-
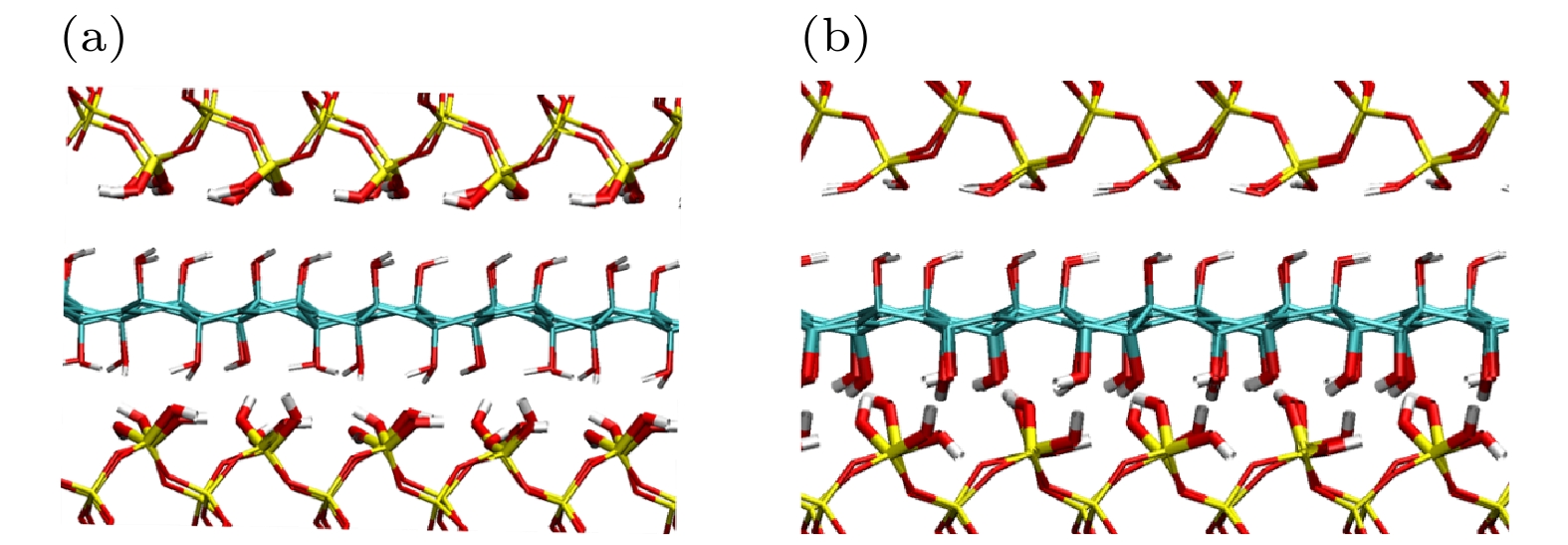
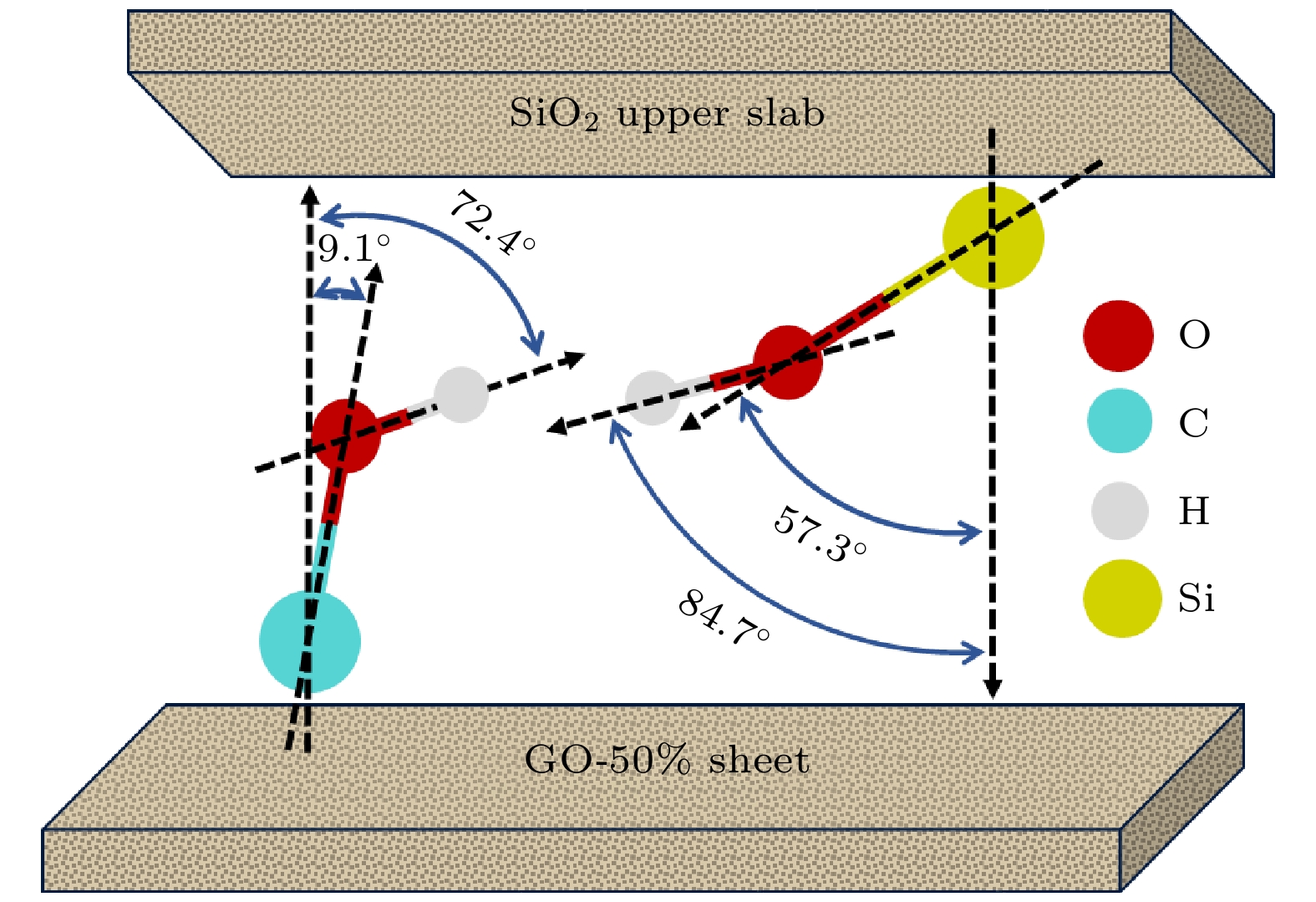
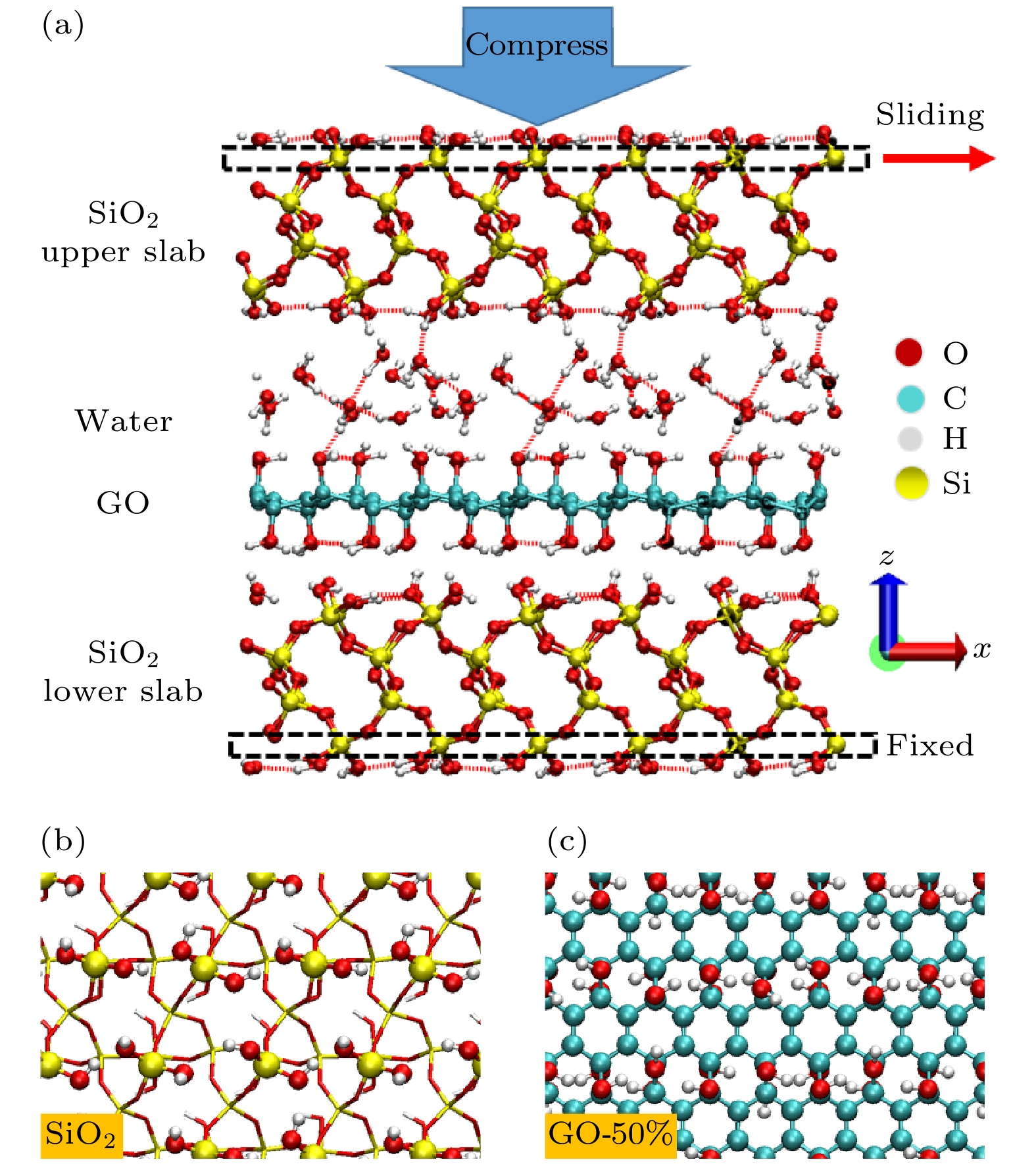
应用从头算分子动力学方法, 模拟了在挤压剪切作用下石墨烯片层作为润滑剂添加于硅基材料界面的摩擦过程, 研究了水分子和石墨烯表面氧化对石墨烯片层运动行为的影响. 干燥环境下, 压强较大时单纯石墨烯片层才会出现滑移, 而氧化石墨烯片层在低压强下就开始滑移. 潮湿环境下, 界面结构影响水分子的整体分布和运动状态, 而水分子的运动状态又影响氧化石墨烯片层的运动行为. 由于二氧化硅表面羟基取向角较大, 使得水分子在挤压剪切作用下偶极矩角变大, 从而导致其与氧化石墨烯片层之间的结合强度削弱, 二者之间出现相对滑移. 石墨烯片层运动行为的改变引起了剪切面的转变. 通过对石墨烯片层沿滑移方向上的速度波动幅度的分析, 发现其与摩擦系数之间存在正相关性.The ab initio molecular dynamics method is used to simulate the friction process of the graphene sheet as lubricant added to the silicon-based material interface under the action of compression and shear, and the influence of water molecules and oxidation of graphene surface on the movement behavior of graphene sheet are studied. In a dry environment, the pristine graphene (PG) sheet will slip only when the pressure is high. Owing to the presence of surface functional groups, a strong force is formed between the graphene oxide (GO) sheet and the substrate. The direction of the hydroxyl groups on the surface of the upper slab is consistent as the upper slab moves at a constant speed, resulting in the fact that the force between the GO sheet and the upper slab is greater and the GO sheet slides forward with the upper slab. Owing to the formation of mechanical interlock between the GO sheet and the lower slab surface, the GO sheet no longer slips when the pressure is high. In a humid environment, the interface structure affects the overall distribution and movement state of water molecules. The water molecules between the PG sheet and the upper slab are adsorbed only on the surface of the upper slab and always remain in a “flat” state, and their motion behavior is consistent with the upper slab’s. Comparing with a dry environment, the PG sheet starts to slip only when the pressure is high. Since the hydroxyl orientation angle on the surface of the upper slab is greater than the hydroxyl orientation angle on the surface of the GO sheet, the water molecules gradually change from the "flat" state to the slightly “upright” state as the pressure increases. The change of the orientation of water molecules makes the bonding strength between water molecules and the GO sheet gradually decrease, leading to a relative slip between them. The change in the movement behavior of the graphene sheet causes the shear plane to change. There is a positive correlation between the velocity fluctuation mean square error of the graphene sheet and the friction coefficient as the oxidation rate of graphene sheet increases under different coverages of water molecules, indicating that the motion behavior of the lubricant affects the interface friction characteristics.
-
Keywords:
- graphene oxide /
- water /
- silica /
- ab initio molecular dynamics
[1] Wang X D, Yu J X, Chen L, Qian L M, Zhou Z R 2011 Wear 271 1681
 Google Scholar
Google Scholar
[2] Chandross M, Lorenz C A, Grest G S, Stevens M J, Webb E B 2005 JOM 57 55
 Google Scholar
Google Scholar
[3] Chen H, Filleter T 2015 Nanotechnology 26 135702
 Google Scholar
Google Scholar
[4] Koenig S P, Boddeti N G, Dunn M L, Bunch J S 2011 Nat. Nanotechnol. 6 543
 Google Scholar
Google Scholar
[5] Li A, Liu Y, Szlufarska I 2014 Tribol. Lett. 56 481
 Google Scholar
Google Scholar
[6] Dong Y L, Wu X W, Martini A 2013 Nanotechnology 24 375701
 Google Scholar
Google Scholar
[7] Zeng X Z, Peng Y T, Yu M C, Lang H J, Cao X A, Zou K 2018 ACS Appl. Mater. Interfaces 10 8214
 Google Scholar
Google Scholar
[8] Ou J F, Wang Y, Wang J Q, Liu S, Li Z P, Yang S R 2011 J. Phys. Chem. C 115 10080
 Google Scholar
Google Scholar
[9] Arif T, Colas G, Filleter T 2018 ACS Appl. Mater. Interfaces 10 22537
 Google Scholar
Google Scholar
[10] Kim B I, Boehm R D, Bonander J R 2013 J. Chem. Phys. 139 054701
 Google Scholar
Google Scholar
[11] Ramin L, Jabbarzadeh A 2013 Langmuir 29 13367
 Google Scholar
Google Scholar
[12] Ootani Y, Xu J X, Hatano T, Kubo M 2018 J. Phys. Chem. C 122 10459
 Google Scholar
Google Scholar
[13] Hasz K, Ye Z J, Martini A, Carpick R W 2018 Phys. Rev. Mater. 2 126001
 Google Scholar
Google Scholar
[14] Rana M K, Chandra A 2013 J. Chem. Phys. 138 204702
 Google Scholar
Google Scholar
[15] Lee H, Ko J H, Choi J S, Hwang J H, Kim Y H, Salmeron M, Park J Y 2017 J. Phys. Chem. Lett. 8 3482
 Google Scholar
Google Scholar
[16] Wang C, Qian C, Li Z, Wei N, Zhang N, Wang Y, He H 2020 Ind. Eng. Chem. Res. 59 8028
 Google Scholar
Google Scholar
[17] Cheng Y, Ma M 2020 Phys. Rev. Mater. 4 113606
 Google Scholar
Google Scholar
[18] van Wijk M M, de Wijn A S, Fasolino A 2016 J. Phys.: Condens. Matter 28 134007
 Google Scholar
Google Scholar
[19] Levita G, Righi M C 2017 ChemPhysChem 18 1475
 Google Scholar
Google Scholar
[20] Car R, Parrinello M 1985 Phys. Rev. Lett. 55 2471
 Google Scholar
Google Scholar
[21] Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti G L, Cococcioni M, Dabo I, Dal Corso A, de Gironcoli S, Fabris S, Fratesi G, Gebauer R, Gerstmann U, Gougoussis C, Kokalj A, Lazzeri M, Martin-Samos L, Marzari N, Mauri F, Mazzarello R, Paolini S, Pasquarello A, Paulatto L, Sbraccia C, Scandolo S, Sclauzero G, Seitsonen A P, Smogunov A, Umari P, Wentzcovitch R M 2009 J. Phys.: Condens. Matter 21 395502
 Google Scholar
Google Scholar
[22] Yue D C, Ma T B, Hu Y Z, Yeon J, van Duin A C T, Wang H, Luo J B 2013 J. Phys. Chem. C 117 25604
 Google Scholar
Google Scholar
[23] Lu N, Yin D, Li Z, Yang J 2011 J. Phys. Chem. C 115 11991
 Google Scholar
Google Scholar
[24] Gongyang Y, Qu C, Zhang S, Ma M, Zheng Q 2018 Carbon 132 444
 Google Scholar
Google Scholar
[25] Humphrey W, Dalke A, Schulten K 1996 J. Mol. Graph. 14 33
 Google Scholar
Google Scholar
[26] Chateauneuf G M, Mikulski P T, Gao G T, Harrison J A 2004 J. Phys. Chem. B 108 16626
 Google Scholar
Google Scholar
[27] Gao W, Xiao P H, Henkelman G, Liechti K M, Huang R 2014 J. Phys. D: Appl. Phys. 47 255301
 Google Scholar
Google Scholar
[28] Lee M J, Choi J S, Kim J, Byun I, Lee D H, Ryu S, Lee C, Park B H 2012 Nano Res. 5 710
 Google Scholar
Google Scholar
[29] McKenzie S, Kang H C 2014 Phys. Chem. Chem. Phys. 16 26004
 Google Scholar
Google Scholar
[30] Yang J J, Wang E G 2006 Phys. Rev. B 73 035406
 Google Scholar
Google Scholar
[31] Bonnaud P A, Coasne B, Pellenq R J M 2010 J. Phys.: Condens. Matter 22 284110
 Google Scholar
Google Scholar
[32] Notman R, Walsh T R 2009 Langmuir 25 1638
 Google Scholar
Google Scholar
[33] Cimas A, Tielens F, Sulpizi M, Gaigeot M P, Costa D 2014 J. Phys.: Condens. Matter 26 244106
 Google Scholar
Google Scholar
[34] Kajita S, Righi M C 2016 Tribol. Lett. 61 17
 Google Scholar
Google Scholar
[35] Lee D, Ahn G, Ryu S 2014 J. Am. Chem. Soc. 136 6634
 Google Scholar
Google Scholar
[36] Lang H, Peng Y, Zeng X, Cao X A, Liu L, Zou K 2018 Carbon 137 519
 Google Scholar
Google Scholar
[37] Peng Y, Wang Z, Zou K 2015 Langmuir 31 7782
 Google Scholar
Google Scholar
-
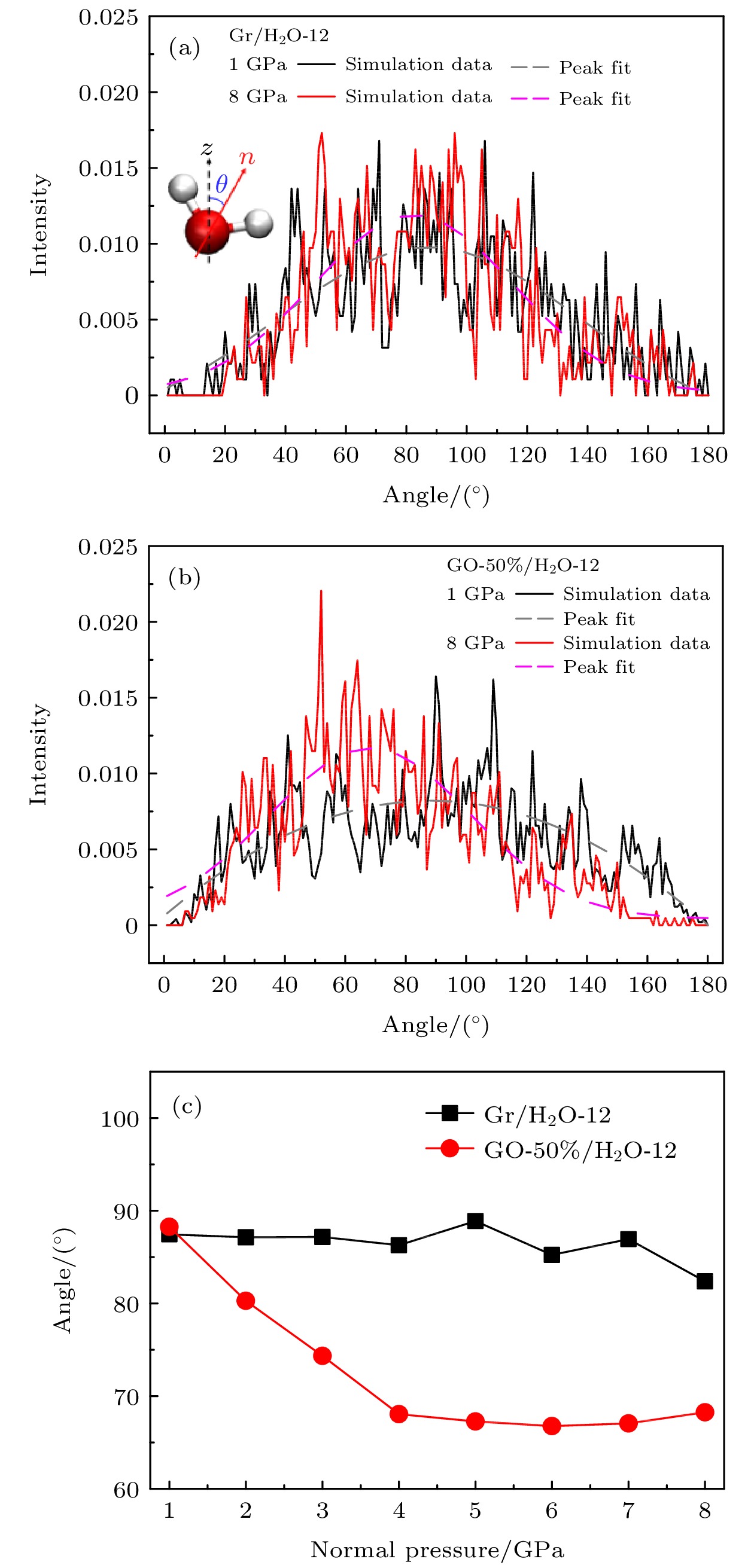
图 5 水分子偶极矩角在不同压强下的概率分布 (a) Gr/H2O-12模型; (b) GO-50%/H2O-12模型; (c)水分子偶极矩角拟合峰值随压强变化
Fig. 5. Probability distribution of the dipole moment angle of water molecules under different pressure in the model: (a) Gr/H2O-12; (b) GO-50%/H2O-12; (c) fitting peak values of the dipole momentangle of water molecule change with pressure.
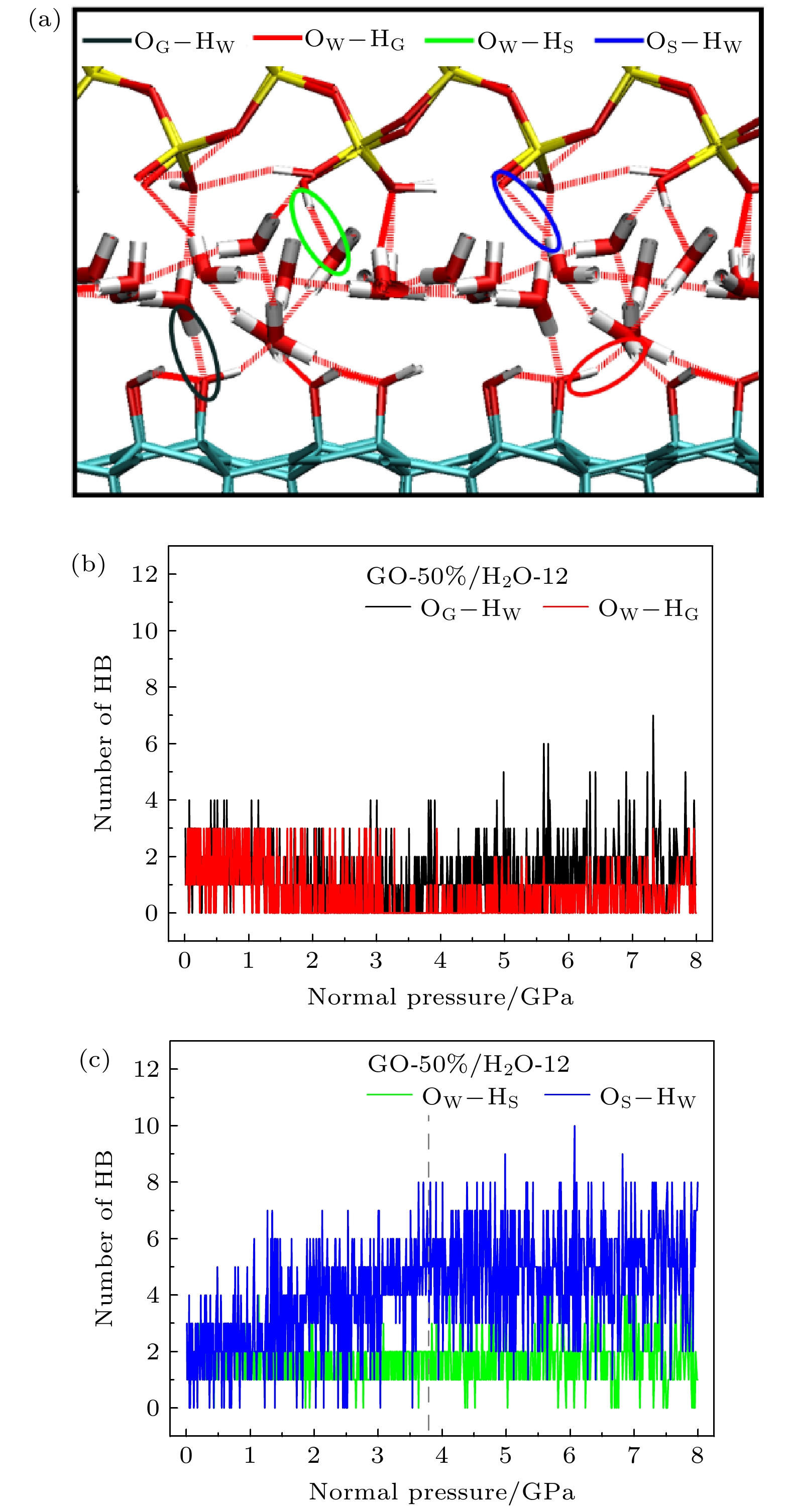
图 6 (a) 潮湿环境下不同类型的界面氢键; GO片层/水分子之间(b)及水分子/SiO2上基底之间(c)氢键数量随压强变化
Fig. 6. (a) Different patterns of interfacial hydrogen bonds in a humid environment; changes of numbers of hydrogen bonds between the GO sheet and water molecules (b) and between water molecules and the SiO2 upper slab (c) with pressure.
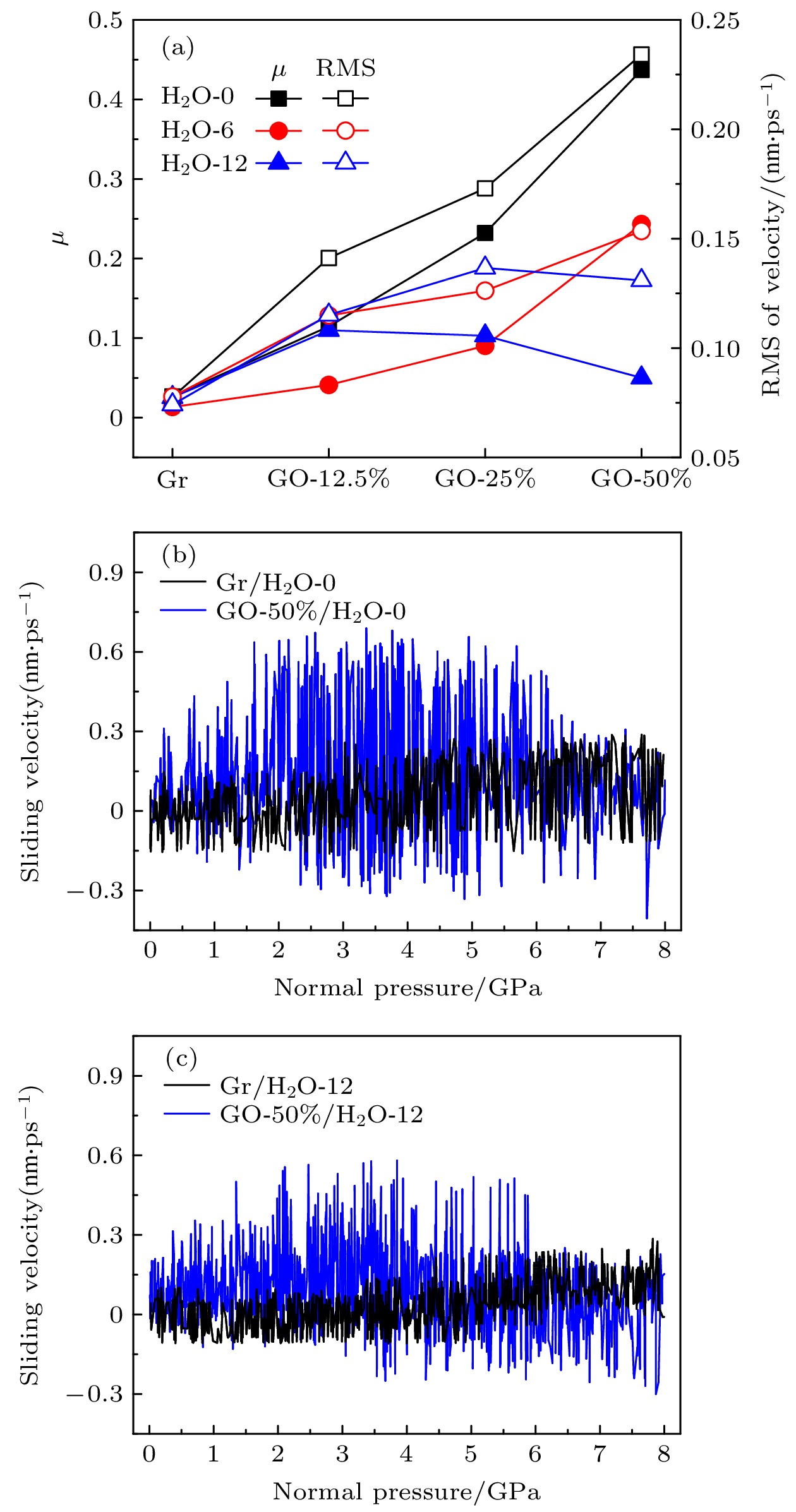
图 9 (a)不同水分子覆盖率下石墨烯片层的速度波动均方差与摩擦系数随石墨烯氧化率变化; 干燥环境(b)和潮湿环境(c)下石墨烯片层速度随压强变化
Fig. 9. (a) Velocity fluctuation mean square error of the graphene sheet and the friction coefficient changes with the graphene oxidation rate under different water molecule coverage; The velocity of graphene sheet changes with pressure in a (b) dry and (c) humid environment.
-
[1] Wang X D, Yu J X, Chen L, Qian L M, Zhou Z R 2011 Wear 271 1681
 Google Scholar
Google Scholar
[2] Chandross M, Lorenz C A, Grest G S, Stevens M J, Webb E B 2005 JOM 57 55
 Google Scholar
Google Scholar
[3] Chen H, Filleter T 2015 Nanotechnology 26 135702
 Google Scholar
Google Scholar
[4] Koenig S P, Boddeti N G, Dunn M L, Bunch J S 2011 Nat. Nanotechnol. 6 543
 Google Scholar
Google Scholar
[5] Li A, Liu Y, Szlufarska I 2014 Tribol. Lett. 56 481
 Google Scholar
Google Scholar
[6] Dong Y L, Wu X W, Martini A 2013 Nanotechnology 24 375701
 Google Scholar
Google Scholar
[7] Zeng X Z, Peng Y T, Yu M C, Lang H J, Cao X A, Zou K 2018 ACS Appl. Mater. Interfaces 10 8214
 Google Scholar
Google Scholar
[8] Ou J F, Wang Y, Wang J Q, Liu S, Li Z P, Yang S R 2011 J. Phys. Chem. C 115 10080
 Google Scholar
Google Scholar
[9] Arif T, Colas G, Filleter T 2018 ACS Appl. Mater. Interfaces 10 22537
 Google Scholar
Google Scholar
[10] Kim B I, Boehm R D, Bonander J R 2013 J. Chem. Phys. 139 054701
 Google Scholar
Google Scholar
[11] Ramin L, Jabbarzadeh A 2013 Langmuir 29 13367
 Google Scholar
Google Scholar
[12] Ootani Y, Xu J X, Hatano T, Kubo M 2018 J. Phys. Chem. C 122 10459
 Google Scholar
Google Scholar
[13] Hasz K, Ye Z J, Martini A, Carpick R W 2018 Phys. Rev. Mater. 2 126001
 Google Scholar
Google Scholar
[14] Rana M K, Chandra A 2013 J. Chem. Phys. 138 204702
 Google Scholar
Google Scholar
[15] Lee H, Ko J H, Choi J S, Hwang J H, Kim Y H, Salmeron M, Park J Y 2017 J. Phys. Chem. Lett. 8 3482
 Google Scholar
Google Scholar
[16] Wang C, Qian C, Li Z, Wei N, Zhang N, Wang Y, He H 2020 Ind. Eng. Chem. Res. 59 8028
 Google Scholar
Google Scholar
[17] Cheng Y, Ma M 2020 Phys. Rev. Mater. 4 113606
 Google Scholar
Google Scholar
[18] van Wijk M M, de Wijn A S, Fasolino A 2016 J. Phys.: Condens. Matter 28 134007
 Google Scholar
Google Scholar
[19] Levita G, Righi M C 2017 ChemPhysChem 18 1475
 Google Scholar
Google Scholar
[20] Car R, Parrinello M 1985 Phys. Rev. Lett. 55 2471
 Google Scholar
Google Scholar
[21] Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti G L, Cococcioni M, Dabo I, Dal Corso A, de Gironcoli S, Fabris S, Fratesi G, Gebauer R, Gerstmann U, Gougoussis C, Kokalj A, Lazzeri M, Martin-Samos L, Marzari N, Mauri F, Mazzarello R, Paolini S, Pasquarello A, Paulatto L, Sbraccia C, Scandolo S, Sclauzero G, Seitsonen A P, Smogunov A, Umari P, Wentzcovitch R M 2009 J. Phys.: Condens. Matter 21 395502
 Google Scholar
Google Scholar
[22] Yue D C, Ma T B, Hu Y Z, Yeon J, van Duin A C T, Wang H, Luo J B 2013 J. Phys. Chem. C 117 25604
 Google Scholar
Google Scholar
[23] Lu N, Yin D, Li Z, Yang J 2011 J. Phys. Chem. C 115 11991
 Google Scholar
Google Scholar
[24] Gongyang Y, Qu C, Zhang S, Ma M, Zheng Q 2018 Carbon 132 444
 Google Scholar
Google Scholar
[25] Humphrey W, Dalke A, Schulten K 1996 J. Mol. Graph. 14 33
 Google Scholar
Google Scholar
[26] Chateauneuf G M, Mikulski P T, Gao G T, Harrison J A 2004 J. Phys. Chem. B 108 16626
 Google Scholar
Google Scholar
[27] Gao W, Xiao P H, Henkelman G, Liechti K M, Huang R 2014 J. Phys. D: Appl. Phys. 47 255301
 Google Scholar
Google Scholar
[28] Lee M J, Choi J S, Kim J, Byun I, Lee D H, Ryu S, Lee C, Park B H 2012 Nano Res. 5 710
 Google Scholar
Google Scholar
[29] McKenzie S, Kang H C 2014 Phys. Chem. Chem. Phys. 16 26004
 Google Scholar
Google Scholar
[30] Yang J J, Wang E G 2006 Phys. Rev. B 73 035406
 Google Scholar
Google Scholar
[31] Bonnaud P A, Coasne B, Pellenq R J M 2010 J. Phys.: Condens. Matter 22 284110
 Google Scholar
Google Scholar
[32] Notman R, Walsh T R 2009 Langmuir 25 1638
 Google Scholar
Google Scholar
[33] Cimas A, Tielens F, Sulpizi M, Gaigeot M P, Costa D 2014 J. Phys.: Condens. Matter 26 244106
 Google Scholar
Google Scholar
[34] Kajita S, Righi M C 2016 Tribol. Lett. 61 17
 Google Scholar
Google Scholar
[35] Lee D, Ahn G, Ryu S 2014 J. Am. Chem. Soc. 136 6634
 Google Scholar
Google Scholar
[36] Lang H, Peng Y, Zeng X, Cao X A, Liu L, Zou K 2018 Carbon 137 519
 Google Scholar
Google Scholar
[37] Peng Y, Wang Z, Zou K 2015 Langmuir 31 7782
 Google Scholar
Google Scholar
计量
- 文章访问数: 9270
- PDF下载量: 119
- 被引次数: 0













 下载:
下载: