-
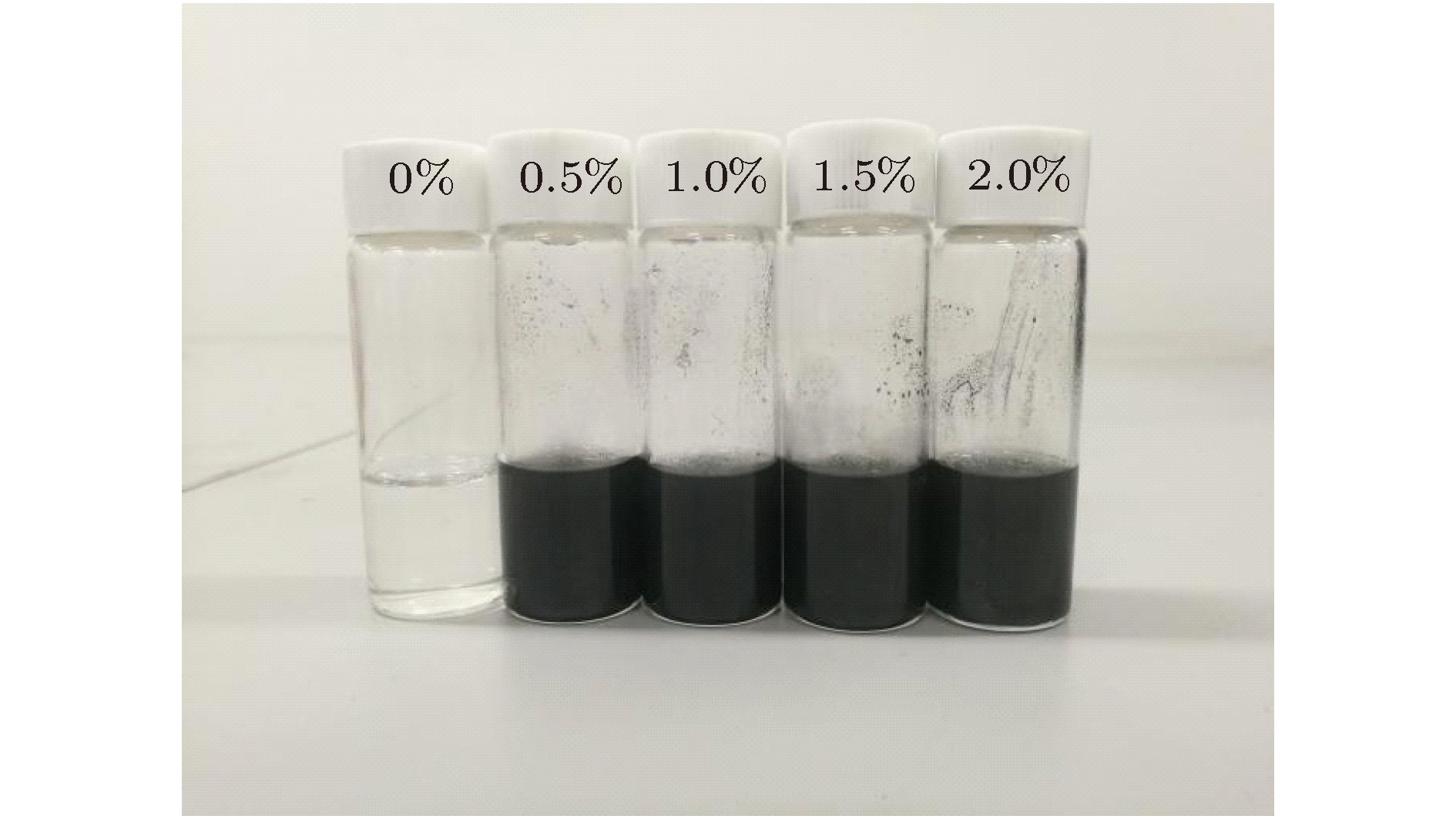
Latent heat storage mainly uses the latent heat of phase change material (PCM) to realize thermal energy storage and utilization, which is the most important thermal energy storage method at present. However, most of PCMs have the disadvantage of low thermal conductivity, which greatly restricts the thermal response rate and system efficiency of the thermal energy storage system. With the development of nanotechnology, it is expected to improve the thermal conductivity of traditional PCMs by adding high thermal conductivity nanoparticles. In this paper, a novel two-dimensional carbon nanomaterial, graphene is selected as an additive for PCM. In this paper, graphene nanoplatelets-octadecane phase change composite materials are prepared with a two-step method and the mass fractions of graphene nanoplatelets are 0%, 0.5%, 1%, 1.5%, and 2%. Their microstructures, morphologies and thermophysical properties are characterized by scanning electron microscopy (SEM), infrared spectroscopy (IR), differential scanning calorimetry (DSC), and thermal conductivity analysis. The effects of the addition quantity of graphene nanoplatelets on the phase transition temperature, enthalpy, specific heat capacity, thermal conductivity and thermal stability of the composite PCM are compared. The experimental results show that the dispersion stability of the graphene nanoplatelets in the composite system is greatly improved by the addition of dispersant, and the system does not produce obvious agglomeration nor sedimentation after multiple phase transformation cycles. The graphene nanoplatelets still maintain good lamellar structure and homogeneous dispersion in the n-octadecane matrix, and no chemical reaction occurs in the composite process. Comparing with the n-octadecane, the melting point of the composite phase change material decreases slightly, and the freezing point increases slightly. With the increase of graphene nanoplatelets, the latent heat value of graphene nanoplatelets-octadecane composite phase change material decreases gradually. For the composite phase change material with 2.0 wt.% graphene nanoplatelets, the melting enthalpy and solidified enthalpy are reduced by 6.01% and 7.35%, respectively. When the mass fractions of graphene nanoplatelets are 0.5%, 1%, 1.5%, and 2%, the thermal conductivity values of phase change composite materials are nearly 32.4%, 77.4%, 83.1%, and 89.4% higher than the thermal conductivity value of pure octadecane, respectively. Comparing with the significant increase in thermal conductivity, the addition of graphene nanoplatelets has little effect on the phase transition temperature and latent heat of PCM, and still exhibits the good heat storage performance. [1] Lin Y X, Alva G, Fang G Y 2018 Renewable Sustainable Energy Rev. 82 2730
 Google Scholar
Google Scholar
[2] Liu L K, Su D, Tang Y J, Fang G Y 2016 Renewable Sustainable Energy Rev. 62 305
 Google Scholar
Google Scholar
[3] Tay N H S, Liu M, Belusko M, Bruno F 2016 Renewable Sustainable Energy Rev. 75 264
[4] Alva G, Lin Y X, Fang G Y 2018 Energy 144 341
 Google Scholar
Google Scholar
[5] Reddy K S, Mudgal V, Mallick T K 2018 J. Energy Storage 15 205
 Google Scholar
Google Scholar
[6] Jaguemont J, Omar N, Den Bossche P V, Mierlo J V 2017 Appl. Therm. Eng. S 1359-4311 31976
[7] Li Y T, Du Y X, Xu T, Wu Huijun, Zhou X Q, Ling Z Y, Zhang Z G 2018 Appl. Therm. Eng. 131 766
 Google Scholar
Google Scholar
[8] Giro-Paloma J, Martinez M, Cabeza L F, Fernandez A L 2016 Renewable Sustainable Energy Rev. 53 1059
 Google Scholar
Google Scholar
[9] Jamekhorshid A, Sadrameli S M, Farid M 2014 Renewable Sustainable Energy Rev. 31 531
 Google Scholar
Google Scholar
[10] Ibrahim N I, Al-Sulaiman F A, Rahman S, Yilbas B S, Sahin A Z 2017 Renewable Sustainable Energy Rev. 74 26
 Google Scholar
Google Scholar
[11] Mohamed N H, Soliman F S, El Maghraby H, Moustfa Y M 2017 Renewable Sustainable Energy Rev. 70 1052
 Google Scholar
Google Scholar
[12] Novoselov K S, Geim A K, Morozov S V 2004 Science 306 666
 Google Scholar
Google Scholar
[13] Ghosh S, Calizo I, Teweldebrhan D, Pokatilov E P 2008 Appl. Phys. Lett. 92 1148
[14] Balandin A A, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F 2008 Nano Lett. 8 902
 Google Scholar
Google Scholar
[15] Chae H K, Siberio-Perez D Y, Kim J 2004 Nature 427 523
 Google Scholar
Google Scholar
[16] Fu Y X, He Z X, Mo D C, Lu S S 2014 Int. J. Therm. Sci. 86 276
 Google Scholar
Google Scholar
[17] Mehrali M, Latibari S T, Mehrali M, Mahlia T M I, Metselaar H S C, Naghavi M S, Sadeghinezhad E, Akhiani A R 2013 Appl. Therm. Eng. 61 633
 Google Scholar
Google Scholar
[18] Amin M, Putra N, Kosasih E A, Prawiro E, Luanto R A, Mahlia T M I 2017 Appl. Therm. Eng. 112 273
 Google Scholar
Google Scholar
[19] Liu X, Rao Z 2017 Thermochim. Acta 647 15
 Google Scholar
Google Scholar
[20] 周艳, 张金辉, 王艳, 路海滨, 李庆领 2013 材料导报 27 8
 Google Scholar
Google Scholar
Zhou Y, Zhang J H, Wang Y, Lu H B, Li Q L 2013 Mater. Rev. 27 8
 Google Scholar
Google Scholar
[21] 吴炳洋, 郑帼, 孙玉, 陈旭 2016 高分子学报 2 242
Wu B Y, Zheng G, Sun Y, Chen X 2016 Acta Polym. Sin. 2 242
[22] Galli G, Sorella S, Spanu L 2009 Physics 103 196401
[23] Holmes N S, Morawska L 2006 Atmos. Environ. 40 5902
 Google Scholar
Google Scholar
-
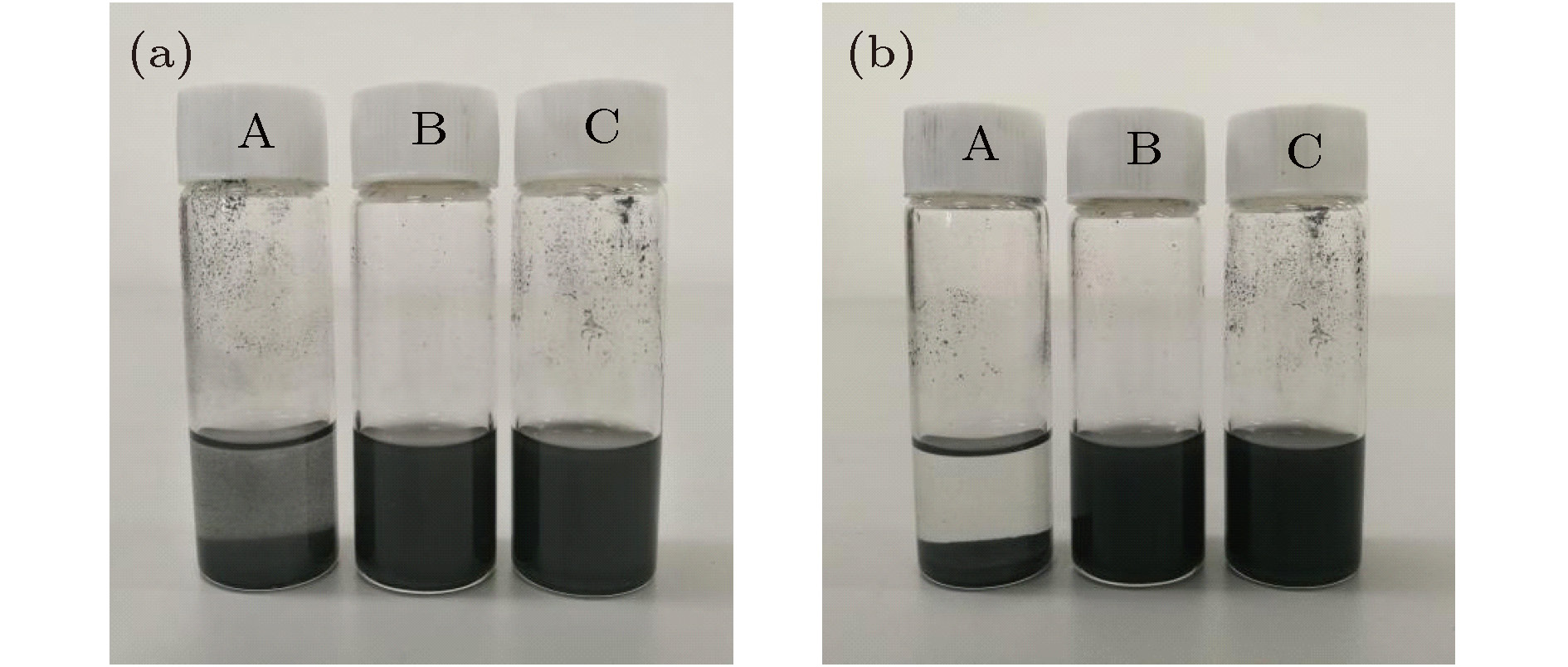
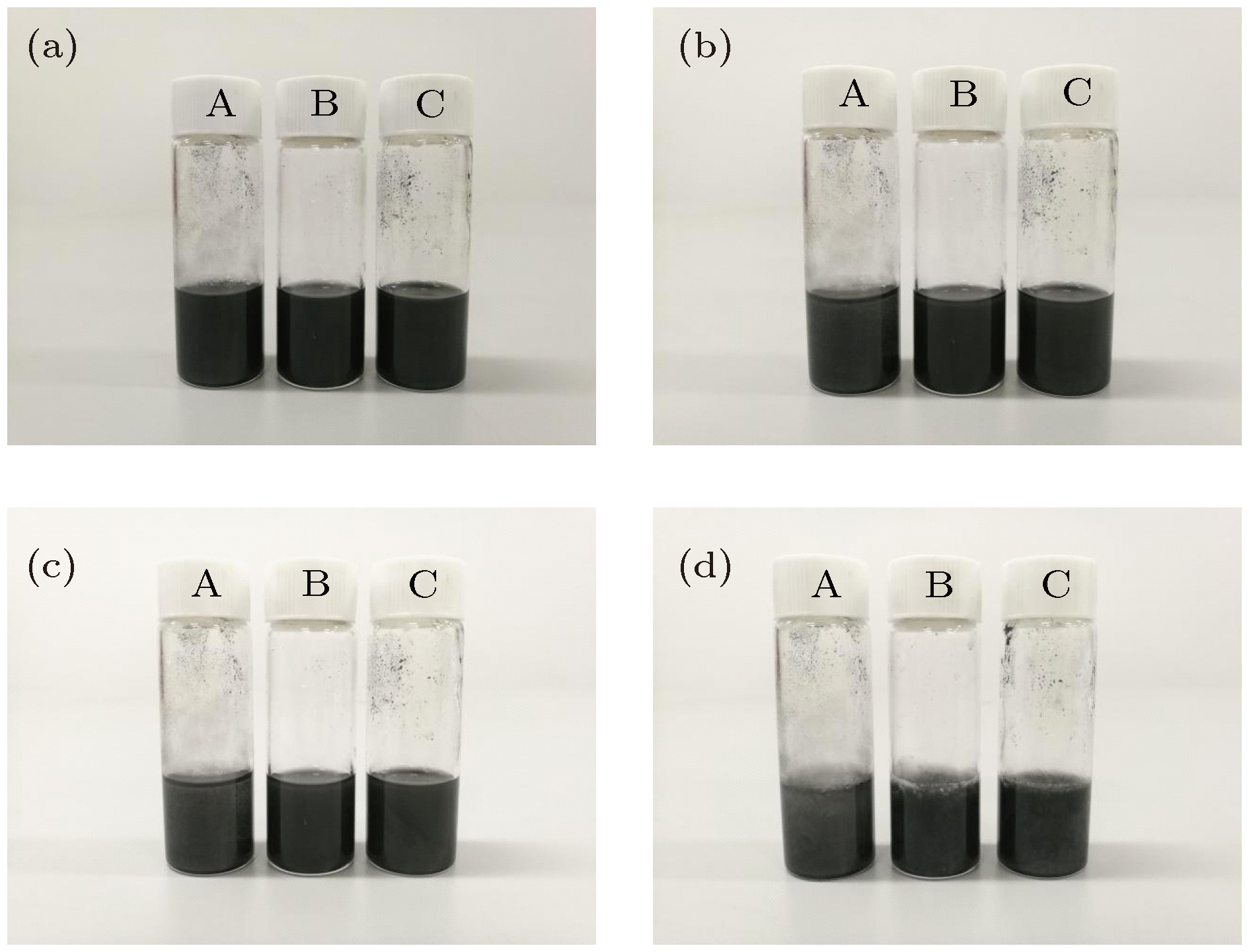
图 1 未添加分散剂与添加不同种类分散剂的复合相变材料(GNP 0.5 wt.%)分散稳定性 (a)初始状态; (b)静置15 min; (c)静置30 min; (d)凝固状态
Figure 1. Dispersion stability of composite phase change material (GNP 0.5 wt.%) without addition of dispersant and with adding different kinds of dispersants: (a) Initial state; (b) let the mixture stand for 15 min; (c) let the mixture stand for 30 min; (c) solidification state.
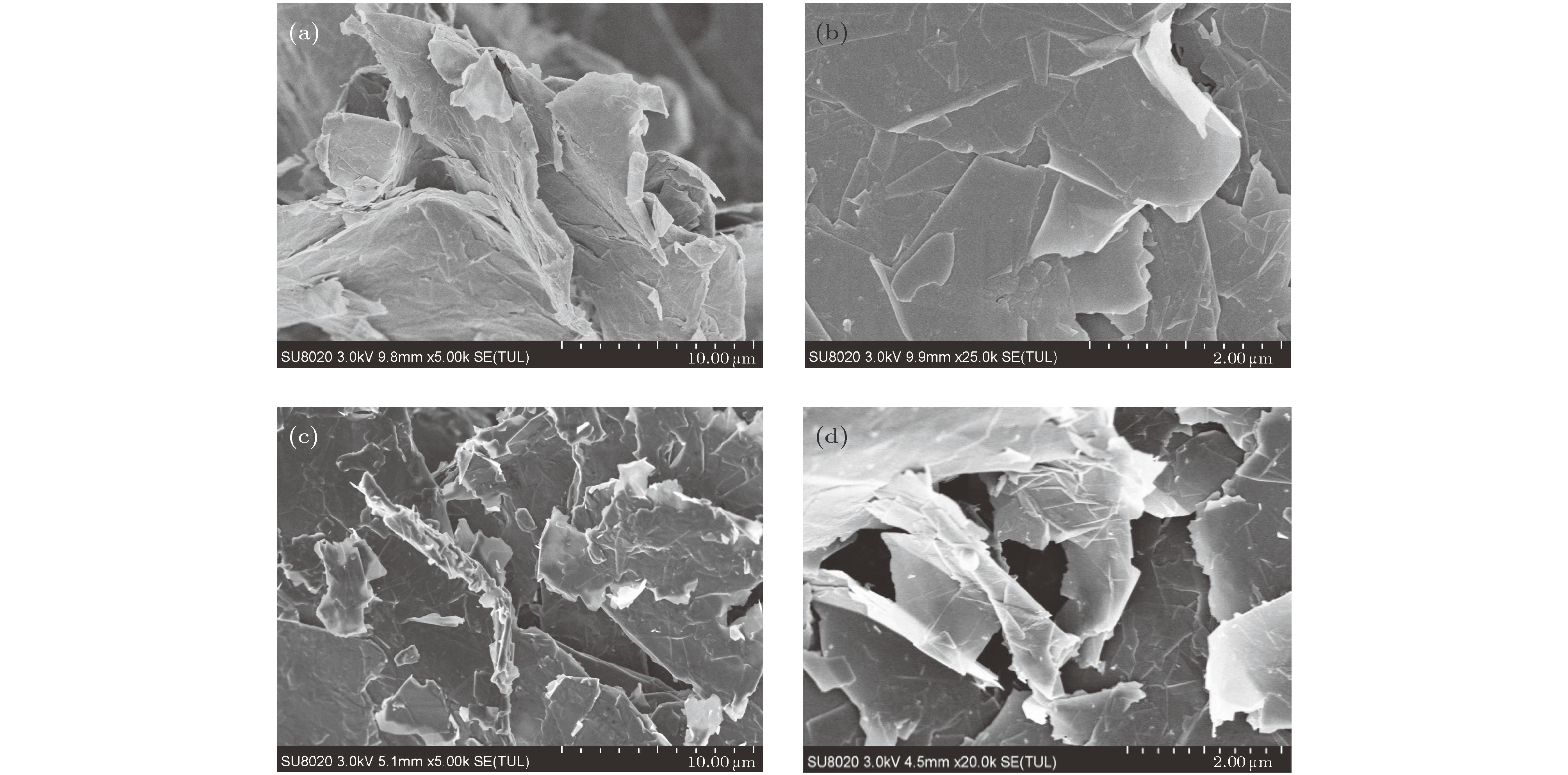
图 4 微观形貌结构 (a)纳米石墨烯片(× 5000); (b)纳米石墨烯片(× 25000); (c)复合相变材料(× 5000); (d)复合相变材料(× 20000)
Figure 4. The microstructure and morphology of (a) graphene nanoplatelets (× 5000); (b) graphene nanoplatelets (× 25000); (c) composite phase change materials (× 5000); (d) composite phase change materials (× 20000).
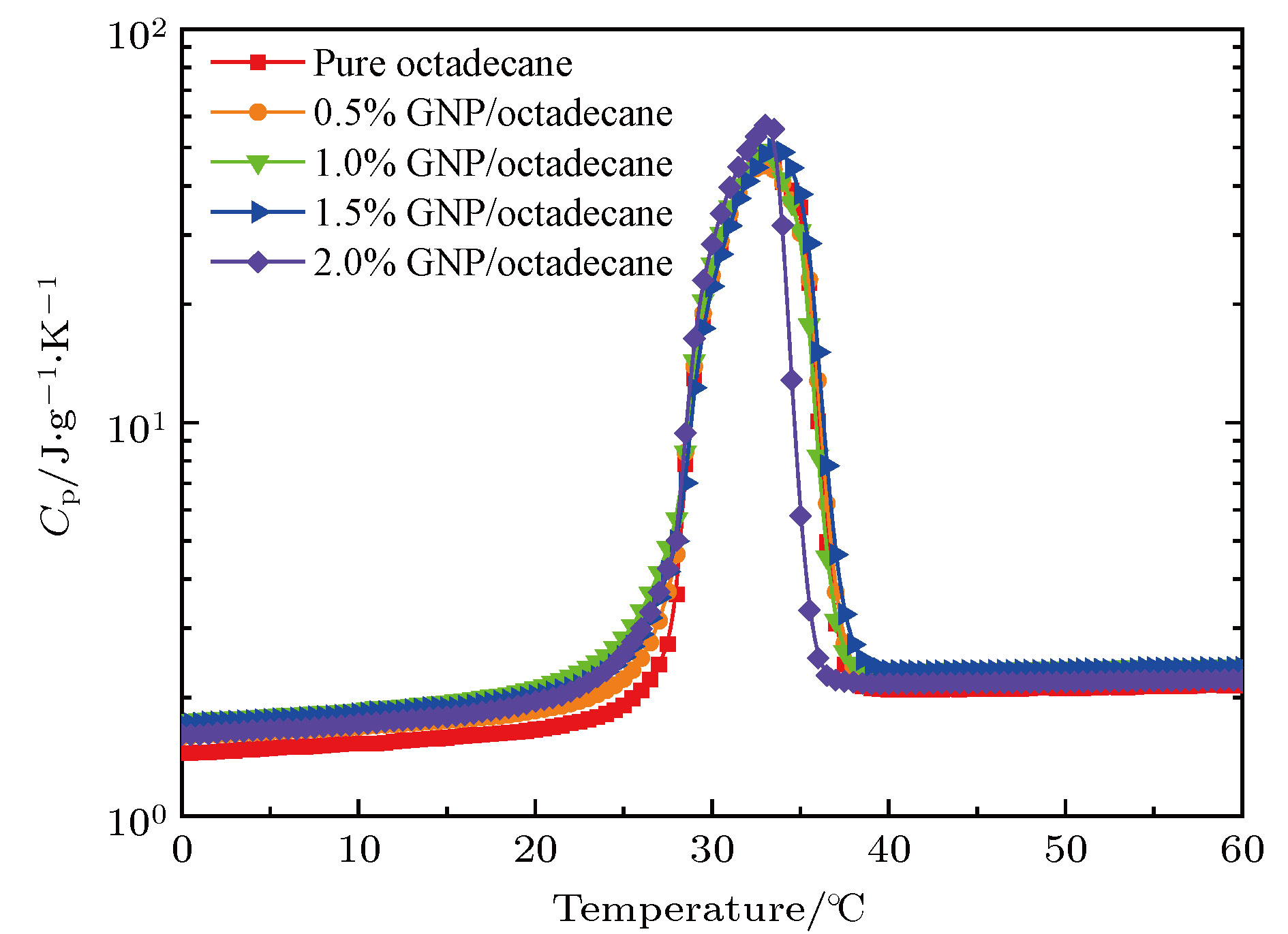
表 1 正十八烷及其复合相变材料熔化过程的相变温度及相变焓
Table 1. Phase transition temperature and enthalpy of n-octadecane and composite phase change materials during melting process.
材料 起始温度 Tms/℃ 峰值 Tmp /℃ 终止温度 Tme/℃ 相变焓 Hm/J·g–1 正十八烷 28.1 33.3 35.9 241.4 0.5%纳米石墨烯片/正十八烷 27.9 33.5 36.5 237.4 1.0%纳米石墨烯片/正十八烷 27.9 32.9 36.0 237.0 1.5%纳米石墨烯片/正十八烷 27.5 33.4 36.2 234.8 2.0%纳米石墨烯片/正十八烷 27.9 33.3 35.7 226.9 表 2 正十八烷及其复合相变材料凝固过程的相变温度及相变焓
Table 2. Phase transition temperature and enthalpy of n-octadecane and composite phase change materials during solidification process.
材料 起始温度 Tss/℃ 峰值 Tsp/℃ 终止温度 Tse/℃ 相变焓 Hs /J·g–1 正十八烷 26.1 21.5 19.8 –240.7 0.5%纳米石墨烯片/正十八烷 26.3 20.9 19.0 –237.8 1.0%纳米石墨烯片/正十八烷 26.5 21.1 19.3 –237.2 1.5%纳米石墨烯片/正十八烷 26.4 21.5 20.0 –233.5 2.0%纳米石墨烯片/正十八烷 26.5 21.1 19.5 –223.0 -
[1] Lin Y X, Alva G, Fang G Y 2018 Renewable Sustainable Energy Rev. 82 2730
 Google Scholar
Google Scholar
[2] Liu L K, Su D, Tang Y J, Fang G Y 2016 Renewable Sustainable Energy Rev. 62 305
 Google Scholar
Google Scholar
[3] Tay N H S, Liu M, Belusko M, Bruno F 2016 Renewable Sustainable Energy Rev. 75 264
[4] Alva G, Lin Y X, Fang G Y 2018 Energy 144 341
 Google Scholar
Google Scholar
[5] Reddy K S, Mudgal V, Mallick T K 2018 J. Energy Storage 15 205
 Google Scholar
Google Scholar
[6] Jaguemont J, Omar N, Den Bossche P V, Mierlo J V 2017 Appl. Therm. Eng. S 1359-4311 31976
[7] Li Y T, Du Y X, Xu T, Wu Huijun, Zhou X Q, Ling Z Y, Zhang Z G 2018 Appl. Therm. Eng. 131 766
 Google Scholar
Google Scholar
[8] Giro-Paloma J, Martinez M, Cabeza L F, Fernandez A L 2016 Renewable Sustainable Energy Rev. 53 1059
 Google Scholar
Google Scholar
[9] Jamekhorshid A, Sadrameli S M, Farid M 2014 Renewable Sustainable Energy Rev. 31 531
 Google Scholar
Google Scholar
[10] Ibrahim N I, Al-Sulaiman F A, Rahman S, Yilbas B S, Sahin A Z 2017 Renewable Sustainable Energy Rev. 74 26
 Google Scholar
Google Scholar
[11] Mohamed N H, Soliman F S, El Maghraby H, Moustfa Y M 2017 Renewable Sustainable Energy Rev. 70 1052
 Google Scholar
Google Scholar
[12] Novoselov K S, Geim A K, Morozov S V 2004 Science 306 666
 Google Scholar
Google Scholar
[13] Ghosh S, Calizo I, Teweldebrhan D, Pokatilov E P 2008 Appl. Phys. Lett. 92 1148
[14] Balandin A A, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F 2008 Nano Lett. 8 902
 Google Scholar
Google Scholar
[15] Chae H K, Siberio-Perez D Y, Kim J 2004 Nature 427 523
 Google Scholar
Google Scholar
[16] Fu Y X, He Z X, Mo D C, Lu S S 2014 Int. J. Therm. Sci. 86 276
 Google Scholar
Google Scholar
[17] Mehrali M, Latibari S T, Mehrali M, Mahlia T M I, Metselaar H S C, Naghavi M S, Sadeghinezhad E, Akhiani A R 2013 Appl. Therm. Eng. 61 633
 Google Scholar
Google Scholar
[18] Amin M, Putra N, Kosasih E A, Prawiro E, Luanto R A, Mahlia T M I 2017 Appl. Therm. Eng. 112 273
 Google Scholar
Google Scholar
[19] Liu X, Rao Z 2017 Thermochim. Acta 647 15
 Google Scholar
Google Scholar
[20] 周艳, 张金辉, 王艳, 路海滨, 李庆领 2013 材料导报 27 8
 Google Scholar
Google Scholar
Zhou Y, Zhang J H, Wang Y, Lu H B, Li Q L 2013 Mater. Rev. 27 8
 Google Scholar
Google Scholar
[21] 吴炳洋, 郑帼, 孙玉, 陈旭 2016 高分子学报 2 242
Wu B Y, Zheng G, Sun Y, Chen X 2016 Acta Polym. Sin. 2 242
[22] Galli G, Sorella S, Spanu L 2009 Physics 103 196401
[23] Holmes N S, Morawska L 2006 Atmos. Environ. 40 5902
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 16411
- PDF Downloads: 257
- Cited By: 0














 DownLoad:
DownLoad: