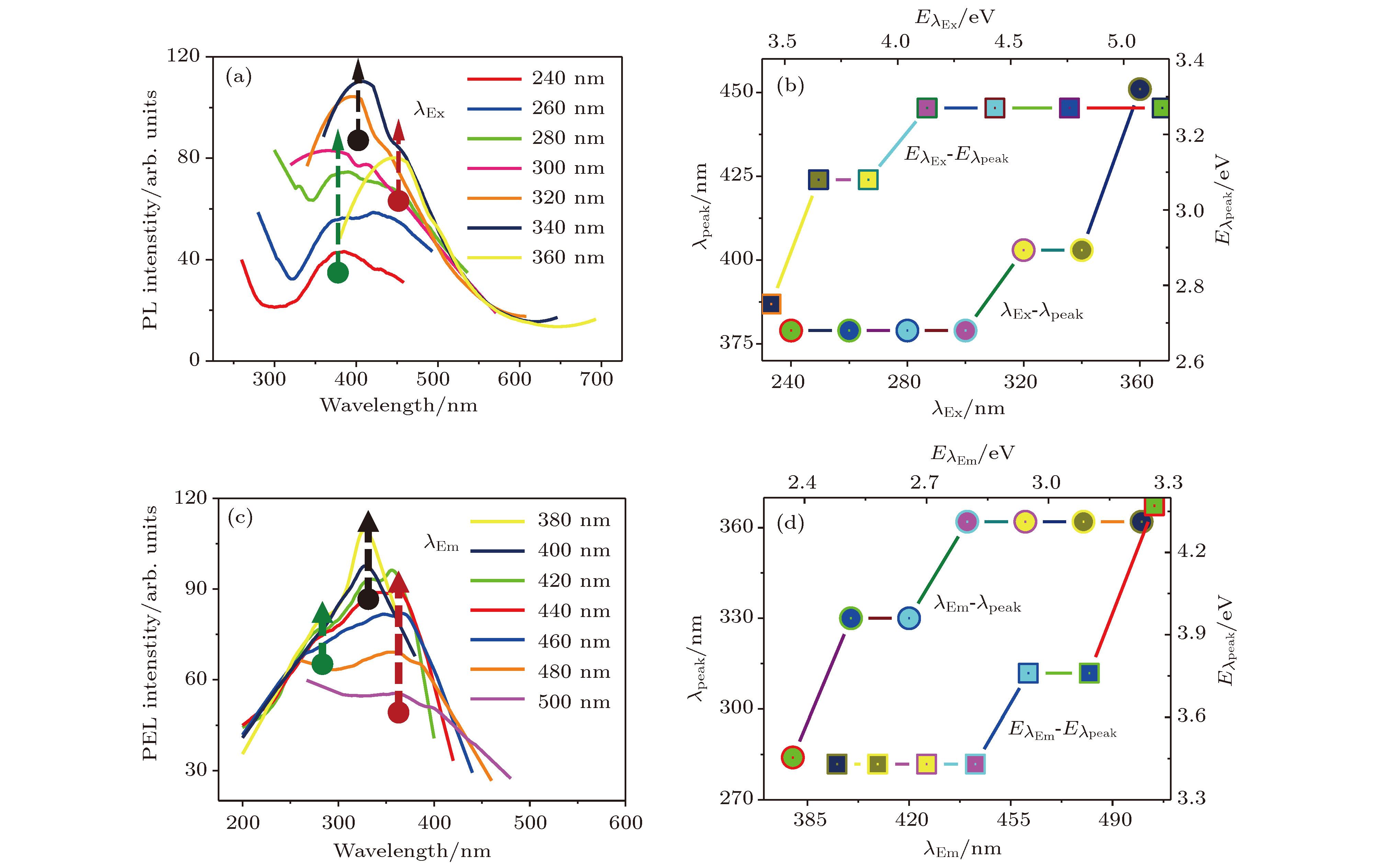
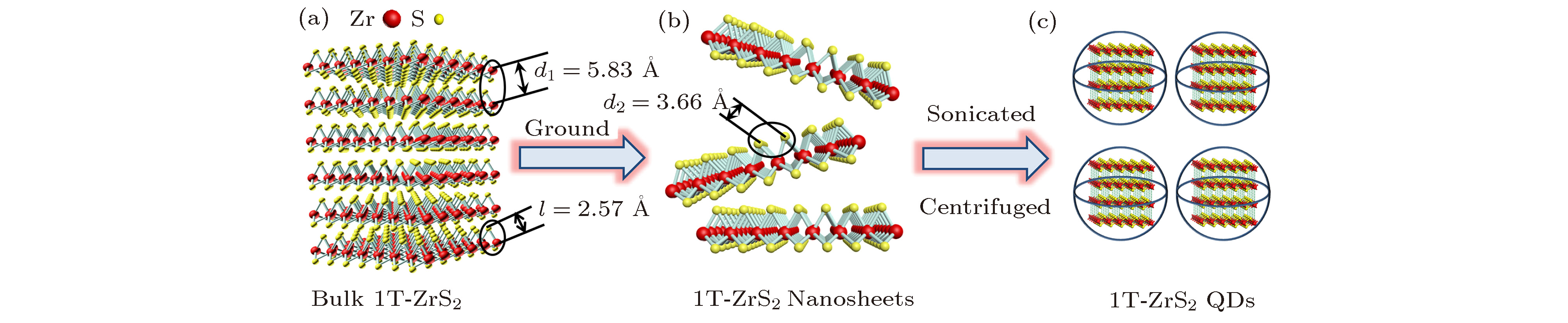
In recent years, transition metal chalcogenides (TMDs) have attracted extensive attention of researchers due to their unique electronic structure and excellent photoelectric properties. In this paper, hexagonal structure 1T-ZrS2 quantum dots (QDs) having a monodisperse grain size of around 3.1 nm is prepared by the ultrasonic exfoliation method. The preparation includes the following steps: ZrS2 powder is ground, followed by ultrasonic exfoliation in 1-methyl-2-pyrrolidone (NMP), and 1T-ZrS2 QDs are collected after centrifugation. The structure, morphology and optical properties of the QDs are studied in detail. The structure, morphology, size distribution, and elemental composition of 1T-ZrS2 QDs are studied by using X-ray diffractometer (XRD), transmission electron microscopy (TEM), atomic force microscopy (AFM), and scanning electron microscopy (SEM). The chemical bonds of 1T-ZrS2 QDs are characterized by X-ray photoelectron microscopy (XPS) and Fourier transform infrared spectrometer (FTIR). The TEM and AFM results show that the 1T-ZrS2 QDs are spherical in shape with uniform size distribution. The sizes of the 1T-ZrS2 QDs follow a Gaussian fitted distribution with an average diameter of WC = 3.1 nm and the FWHM is 1.3 nm. The XRD diffraction pattern of 1T-ZrS2 QDs show wide dispersed diffraction peaks, which is the characteristic of QDs. The diffraction peak at 2θ = 32.3° (d = 0.278 nm) corresponds to the (101) crystal plane, and the weak diffraction peak at 2θ = 56.8°(d = 0.167 nm) belongs to the (103) crystal plane. The grain size is also calculated by using the Debye-Scherrer formula, and the calculated value (2.9 nm) is consistent with the result of TEM (3.1 nm). Two Raman vibration modes (E1g and A1g) are observed. The E1g (507.3 cm–1) and A1g (520.1 cm–1) modes relate to the in-plane and out-of-plane vibration respectively. The Raman intensity of the A1g vibration mode is stronger than that of E1g. The UV-Vis and photoluminescence (PL and PLE) characterizations exhibit that the 1T-ZrS2 QDs have two UV absorption peaks at 283 nm and 336 nm, respectively. The Stokes shift is ~130 nm, the fluorescence quantum yield reaches up to 53.3%. The results show that the 1T-ZrS2 QDs have the excellent fluorescence performance and unique optical properties, which make the 1T-ZrS2 QDs an important material for developing photodetectors, multi-color luminescent devices, and other devices.
[1] 黄静雯, 罗利琼, 金波, 楚士晋, 彭汝芳 2017 66 137801
 Google Scholar
Google Scholar
Huang J W, Luo L Q, Jin B, Chu S J, Peng R F 2017 Acta Phys. Sin. 66 137801
 Google Scholar
Google Scholar
[2] Duan X, Wang C, Pan A, Yu R, Duan X 2015 Chem. Soc. Rev. 44 8859
 Google Scholar
Google Scholar
[3] Zhen Y X, Yang M, Zhang H, Fu G S, Wang J L, Wang S F, Wang R N 2017 Sci. Bull. 62 1530
 Google Scholar
Google Scholar
[4] Zhao X, Wang T, Wei S, Dai X, Yang L 2017 J. Alloys Compd. 695 2048
 Google Scholar
Google Scholar
[5] Fiori G, Bonaccorso F, Iannaccone G, Palacios T, Neumaier D, Seabaugh A 2014 Nat. Nanotechnol. 9 768
 Google Scholar
Google Scholar
[6] Huang Z S, Zhang W X, Zhang W L, Li Y 2014 Nano Res. 7 1731
 Google Scholar
Google Scholar
[7] Li L, Wang H, Fang X 2011 Energy Environ. Sci. 4 2586
 Google Scholar
Google Scholar
[8] Li S, Wang C, Qiu H 2015 Int. J. Hydrogen Energy 40 15503
 Google Scholar
Google Scholar
[9] Wen Y, Zhu Y, Zhang S 2015 RSC Adv. 5 66082
 Google Scholar
Google Scholar
[10] Si Y, Wu H Y, Yang H M 2016 Nanoscale Res. Lett. 11 495
 Google Scholar
Google Scholar
[11] Li L, Fang X, Zhai T 2010 Adv. Mater. 22 4151
 Google Scholar
Google Scholar
[12] 张慧珍, 李金涛, 吕文刚, 杨海方, 唐成春, 顾长志 2017 66 217301
 Google Scholar
Google Scholar
Zhang H Z, Li J T, Lü W G, Yang H F, Tang C C, Gu C Z 2017 Acta Phys. Sin. 66 217301
 Google Scholar
Google Scholar
[13] Zhang X, Cheng H, Zhang H 2017 Adv. Mater. 29 1701704
 Google Scholar
Google Scholar
[14] Tan C, Cao X, Wu X J, He Q, Yang J, Zhang X, Sindoro M 2017 Chem. Rev. 117 6225
 Google Scholar
Google Scholar
[15] Yang W, Zhang B, Ding N 2016 Ultrason. Sonochem. 30 103
 Google Scholar
Google Scholar
[16] Kočišová E, Petr M, Šípová H, Kylián O, Procházka M 2017 Phys. Chem. Chem. Phys. 19 388
 Google Scholar
Google Scholar
[17] Esro M, Vourlias G, Somerton C, Milne W I, Adamopoulos G 2015 Adv. Funct. Mater. 25 134
 Google Scholar
Google Scholar
[18] Wang Z, Zhao B, Li J 2017 Color Res. Appl. 42 10
 Google Scholar
Google Scholar
[19] Gentili P L 2014 Dyes Pigments 110 235
 Google Scholar
Google Scholar
[20] Yu X, Prévot M S, Guijarro N 2015 Nat. Comm. 6 7596
 Google Scholar
Google Scholar
[21] Qian F L, Li X M, Tang L B, Lai S K, Lu C Y, Lau S P 2016 AIP Adv. 6 075116
-
图 2 1T-ZrS2 QDs的制备、结构、形貌及组分表征 (a)薄膜的制备流程图; (b) TEM图及粒径分布图; (c) HR-TEM图(插图为FFT图); (d) HR-TEM图 (插图为Line-Profile); (e) SEM图; (f) EDS能谱图; (g) AFM图
Figure 2. The preparation, structure, morphology, and component characterizations of 1T-ZrS2 QDs: (a) The flow chart of thin film preparation; (b) SEM image and particle size distribution diagram; (c) HR-TEM image (inset: FFT image); (d) HR-TEM image (inset: Line-Profile); (e) SEM image; (f) EDS spectrum; (g) AFM image.
图 3 1T-ZrS2 QDs的物相、XPS能谱及光学性质 (a) XPS图; (b) Zr 3d XPS图; (c) S 2p XPS图; (d) XRD衍射图; (e) FTIR图; (f) Raman图; (g) UV-Vis吸收光谱图 (插图为自然光和紫外灯照射下的量子点溶液图像); (h) 色坐标图; (i) Tauc图
Figure 3. The phase, XPS spectra and optical properties of 1T-ZrS2 QDs: (a) Full-scan XPS spectrum; (b) Zr 3d XPS spectrum; (c) S 2p XPS spectrum; (d) XRD diffraction pattern; (e) FTIR spectrum; (f) Raman spectrum; (g) UV-Vis absorption spectra; (h) color coordinate; (i) Tauc plot.
表 1 1T-ZrS2的晶体结构参数
Table 1. The crystal parameters of 1T-ZrS2.
名称 PDF#00-011-0679数据 晶系 六方晶系 空间群 $P \overline 3m1 $(164) 晶格常数 a = 3.66 Å, b = 3.66 Å, c = 5.83 Å 角度 α = 90°, β = 90°, γ = 120° 波长 1.5406 nm 单个晶胞分子数Z 1 -
[1] 黄静雯, 罗利琼, 金波, 楚士晋, 彭汝芳 2017 66 137801
 Google Scholar
Google Scholar
Huang J W, Luo L Q, Jin B, Chu S J, Peng R F 2017 Acta Phys. Sin. 66 137801
 Google Scholar
Google Scholar
[2] Duan X, Wang C, Pan A, Yu R, Duan X 2015 Chem. Soc. Rev. 44 8859
 Google Scholar
Google Scholar
[3] Zhen Y X, Yang M, Zhang H, Fu G S, Wang J L, Wang S F, Wang R N 2017 Sci. Bull. 62 1530
 Google Scholar
Google Scholar
[4] Zhao X, Wang T, Wei S, Dai X, Yang L 2017 J. Alloys Compd. 695 2048
 Google Scholar
Google Scholar
[5] Fiori G, Bonaccorso F, Iannaccone G, Palacios T, Neumaier D, Seabaugh A 2014 Nat. Nanotechnol. 9 768
 Google Scholar
Google Scholar
[6] Huang Z S, Zhang W X, Zhang W L, Li Y 2014 Nano Res. 7 1731
 Google Scholar
Google Scholar
[7] Li L, Wang H, Fang X 2011 Energy Environ. Sci. 4 2586
 Google Scholar
Google Scholar
[8] Li S, Wang C, Qiu H 2015 Int. J. Hydrogen Energy 40 15503
 Google Scholar
Google Scholar
[9] Wen Y, Zhu Y, Zhang S 2015 RSC Adv. 5 66082
 Google Scholar
Google Scholar
[10] Si Y, Wu H Y, Yang H M 2016 Nanoscale Res. Lett. 11 495
 Google Scholar
Google Scholar
[11] Li L, Fang X, Zhai T 2010 Adv. Mater. 22 4151
 Google Scholar
Google Scholar
[12] 张慧珍, 李金涛, 吕文刚, 杨海方, 唐成春, 顾长志 2017 66 217301
 Google Scholar
Google Scholar
Zhang H Z, Li J T, Lü W G, Yang H F, Tang C C, Gu C Z 2017 Acta Phys. Sin. 66 217301
 Google Scholar
Google Scholar
[13] Zhang X, Cheng H, Zhang H 2017 Adv. Mater. 29 1701704
 Google Scholar
Google Scholar
[14] Tan C, Cao X, Wu X J, He Q, Yang J, Zhang X, Sindoro M 2017 Chem. Rev. 117 6225
 Google Scholar
Google Scholar
[15] Yang W, Zhang B, Ding N 2016 Ultrason. Sonochem. 30 103
 Google Scholar
Google Scholar
[16] Kočišová E, Petr M, Šípová H, Kylián O, Procházka M 2017 Phys. Chem. Chem. Phys. 19 388
 Google Scholar
Google Scholar
[17] Esro M, Vourlias G, Somerton C, Milne W I, Adamopoulos G 2015 Adv. Funct. Mater. 25 134
 Google Scholar
Google Scholar
[18] Wang Z, Zhao B, Li J 2017 Color Res. Appl. 42 10
 Google Scholar
Google Scholar
[19] Gentili P L 2014 Dyes Pigments 110 235
 Google Scholar
Google Scholar
[20] Yu X, Prévot M S, Guijarro N 2015 Nat. Comm. 6 7596
 Google Scholar
Google Scholar
[21] Qian F L, Li X M, Tang L B, Lai S K, Lu C Y, Lau S P 2016 AIP Adv. 6 075116
Catalog
Metrics
- Abstract views: 11888
- PDF Downloads: 273
- Cited By: 0














 DownLoad:
DownLoad: