-
Miscible mixtures of polymer blends have physical properties that are often linked simply to the blend composition, thus offering an inexpensive and convenient method to achieve new high performance polymers. Confinement effect has been found in various polymer blend systems by the ensemble methods, but further understanding the confinement effect still requires large efforts both in experiment and in theory. Single molecule spectroscopy has the potential to provide an in-depth insight to the dynamic information by directly coupling their reorientation to the segmental relaxation of the surrounding polymer matrix. We investigate the confinement effects in polystyrene and oligostyrene blend films by using single-molecule defocused wide-field fluorescence microscopy. According to the observation for dynamic behaviors of probe molecules in the blend films of 75 wt.% and 25 wt.% polystyrene, we find that there are two types of single molecules in the blend films: rotational molecules and immobile molecules. The experimental temperature of 296 K is between the glass transition temperature (Tg) values of two pure components and also is far from the two Tg values. At the temperature, oligostyrene component is trapped by the frozen polystyrene component, but they still move locally. Therefore, the rotational and immobile molecules should couple to the oligostyrene component and polystyrene component, respectively. The distribution of rotational single molecules reveals that the confined regions randomly distribute across miscible polymer blends. The length scale of confined region is estimated to be close to that of the probe molecule by taking into account the rotational dynamics of single molecules. The local relaxation of blend film is also investigated by the rotational correlation time which can be estimated by fitting the autocorrelation curve of 〈cos(Φ)〉 with a Kohlrausch-Williams-Watts stretched exponential function. The histograms of the rotational correlation times in the blend films of 75 wt.% and 25 wt.% polystyrene are obtained respectively, which reveal the characteristic of local dynamic distribution in the confined nano-regions. We find that the dynamic behavior in the blend film of 75 wt.% polystyrene is faster than that of 25 wt.% polystyrene, indicating there is a confinement effect in the blend due to the increased constraints imposed by the polystyrene component at a higher concentration of polystyrene. All results observed in the experiment can be explained qualitatively by the self-concentration model. Our work indicates that the single molecule defocused wide-field fluorescence microscopy is a powerful tool to study the complex dynamic features in the polymer blends.
-
Keywords:
- miscible polymer blends /
- confined nano-regions /
- single molecule spectroscopy /
- defocused imaging
[1] Alegria A, Colmenero J 2016 Soft Matter 12 7709
 Google Scholar
Google Scholar
[2] Maranas J K 2007 Curr. Opin. Colloid Interface Sci. 12 29
 Google Scholar
Google Scholar
[3] Colmenero J, Arbe A 2007 Soft Matter 3 1474
 Google Scholar
Google Scholar
[4] Heriot S Y, Jones R A L 2005 Nat. Mater. 4 782
 Google Scholar
Google Scholar
[5] Ebbens S, Hodgkinson R, Parnell A J, Dunbar A, Martin S J, Topham P D, Clarke N, Howse J R 2011 ACS Nano 5 5124
 Google Scholar
Google Scholar
[6] Gambino T, Alegria A, Arbe A, Colmenero J, Malicki N, Dronet S, Schnell B, Lohstroh W, Nemkovski K 2018 Macromolecules 51 6692
 Google Scholar
Google Scholar
[7] Evans C M, Narayanan S, Jiang Z, Torkelson J M 2012 Phys. Rev. Lett. 51 038302
 Google Scholar
Google Scholar
[8] Gooneie A, Schuschnigg S, Holzer C 2017 Polymers 9 16
 Google Scholar
Google Scholar
[9] 李冬梅, 袁晓娟, 周加强 2013 62 167202
 Google Scholar
Google Scholar
Li D M, Yuan X J, Zhou J Q 2013 Acta Phys. Sin. 62 167202
 Google Scholar
Google Scholar
[10] 袁晓娟, 袁慧敏, 张成强, 王文静, 于元勋, 刘德胜 2015 64 067201
Yuan X J, Yuan H M, Zhang C Q, Wang W J, Yu Y X, Liu D S 2015 Acta Phys. Sin. 64 067201
[11] Evans C M, Torkelson J M 2012 Polymer 53 6118
 Google Scholar
Google Scholar
[12] Dudowicz J, Douglas J F, Freed K F 2014 J. Chem. Phys. 140 244905
 Google Scholar
Google Scholar
[13] Zhao J S, Ediger M D, Sun Y, Yu L 2009 Macromolecules 42 6777
 Google Scholar
Google Scholar
[14] Yang H X, Green P F 2013 Macromolecules 46 9390
 Google Scholar
Google Scholar
[15] Sharma R P, Green P F 2017 Macromolecules 50 6617
 Google Scholar
Google Scholar
[16] Harmandaris V A, Kremer K, Floudas G 2013 Phys. Rev. Lett. 110 165701
 Google Scholar
Google Scholar
[17] Nassar S F, Domenek S, Guinault A, Stoclet G, Delpouve N, Sollogoub C 2018 Macromolecules 51 128
 Google Scholar
Google Scholar
[18] Harmandaris V, Doxastakis M 2013 J. Chem. Phys. 139 034904
 Google Scholar
Google Scholar
[19] Liu W J, Bedrov D, Kumar S K, Veytsman B, Colby R H 2009 Phys. Rev. Lett. 103 037801
 Google Scholar
Google Scholar
[20] Lodge T P, McLeish T C B 2000 Macromolecules 33 5278
 Google Scholar
Google Scholar
[21] Adrjanowicz K, Kaminski K, Tarnacka M, Szklarz G, Paluch M 2017 J. Phys. Chem. Lett. 8 696
 Google Scholar
Google Scholar
[22] Zhao L, Wang C L, Liu J, Wen B H, Tu Y S, Wang Z W, Fang H P 2014 Phys. Rev. Lett. 112 078301
 Google Scholar
Google Scholar
[23] 李灵栋, 叶安娜, 周胜林, 张晓华, 杨朝晖 2019 68 026402
Li L D, Ye A N, Zhou S L, Zhang X H, Yang Z H 2019 Acta Phys. Sin. 68 026402
[24] Orrit M, Ha T, Sandoghdar V 2014 Chem. Soc. Rev. 43 973
 Google Scholar
Google Scholar
[25] Li Y, Chen R, Zhou H, Shi Y, Qin C, Gao Y, Zhang G, Gao Y, Xiao L, Jia S 2018 J. Phys. Chem. Lett. 9 5207
 Google Scholar
Google Scholar
[26] Yuan H, Gaiduk A, Siekierzycka J R, Fujiyoshi S, Matsushita M, Nettels D, Schuler B, Seidel C A M, Orrit M 2015 Phys. Chem. Chem. Phys. 17 6532
 Google Scholar
Google Scholar
[27] 石莹, 李耀, 周海涛, 陈瑞云, 张国峰, 秦成兵, 高岩, 肖连团, 贾锁堂 2019 68 048201
Shi Y, Li Y, Zhou H T, Chen R Y, Zhang G F, Qin C B, Gao Y, Xiao L T, Jia S T 2019 Acta Phys. Sin. 68 048201
[28] 秦亚强, 陈瑞云, 石莹, 周海涛, 张国峰, 秦成兵, 高岩, 肖连团, 贾锁堂 2017 66 248201
 Google Scholar
Google Scholar
Qin Y Q, Chen R Y, Shi Y, Zhou H T, Zhang G F, Qin C B, Gao Y, Xiao L T, Jia S T 2017 Acta Phys. Sin. 66 248201
 Google Scholar
Google Scholar
[29] Zhang G F, Xiao L T, Zhang F, Wang X B, Jia S T 2010 Phys. Chem. Chem. Phys. 12 2308
 Google Scholar
Google Scholar
[30] Zheng Z L, Kuang F Y, Zhao J 2010 Macromolecules 43 3165
 Google Scholar
Google Scholar
[31] 张国峰, 张芳, 程峰钰, 孙建虎, 肖连团, 贾锁堂 2009 58 2364
 Google Scholar
Google Scholar
Zhang G F, Zhang F, Cheng F Y, Sun J H, Xiao L T, Jia S T 2009 Acta Phys. Sin. 58 2364
 Google Scholar
Google Scholar
[32] Hutchison J A, Uji-i H, Deres A, Vosch T, Rocha S, Müller S, Bastian A A, Enderlein J, Nourouzi H, Li C, Herrmann A, Müllen K, de Schryver F, Hofkens J 2014 Nat. Nanotechnol. 9 131
 Google Scholar
Google Scholar
[33] Krajnik B, Chen J W, Watson M A, Cockroft S L, Feringa B L, Hofkens J 2017 J. Am. Chem. Soc. 139 7156
 Google Scholar
Google Scholar
[34] Deres A, Floudas G A, Müllen K, van der Auweraer M, de Schryver F, Enderlein J, Uji-i H, Hofkens J 2011 Macromolecules 44 9703
 Google Scholar
Google Scholar
[35] Dedecker P, Muls B, Deres A, Uji-i H, Hotta J, Sliwa M, Soumillion J P, Müllen K, Enderlein J, Hofkens J 2009 Adv. Mater. 21 1079
 Google Scholar
Google Scholar
[36] 李斌, 张国峰, 景明勇, 陈瑞云, 秦成兵, 高岩, 肖连团, 贾锁堂 2016 68 218201
 Google Scholar
Google Scholar
Bin L, Zhang G F, Jing M Y, Chen R Y, Qin C B, Gao Y, Xiao L T, Jia S T 2016 Acta Phys. Sin. 68 218201
 Google Scholar
Google Scholar
-
图 1 含有PDI单分子光学探针的苯乙烯高聚物与苯乙烯寡聚物形成的易混聚合物薄膜样品示意图, 上方插图为苯乙烯高聚物(PS, n ≈ 65)、苯乙烯寡聚物(OS, n ≈ 3)和PDI单分子的分子结构
Figure 1. Schematic view of experimental samples of polystyrene/oligostyrene blend films with PDI single molecules. The above inserts are the chemical structures of polystyrene, oligostyrene and PDI dye molecule, respectively
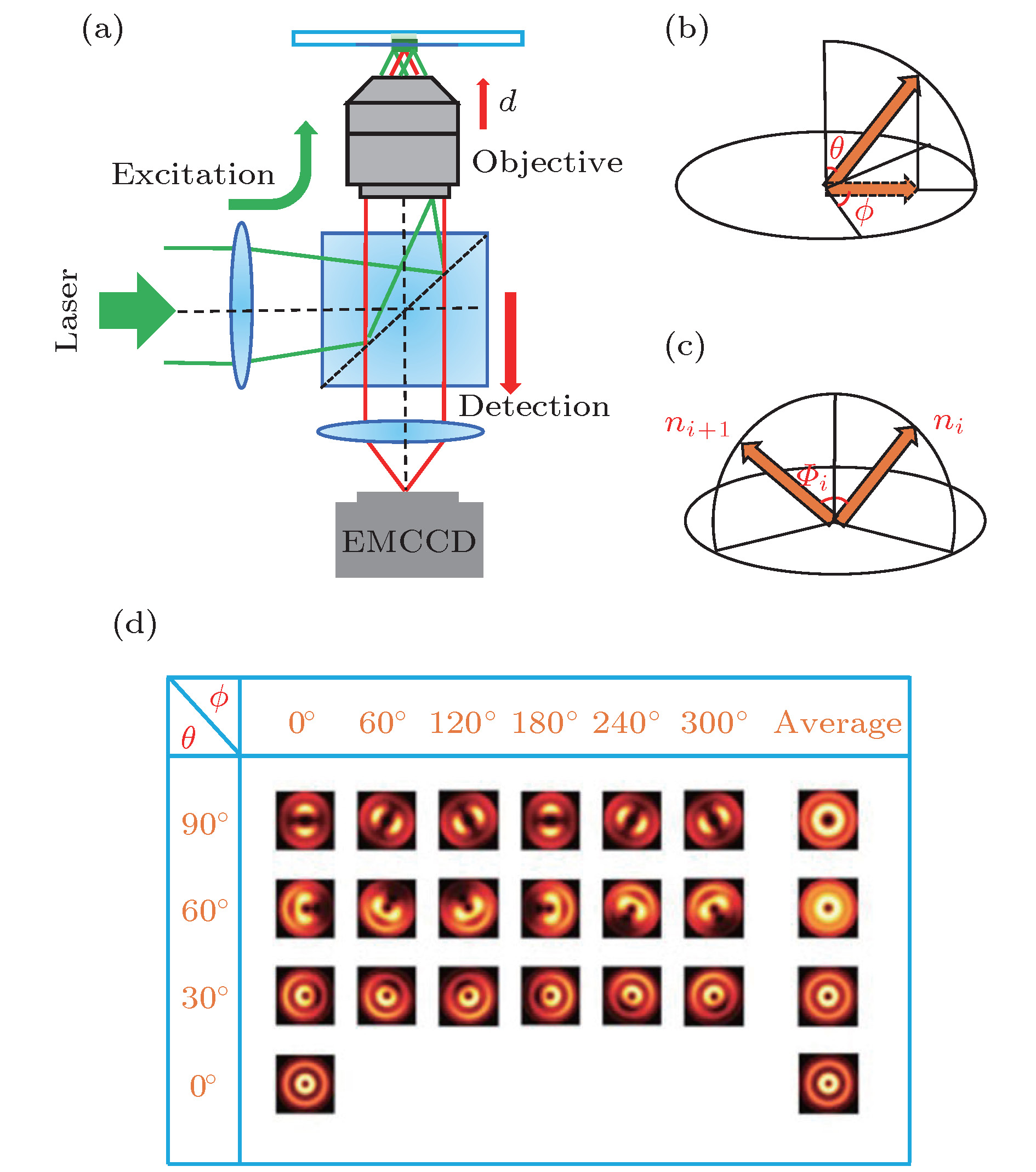
图 2 (a)单分子散焦宽场荧光成像显微镜实验装置示意图; (b)单分子的跃迁偶极矩可以由面内角(ϕ)和面外角(θ)来表示; (c)单分子的跃迁偶极矩的三维角位移(Φ); (d)不同ϕ和θ所对应的单分子散焦成像和每一行内的平均散焦成像
Figure 2. (a) Schematic of single-molecule defocused wide-field fluorescence microscopy; (b) transition dipole moment of single PDI molecule presented by using in-plane (ϕ) and out-of-plane (θ) angles; (c) 3D angular displacement (Φ); (d) defocused patterns of single molecules for different ϕ and θ angles as well as an averaged image for each row.
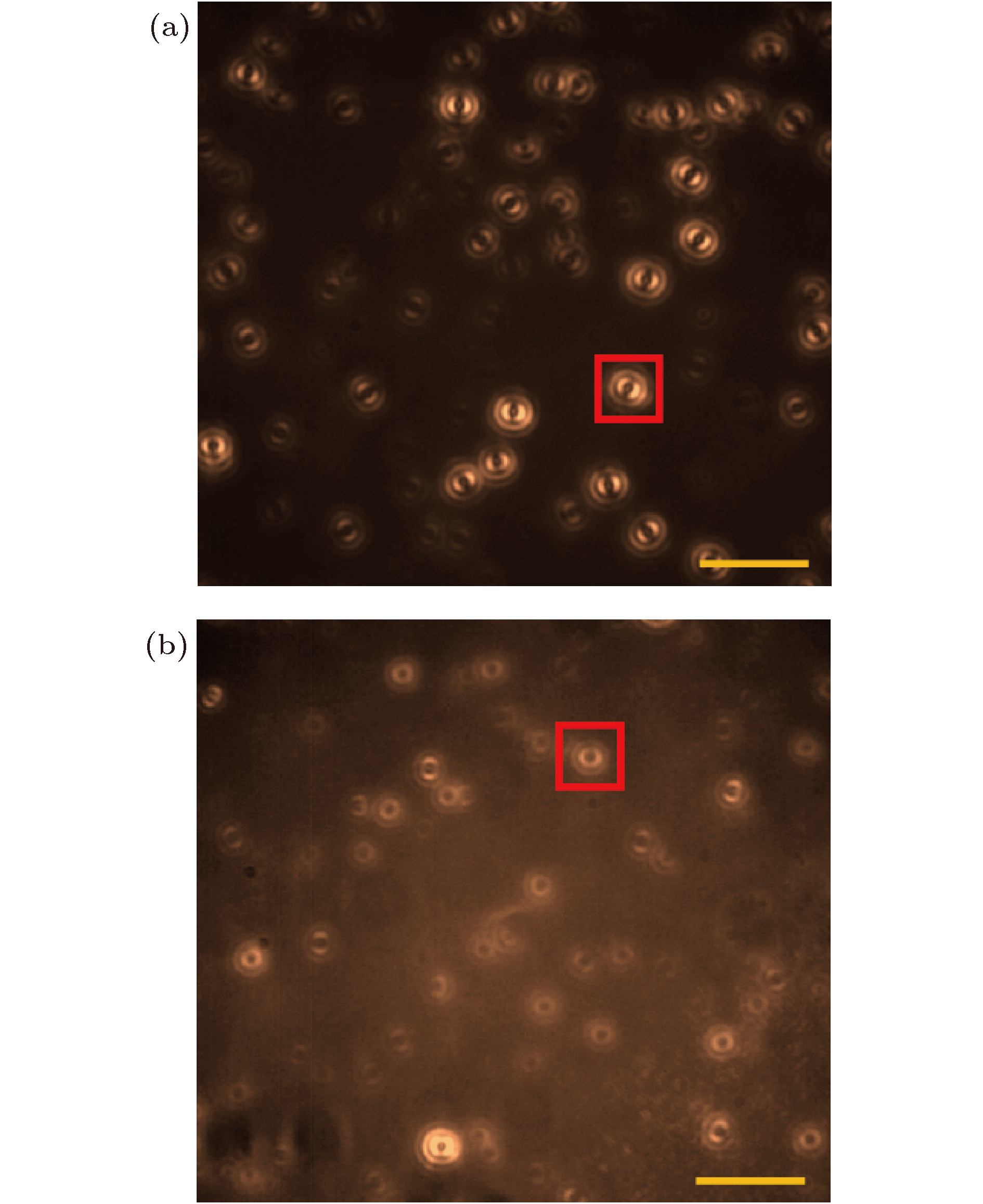
图 3 (a) 苯乙烯高聚物薄膜中的单分子散焦宽场荧光成像; (b)苯乙烯寡聚物薄膜中的单分子散焦宽场荧光成像; 以上的两个散焦成像都由100个单帧的成像叠加形成的, 可以直观地显示单分子在苯乙烯高聚物和苯乙烯寡聚物薄膜中的不同的动力学行为(图中标尺为4 μm)
Figure 3. Defocused images of single molecules in a pure polystyrene film (a) and a pure oligostyrene film (b). Each image is formed by accumulating 100 frames of a defocused imaging sequences (scale bar is 4 μm)
图 4 (a)苯乙烯高聚物与苯乙烯寡聚物的易混聚合物薄膜中的单分子散焦宽场荧光成像, 该散焦成像都由100个单帧的成像叠加而成, 成像图中存在两类动力学形式的单分子, 分别用方框和圆圈所标记(图中标尺为6 μm); (b) 方框所标记的单分子ϕ, θ和Φ角的时间轨迹曲线, 表示为固定不动的单分子; (c) 圆圈所标记的单分子的ϕ, θ和Φ角的时间轨迹曲线, 表示为连续转动的单分子
Figure 4. (a) Defocused images of single molecules in a polystyrene/oligostyrene blend film, and the image was formed by accumulating 100 frames of a defocused imaging sequences, two typical rotational diffusion behaviors of single molecules in the polystyrene/oligostyrene blend films were marked with square and circle, respectively (scale bar is 6 μm); (b) ϕ, θ, and Φ as a function of time indicating the behavior of an immobile molecule; (c) ϕ, θ, and Φ as a function of time indicating the behavior of a rotational molecule
图 5 (a)在混聚物薄膜中的转动单分子的转动自关联函数及其拟合曲线; (b) 在苯乙烯高聚物占比为75 wt.%的易混聚合物薄膜中的单分子的转动关联时间(τc)的统计柱状图; (c) 在苯乙烯高聚物占比为25 wt%的易混聚合物薄膜中的单分子的τc的统计柱状图
Figure 5. (a) Rotational autocorrelation function of a single molecule in the polystyrene/ oligostyrene blend films, and a fitting with KWW stretched exponential function; (b) histogram of correlation times (τc) of single molecules in blend film with 75 wt.%; (c) histogram of τc of single molecules in blend film with 25 wt.%
-
[1] Alegria A, Colmenero J 2016 Soft Matter 12 7709
 Google Scholar
Google Scholar
[2] Maranas J K 2007 Curr. Opin. Colloid Interface Sci. 12 29
 Google Scholar
Google Scholar
[3] Colmenero J, Arbe A 2007 Soft Matter 3 1474
 Google Scholar
Google Scholar
[4] Heriot S Y, Jones R A L 2005 Nat. Mater. 4 782
 Google Scholar
Google Scholar
[5] Ebbens S, Hodgkinson R, Parnell A J, Dunbar A, Martin S J, Topham P D, Clarke N, Howse J R 2011 ACS Nano 5 5124
 Google Scholar
Google Scholar
[6] Gambino T, Alegria A, Arbe A, Colmenero J, Malicki N, Dronet S, Schnell B, Lohstroh W, Nemkovski K 2018 Macromolecules 51 6692
 Google Scholar
Google Scholar
[7] Evans C M, Narayanan S, Jiang Z, Torkelson J M 2012 Phys. Rev. Lett. 51 038302
 Google Scholar
Google Scholar
[8] Gooneie A, Schuschnigg S, Holzer C 2017 Polymers 9 16
 Google Scholar
Google Scholar
[9] 李冬梅, 袁晓娟, 周加强 2013 62 167202
 Google Scholar
Google Scholar
Li D M, Yuan X J, Zhou J Q 2013 Acta Phys. Sin. 62 167202
 Google Scholar
Google Scholar
[10] 袁晓娟, 袁慧敏, 张成强, 王文静, 于元勋, 刘德胜 2015 64 067201
Yuan X J, Yuan H M, Zhang C Q, Wang W J, Yu Y X, Liu D S 2015 Acta Phys. Sin. 64 067201
[11] Evans C M, Torkelson J M 2012 Polymer 53 6118
 Google Scholar
Google Scholar
[12] Dudowicz J, Douglas J F, Freed K F 2014 J. Chem. Phys. 140 244905
 Google Scholar
Google Scholar
[13] Zhao J S, Ediger M D, Sun Y, Yu L 2009 Macromolecules 42 6777
 Google Scholar
Google Scholar
[14] Yang H X, Green P F 2013 Macromolecules 46 9390
 Google Scholar
Google Scholar
[15] Sharma R P, Green P F 2017 Macromolecules 50 6617
 Google Scholar
Google Scholar
[16] Harmandaris V A, Kremer K, Floudas G 2013 Phys. Rev. Lett. 110 165701
 Google Scholar
Google Scholar
[17] Nassar S F, Domenek S, Guinault A, Stoclet G, Delpouve N, Sollogoub C 2018 Macromolecules 51 128
 Google Scholar
Google Scholar
[18] Harmandaris V, Doxastakis M 2013 J. Chem. Phys. 139 034904
 Google Scholar
Google Scholar
[19] Liu W J, Bedrov D, Kumar S K, Veytsman B, Colby R H 2009 Phys. Rev. Lett. 103 037801
 Google Scholar
Google Scholar
[20] Lodge T P, McLeish T C B 2000 Macromolecules 33 5278
 Google Scholar
Google Scholar
[21] Adrjanowicz K, Kaminski K, Tarnacka M, Szklarz G, Paluch M 2017 J. Phys. Chem. Lett. 8 696
 Google Scholar
Google Scholar
[22] Zhao L, Wang C L, Liu J, Wen B H, Tu Y S, Wang Z W, Fang H P 2014 Phys. Rev. Lett. 112 078301
 Google Scholar
Google Scholar
[23] 李灵栋, 叶安娜, 周胜林, 张晓华, 杨朝晖 2019 68 026402
Li L D, Ye A N, Zhou S L, Zhang X H, Yang Z H 2019 Acta Phys. Sin. 68 026402
[24] Orrit M, Ha T, Sandoghdar V 2014 Chem. Soc. Rev. 43 973
 Google Scholar
Google Scholar
[25] Li Y, Chen R, Zhou H, Shi Y, Qin C, Gao Y, Zhang G, Gao Y, Xiao L, Jia S 2018 J. Phys. Chem. Lett. 9 5207
 Google Scholar
Google Scholar
[26] Yuan H, Gaiduk A, Siekierzycka J R, Fujiyoshi S, Matsushita M, Nettels D, Schuler B, Seidel C A M, Orrit M 2015 Phys. Chem. Chem. Phys. 17 6532
 Google Scholar
Google Scholar
[27] 石莹, 李耀, 周海涛, 陈瑞云, 张国峰, 秦成兵, 高岩, 肖连团, 贾锁堂 2019 68 048201
Shi Y, Li Y, Zhou H T, Chen R Y, Zhang G F, Qin C B, Gao Y, Xiao L T, Jia S T 2019 Acta Phys. Sin. 68 048201
[28] 秦亚强, 陈瑞云, 石莹, 周海涛, 张国峰, 秦成兵, 高岩, 肖连团, 贾锁堂 2017 66 248201
 Google Scholar
Google Scholar
Qin Y Q, Chen R Y, Shi Y, Zhou H T, Zhang G F, Qin C B, Gao Y, Xiao L T, Jia S T 2017 Acta Phys. Sin. 66 248201
 Google Scholar
Google Scholar
[29] Zhang G F, Xiao L T, Zhang F, Wang X B, Jia S T 2010 Phys. Chem. Chem. Phys. 12 2308
 Google Scholar
Google Scholar
[30] Zheng Z L, Kuang F Y, Zhao J 2010 Macromolecules 43 3165
 Google Scholar
Google Scholar
[31] 张国峰, 张芳, 程峰钰, 孙建虎, 肖连团, 贾锁堂 2009 58 2364
 Google Scholar
Google Scholar
Zhang G F, Zhang F, Cheng F Y, Sun J H, Xiao L T, Jia S T 2009 Acta Phys. Sin. 58 2364
 Google Scholar
Google Scholar
[32] Hutchison J A, Uji-i H, Deres A, Vosch T, Rocha S, Müller S, Bastian A A, Enderlein J, Nourouzi H, Li C, Herrmann A, Müllen K, de Schryver F, Hofkens J 2014 Nat. Nanotechnol. 9 131
 Google Scholar
Google Scholar
[33] Krajnik B, Chen J W, Watson M A, Cockroft S L, Feringa B L, Hofkens J 2017 J. Am. Chem. Soc. 139 7156
 Google Scholar
Google Scholar
[34] Deres A, Floudas G A, Müllen K, van der Auweraer M, de Schryver F, Enderlein J, Uji-i H, Hofkens J 2011 Macromolecules 44 9703
 Google Scholar
Google Scholar
[35] Dedecker P, Muls B, Deres A, Uji-i H, Hotta J, Sliwa M, Soumillion J P, Müllen K, Enderlein J, Hofkens J 2009 Adv. Mater. 21 1079
 Google Scholar
Google Scholar
[36] 李斌, 张国峰, 景明勇, 陈瑞云, 秦成兵, 高岩, 肖连团, 贾锁堂 2016 68 218201
 Google Scholar
Google Scholar
Bin L, Zhang G F, Jing M Y, Chen R Y, Qin C B, Gao Y, Xiao L T, Jia S T 2016 Acta Phys. Sin. 68 218201
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 9312
- PDF Downloads: 66
- Cited By: 0















 DownLoad:
DownLoad: