-
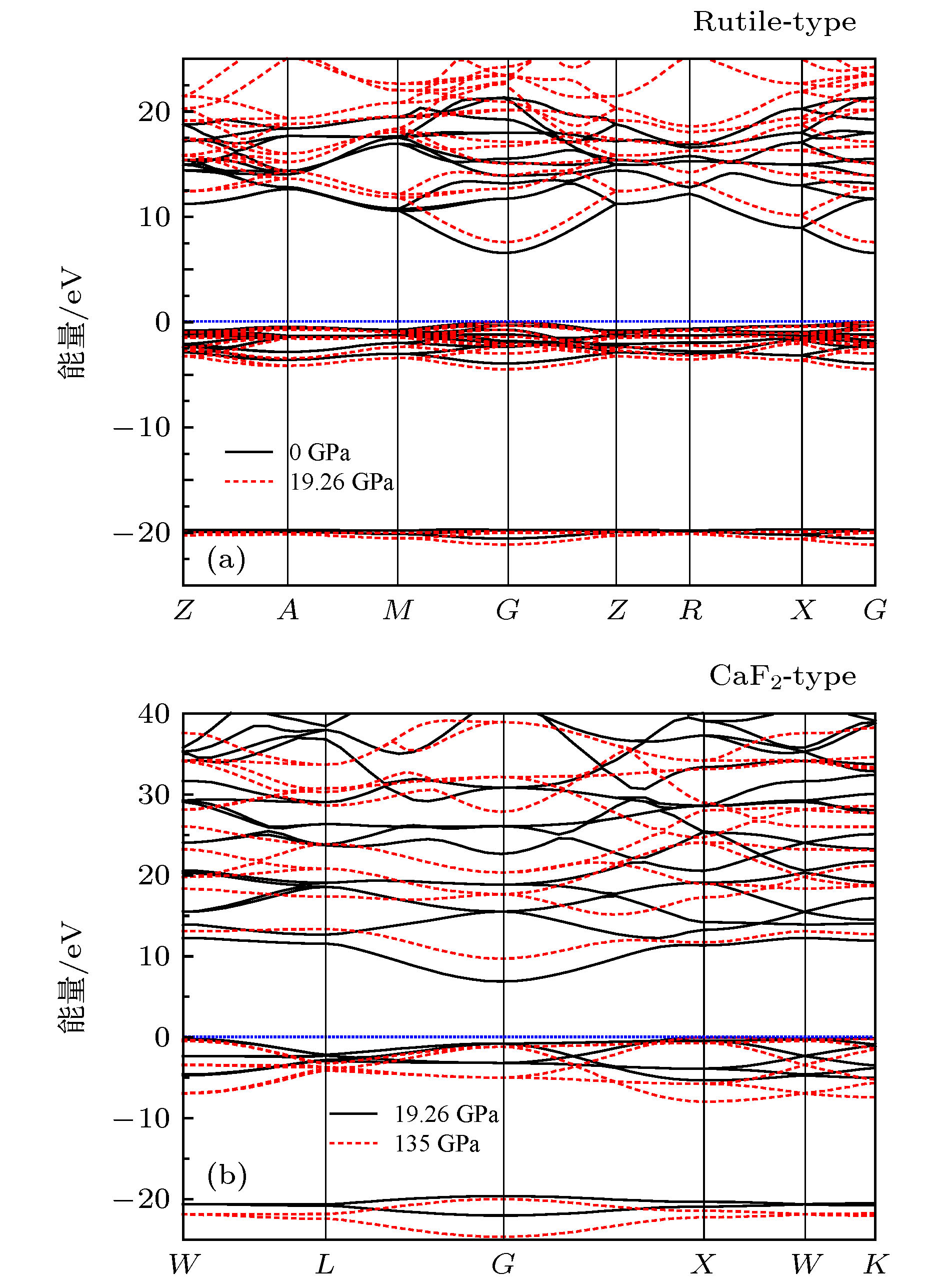
MgF2 is an important member of alkaline-earth fluorides and has a wide range of applications in industry. Meanwhile, MgF2 occurs naturally as a mineral sellaite. Compared with the study of its electronic structure and optical properties, the researches of the behavior under high pressure of MgF2, especially the thermodynamic properties are still limited. The high-pressure melting, volume thermal expansion coefficient, and thermoelastic parameter of the Earth’s lower mantle mineral, like MgF2, are of interest and importance for understanding the physical nature of the functional material and for recognizing the structural compositions, dynamics, evolution and origin of the earth. Using the first-principles calculations based on density functional theory, the thermodynamic, mechanical, and dynamic stability of the fluorite-type structure for MgF2 are systematically studied. The calculations indicate that the fluorite-type structure is a high-pressure phase and it is stable at least up to 135 GPa. According to the principle of equal enthalpies, the phase transition pressure of MgF2 crystal from stable rutile structure to high pressure fluorite structure is determined to be 19.26 GPa and 18.15 GPa based on the the generalized gradient approximation and local density approximation calculations, respectively. The high-temperature structural stability of MgF2 with the fluorite-type structure is investigated and confirmed by using the classical molecular dynamics (MD) simulations by taking into account the molar volume and total energy change behavior in a temperature range from 300 to 6000 K. On the basis of previous research, the volume thermal expansion coefficient, isothermal bulk modulus, and thermoelastic parameter of MgF2 with the CaF2-type fluorite structure are predicted systematically in a temperature range from 300 to 1500 K and in a pressure range from 0 to 135 GPa with the help of the generalized gradient approximation of the revised Perdew-Burke-Ernzerhof form combined with quasiharmonic Debye model calculations and the molecular dynamics method combined with reliable interatomic potentials. An important discovery is that the thermoelastic parameter of this material under low temperature and low pressure is not a constant as assumed usually in previous studies of the equation of states, but it approaches to a constant under both high temperature and high pressure.
-
Keywords:
- MgF2 /
- structural phase transition /
- thermodynamics properties /
- high temperature and pressure
[1] Appel R, Dyer C D, Lockwood J N 2002 Appl. Opt. 41 2470
 Google Scholar
Google Scholar
[2] Arroussi A, Ghezali M 2018 Optik 164 16
[3] Wojciechowska M, Zieliński M, Pietrowski M 2003 J. Fluorine Chem. 120 1
 Google Scholar
Google Scholar
[4] Sun X W, Liu Z J, Song T, Quan W L, Chen Q F 2012 Phys. Scr. 85 065707
 Google Scholar
Google Scholar
[5] Haines J, Léger J M, Gorelli F, Klug D D, Tse J S, Li Z Q 2001 Phys. Rev. B 64 134110
 Google Scholar
Google Scholar
[6] Ming L C, Manghani M H 1979 Geophys. Res. Lett. 6 13
 Google Scholar
Google Scholar
[7] Öztürk H, Kürkçü C, Kürkçü C 2014 J. Alloys Compd. 609 185
 Google Scholar
Google Scholar
[8] Nelson J R, Needs R J, Pickard C J 2017 Phys. Rev. B 95 054118
 Google Scholar
Google Scholar
[9] Allan N L, Hines R I, Towler M D, Mackrodt W C 1994 J. Chem. Phys. 100 4710
 Google Scholar
Google Scholar
[10] Nishidate K, Baba M, Sato T, Nishikawa K 1995 Phys. Rev. B 52 3170
[11] Catti M, Pavese A, Dovesi R, Roetti C, Causà M, 1991 Phys. Rev. B 44 3509
 Google Scholar
Google Scholar
[12] Nga Y A, Ong C K, 1993 J. Chem. Phys. 98 3240
 Google Scholar
Google Scholar
[13] Barrera G D, Taylor M B, Allan N L, Barron T H K, Kantorovich L N, Mackrodt W C 1997 J. Chem. Phys. 107 4337
 Google Scholar
Google Scholar
[14] Tian J H, Song T, Sun X W, Liu Z J, Quan W L, Guo P 2012 Physica B 407 551
 Google Scholar
Google Scholar
[15] Sun X W, Song T, Wei X P, Quan W L, Liu X B, Su W F 2014 Mater. Res. Bull. 52 151
 Google Scholar
Google Scholar
[16] Lin J F, Speziale S, Mao Z, Marquardt H 2013 Rev. Geophys. 51 244
 Google Scholar
Google Scholar
[17] Segall M D, Lindan P J, Probert M J, Pickard1C J, Hasnip P J, Clark S J, Payne M C 2002 J. Phys. Condens. Matter 14 2717
 Google Scholar
Google Scholar
[18] Ceperley D M, Alder B 1980 Phys. Rev. Lett. 45 566
 Google Scholar
Google Scholar
[19] Perdew J P, Zunger A 1981 Phys. Rev. B 23 5048
 Google Scholar
Google Scholar
[20] Perdew J P, Ruzsinszky A, Csonka G I, Vydrov O A, Scuseria G E, Constantin L A, Zhou X, Burke K 2008 Phys. Rev. Lett. 100 136406
 Google Scholar
Google Scholar
[21] Vanderbilt D 1990 Phys. Rev. B 41 7892
 Google Scholar
Google Scholar
[22] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[23] Fischer T H, Almlof J 1992 J. Phys. Chem. 96 9768
 Google Scholar
Google Scholar
[24] Gonze X, Lee C 1997 Phys. Rev. B 55 10355
 Google Scholar
Google Scholar
[25] Karki B B, Ackland G J, Crain J 1997 J. Phys. Condens. Matter 9 8579
 Google Scholar
Google Scholar
[26] Fincham D 1992 Mol. Simul. 8 165
 Google Scholar
Google Scholar
[27] 宋婷, 孙小伟, 魏小平, 欧阳玉花, 张春林, 郭鹏, 赵炜 2019 68 126201
 Google Scholar
Google Scholar
Song T, Sun X W, Wei X P, Ouyang Y H, Zhang C L, Guo P, Zhao W 2019 Acta Phys. Sin. 68 126201
 Google Scholar
Google Scholar
[28] Cazorla C, Errandonea D 2013 J. Phys. Chem. C 117 11292
[29] Song T, Sun X W, Liu Z J, Li J F, Tian J H 2012 Chin. Phys. B 21 037103
 Google Scholar
Google Scholar
[30] 孙小伟, 褚衍东, 刘子江, 刘玉孝, 王成伟, 刘维民 2005 54 5830
 Google Scholar
Google Scholar
Sun X W, Chu Y D, Liu Z J, Liu Y X, Wang C W, Liu W M 2005 Acta Phys. Sin. 54 5830
 Google Scholar
Google Scholar
[31] 张计划, 丁建文, 卢章辉 2009 58 1901
 Google Scholar
Google Scholar
Zhang J H, Ding J W, Lu Z H 2009 Acta Phys. Sin. 58 1901
 Google Scholar
Google Scholar
[32] Simanovskii D M, Schwettman H A 2003 Phys. Rev. Lett. 91 107601
 Google Scholar
Google Scholar
[33] Wang J, Yip S, Phillpot S R, Wolf D 1993 Phys. Rev. Lett. 71 4182
 Google Scholar
Google Scholar
[34] Blanco M, Francisco E, Luana V 2004 Comput. Phys. Commun. 158 57
 Google Scholar
Google Scholar
[35] Liu M, Lee C, Kaneko M, Nakahira K, Takano Y 2006 Appl. Opt. 45 1368
 Google Scholar
Google Scholar
[36] Sun X W, Liu Z J, Chen Q F, Quan W L, Chen Z G, Li Y H 2009 Mater. Res. Bull. 44 1729
 Google Scholar
Google Scholar
-
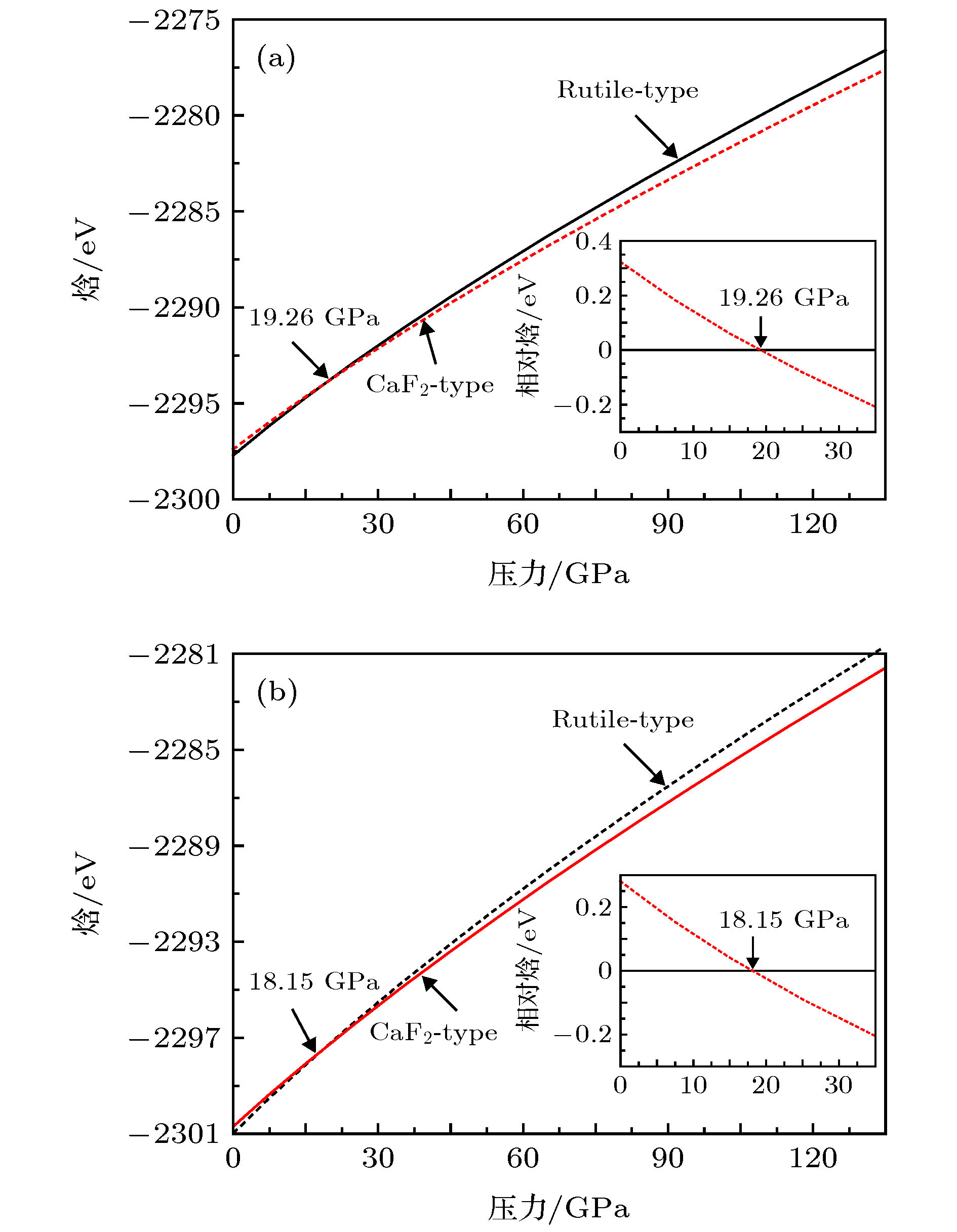
图 2 利用(a) GGA和(b) LDA方法分别计算的MgF2晶体金红石结构和萤石结构零温下的焓随压力的关系, 内插图分别为两种结构的MgF2每个分子式的相对焓随压力的变化
Figure 2. Calculated enthalpy as a function of pressure in the framework of (a) GGA and (b) LDA for MgF2 with the rutile-type and fluorite-type structures at zero temperature. In the inset, the relative enthalpy versus pressure is presented.
图 6 利用分子动力学模拟和第一性原理计算得到的MgF2萤石结构 (a) 在300 K下的体积比率随压力的变化和(b) 在50 GPa下的体积比率随温度的变化, 内插图为0.1 MPa下的模拟结果
Figure 6. Volume ratios of MgF2 with the fluorite-type structure obtained from molecular dynamics simulations and first-principles calculations: (a) Volume ratios under different pressures at 300 K; (b) volume ratios under different temperatures at 50 GPa, where in the inset, the data at 0.1 MPa is presented.
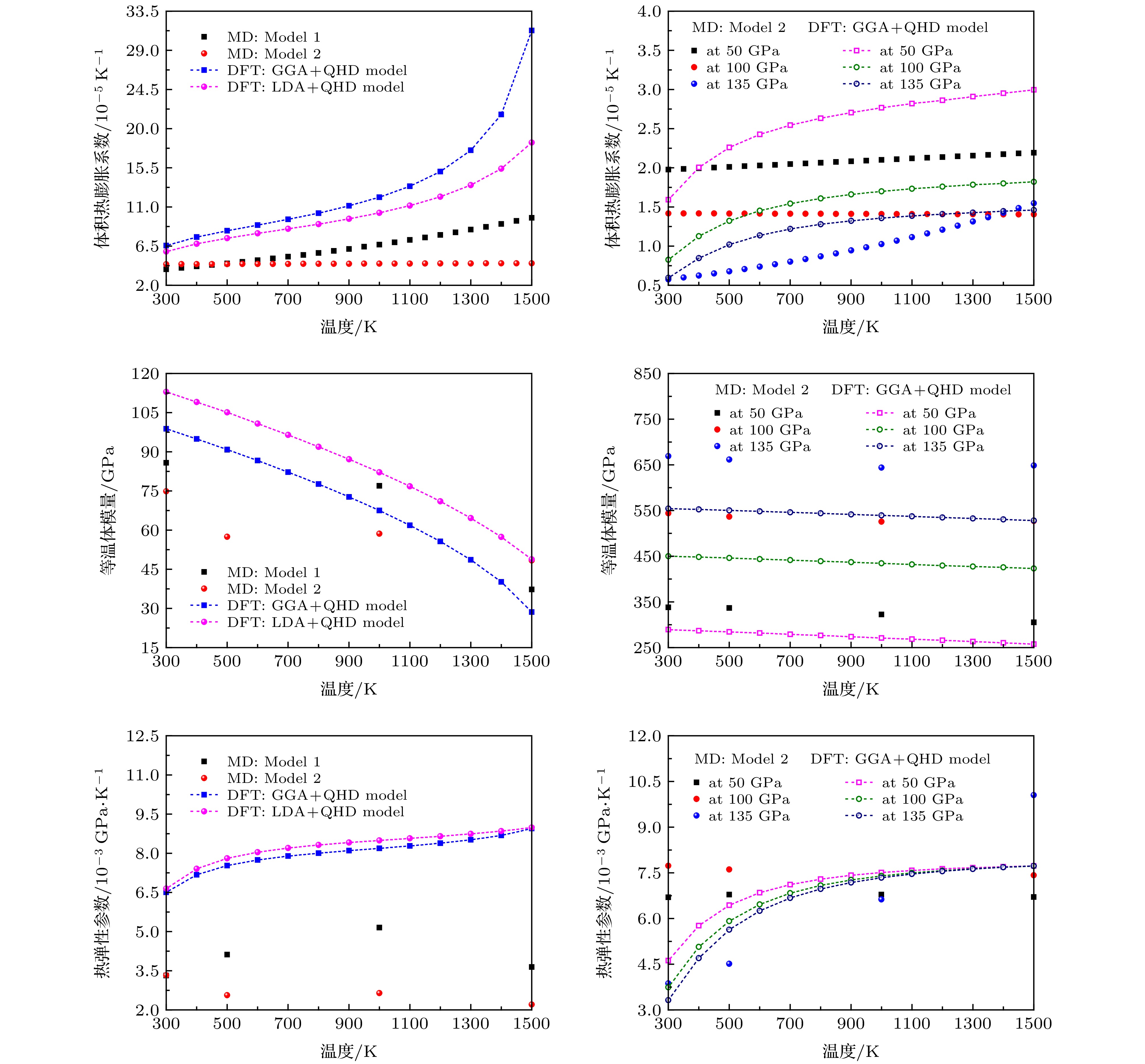
图 10 模拟得到的300 K及其他不同高温(500, 1000和1500 K)下的MgF2萤石结构的体积热膨胀系数、等温体模量、热弹性参数随压力的变化
Figure 10. Predicted volume thermal expansion coefficient α, isothermal bulk modulus KT, and thermoelastic parameter αKT of MgF2 with the fluorite-type structure as a function of pressure at 300 K and other different temperatures (500, 1000 and 1500 K).
图 11 模拟得到的环境压力下及其他不同高压(50, 100和135 GPa)下的MgF2萤石结构的体积热膨胀系数、等温体模量、热弹性参数随温度的变化
Figure 11. Predicted volume thermal expansion coefficient α, isothermal bulk modulus KT, and thermoelastic parameter αKT of MgF2 with the fluorite-type structure as a function of temperature at 0.1 MPa and other different pressures (50, 100 and 135 GPa).
-
[1] Appel R, Dyer C D, Lockwood J N 2002 Appl. Opt. 41 2470
 Google Scholar
Google Scholar
[2] Arroussi A, Ghezali M 2018 Optik 164 16
[3] Wojciechowska M, Zieliński M, Pietrowski M 2003 J. Fluorine Chem. 120 1
 Google Scholar
Google Scholar
[4] Sun X W, Liu Z J, Song T, Quan W L, Chen Q F 2012 Phys. Scr. 85 065707
 Google Scholar
Google Scholar
[5] Haines J, Léger J M, Gorelli F, Klug D D, Tse J S, Li Z Q 2001 Phys. Rev. B 64 134110
 Google Scholar
Google Scholar
[6] Ming L C, Manghani M H 1979 Geophys. Res. Lett. 6 13
 Google Scholar
Google Scholar
[7] Öztürk H, Kürkçü C, Kürkçü C 2014 J. Alloys Compd. 609 185
 Google Scholar
Google Scholar
[8] Nelson J R, Needs R J, Pickard C J 2017 Phys. Rev. B 95 054118
 Google Scholar
Google Scholar
[9] Allan N L, Hines R I, Towler M D, Mackrodt W C 1994 J. Chem. Phys. 100 4710
 Google Scholar
Google Scholar
[10] Nishidate K, Baba M, Sato T, Nishikawa K 1995 Phys. Rev. B 52 3170
[11] Catti M, Pavese A, Dovesi R, Roetti C, Causà M, 1991 Phys. Rev. B 44 3509
 Google Scholar
Google Scholar
[12] Nga Y A, Ong C K, 1993 J. Chem. Phys. 98 3240
 Google Scholar
Google Scholar
[13] Barrera G D, Taylor M B, Allan N L, Barron T H K, Kantorovich L N, Mackrodt W C 1997 J. Chem. Phys. 107 4337
 Google Scholar
Google Scholar
[14] Tian J H, Song T, Sun X W, Liu Z J, Quan W L, Guo P 2012 Physica B 407 551
 Google Scholar
Google Scholar
[15] Sun X W, Song T, Wei X P, Quan W L, Liu X B, Su W F 2014 Mater. Res. Bull. 52 151
 Google Scholar
Google Scholar
[16] Lin J F, Speziale S, Mao Z, Marquardt H 2013 Rev. Geophys. 51 244
 Google Scholar
Google Scholar
[17] Segall M D, Lindan P J, Probert M J, Pickard1C J, Hasnip P J, Clark S J, Payne M C 2002 J. Phys. Condens. Matter 14 2717
 Google Scholar
Google Scholar
[18] Ceperley D M, Alder B 1980 Phys. Rev. Lett. 45 566
 Google Scholar
Google Scholar
[19] Perdew J P, Zunger A 1981 Phys. Rev. B 23 5048
 Google Scholar
Google Scholar
[20] Perdew J P, Ruzsinszky A, Csonka G I, Vydrov O A, Scuseria G E, Constantin L A, Zhou X, Burke K 2008 Phys. Rev. Lett. 100 136406
 Google Scholar
Google Scholar
[21] Vanderbilt D 1990 Phys. Rev. B 41 7892
 Google Scholar
Google Scholar
[22] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[23] Fischer T H, Almlof J 1992 J. Phys. Chem. 96 9768
 Google Scholar
Google Scholar
[24] Gonze X, Lee C 1997 Phys. Rev. B 55 10355
 Google Scholar
Google Scholar
[25] Karki B B, Ackland G J, Crain J 1997 J. Phys. Condens. Matter 9 8579
 Google Scholar
Google Scholar
[26] Fincham D 1992 Mol. Simul. 8 165
 Google Scholar
Google Scholar
[27] 宋婷, 孙小伟, 魏小平, 欧阳玉花, 张春林, 郭鹏, 赵炜 2019 68 126201
 Google Scholar
Google Scholar
Song T, Sun X W, Wei X P, Ouyang Y H, Zhang C L, Guo P, Zhao W 2019 Acta Phys. Sin. 68 126201
 Google Scholar
Google Scholar
[28] Cazorla C, Errandonea D 2013 J. Phys. Chem. C 117 11292
[29] Song T, Sun X W, Liu Z J, Li J F, Tian J H 2012 Chin. Phys. B 21 037103
 Google Scholar
Google Scholar
[30] 孙小伟, 褚衍东, 刘子江, 刘玉孝, 王成伟, 刘维民 2005 54 5830
 Google Scholar
Google Scholar
Sun X W, Chu Y D, Liu Z J, Liu Y X, Wang C W, Liu W M 2005 Acta Phys. Sin. 54 5830
 Google Scholar
Google Scholar
[31] 张计划, 丁建文, 卢章辉 2009 58 1901
 Google Scholar
Google Scholar
Zhang J H, Ding J W, Lu Z H 2009 Acta Phys. Sin. 58 1901
 Google Scholar
Google Scholar
[32] Simanovskii D M, Schwettman H A 2003 Phys. Rev. Lett. 91 107601
 Google Scholar
Google Scholar
[33] Wang J, Yip S, Phillpot S R, Wolf D 1993 Phys. Rev. Lett. 71 4182
 Google Scholar
Google Scholar
[34] Blanco M, Francisco E, Luana V 2004 Comput. Phys. Commun. 158 57
 Google Scholar
Google Scholar
[35] Liu M, Lee C, Kaneko M, Nakahira K, Takano Y 2006 Appl. Opt. 45 1368
 Google Scholar
Google Scholar
[36] Sun X W, Liu Z J, Chen Q F, Quan W L, Chen Z G, Li Y H 2009 Mater. Res. Bull. 44 1729
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 13462
- PDF Downloads: 163
- Cited By: 0















 DownLoad:
DownLoad: